Abstract
Aims
Increased proinflammatory cytokines after myocardial infarction augment the progression of heart failure (HF) and are of prognostic significance. Recently, we demonstrated that increased proinflammatory cytokines in the brains of HF rats increased paraventricular nucleus (PVN) superoxide and down-regulated neuronal nitric oxide synthase (nNOS), contributing to sympathoexcitation. In this study, we explored the possible roles of brain proinflammatory cytokines and their effects on modulating PVN neurotransmitters in the exaggerated sympathetic activity in HF.
Methods and results
Sprague–Dawley rats with HF or sham-operated control (SHAM) rats were treated for 4 weeks with a continuous intracerebroventricular (ICV) infusion of the cytokine blockers—pentoxifylline (PTX, 10 µg/h and 40 µg/h), etanercept (ETN, 5 µg/h and 10 µg/h), or vehicle. Another set of HF and SHAM rats were treated with intraperitoneal (ip) infusion of a similar dose of PTX or ETN. HF rats had increased neuronal excitation accompanied by higher levels of glutamate, norepinephrine (NE), and tyrosine hydroxylase (TH), and lower levels of γ-aminobutyric acid (GABA), nNOS, and 67-kDa isoform of glutamate decarboxylase (GAD67) in the PVN when compared with SHAM rats. Plasma cytokines, NE, epinephrine, angiotensin II, and renal sympathetic nerve activity (RSNA) were also increased in HF rats. ICV treatment with low doses of PTX or ETN attenuated, and high doses prevented, increases in levels of glutamate, NE, and TH, and decreases in levels of GABA, nNOS, and GAD67 in the PVN in HF rats. The same ICV treatments also attenuated the increased RSNA seen in HF rats. IP treatment with similar doses of PTX or ETN did not affect glutamate, NE, TH, GABA, nNOS, and GAD67 in the PVN and had no effect on RSNA of HF rats.
Conclusion
This study, for the first time, demonstrates that proinflammatory cytokines modulate neurotransmitters in the PVN and contribute to sympathoexcitation in HF.
Keywords: Neurotransmitters, Paraventricular nucleus of hypothalamus, Cytokines, Sympathetic nervous system, Heart failure
1. Introduction
One of the pathophysiological characteristics of chronic heart failure (HF) is increased sympathetic drive, which is a major contributor to the morbidity and mortality of HF patients. Recent evidence points to a central nervous system mechanism that contributes to the sympathetic abnormality in HF. In the brain, the paraventricular nucleus (PVN) of the hypothalamus is reciprocally connected to other areas of the central nervous system that are involved in cardiovascular function,1 and is an important central site for integration of sympathetic nerve activity2 and regulation of cardiovascular function.3
A number of excitatory and inhibitory neurotransmitters converge in the PVN to influence its neuronal activity,2 including glutamate, norepinephrine (NE), and gamma-aminobutyric acid (GABA). Glutamate is a well-known excitatory neurotransmitter in the central nervous system and it has been reported that functional glutamate receptors expressed in the PVN4,5 are involved in the control of cardiovascular reflexes.6,7 It has also been shown that sympathetic hyperactivity in rats with HF is associated with increased extracellular NE in the PVN.8,9 GABA is a well-known inhibitory neurotransmitter in the central nervous system and is a dominant inhibitory neurotransmitter within the PVN.10,11 Previous work by Patel and coworkers12 demonstrated a GABA-mediated inhibitory mechanism within the PVN contributing to sympathoexcitation in HF rats.
Our recent studies suggest that, in addition to the neurohormones, proinflammatory cytokines (PICs) are upregulated in the PVN and contribute to sympathoexcitation in HF.13,14 Tumour necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-6 share a common property of activating the hypothalamic–pituitary–adrenal axis15 and increasing sympathetic nerve activity. However, the mechanisms by which PICs modulate sympathetic activity in HF are not clear. Therefore, we hypothesized that increased PICs in the brains of HF rats caused an imbalance in the excitatory and inhibitory neurotransmitters in the PVN, contributing to increased sympathoexcitation in HF. To test this hypothesis, we inhibited brain TNF-α with centrally infused pentoxifylline (PTX), a cytokine synthesis inhibitor, or etanercept (ETN), a recombinant TNF-α receptor fusion protein, and measured neurotransmitters (glutamate, NE, and GABA) in the PVN in rats with ischaemia-induced HF and sham-operated controls.
2. Methods
2.1. Animals
Adult male Sprague–Dawley rats weighing 275–300 g were obtained from Harlan Sprague–Dawley (Indianapolis, IN, USA). Animals were housed in temperature (23 ± 2°C) and light-controlled (12 h light/dark cycle) animal quarters and were provided with tap water and rat chow ad libitum. All experimental procedures were approved by the Institutional Animal Care and Use Committees of Louisiana State University and Shanxi Medical University. This investigation conforms to the ‘Guide for the Care and Use of Laboratory Animals’ published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
2.2. Coronary ligation and cannula implantation
Rats underwent sterile surgery under anaesthesia (90 mg/kg ketamine+7.5 mg/kg xylazine, ip) for induction of HF by ligation of the left anterior descending coronary artery, or the same surgery without ligating the vessel (SHAM), as previously described.16 While still under anaesthesia, each rat had a cannula implanted in the right lateral cerebral ventricle for ICV infusion using stereotaxic coordinates. Animals received subcutaneous (sc) injection of buprenorphine (0.01 mg/kg) immediately after surgery and 12 h later.
2.3. Echocardiographic assessment of left ventricular function
Echocardiography was performed under ketamine (25 mg/kg, ip) sedation to assess left ventricular (LV) function as previously described.13,14,16 Ischaemic zone (IZ) was estimated by planimetry of the region of the LV endocardial silhouette, which demonstrated akinesis or dyskinesis, and expressed as a percentage of the whole (%IZ). From these measurements, %IZ, LV ejection fraction (LVEF), and LV end-diastolic volume (LVEDV), indices of severity of congestive HF were reported.
2.4. Drug infusion
Within 24 h of coronary ligation or sham operation, rats were anaesthetized (60 mg/kg ketamine+5 mg/kg xylazine, ip) and osmotic mini-pumps (Alzet) implanted sc. The mini-pump was connected to the lateral ventricle cannula for continuous infusion (0.25 µL/h) of PTX (10 or 40 µg/h), which is known to inhibit the production of TNF-α; ETN (5 or 10 µg/h), which binds with circulating TNF-α, thereby blocking the binding of TNF-α to its receptor; or artificial cerebrospinal fluid (aCSF) over a 4-week treatment period. A higher dose of ETN (20 µg/h) was also investigated, however, this dose caused mortality in all animals (n = 7) by the end of 2 weeks, hence only 5 and 10 µg/h doses were used in this study. The doses used in this study are based on a previous study14 and by performing pilot studies. Another set of HF and SHAM rats were treated for 4 weeks with a continuous ip infusion of 40 µg/h PTX, 10 µg/h ETN, or saline.
2.5. Tissue microdissection
Palkovits's microdissection procedure was used to isolate the PVN as described before.17 Briefly, the brain was sectioned serially in 300 µm increments from the bregma to lambda using a cryostat. The sections were transferred to coverslips and placed on a cold stage maintained at −10°C. The PVN was punched with the help of a stereotaxic atlas. Some of the microdissected PVN tissue was stored at −70°C until analysed for cytokines using enzyme-linked immunosorbent assay (ELISA). Some of the PVN tissue samples were thawed and homogenized in 150 µL of 0.1 M HClO4 using a microultrasonic cell disruptor and centrifuged at 10 000 g for 10 min, and the supernatant were extracted and stored at −70°C until analysed for neurotransmitters using high-performance liquid chromatography (HPLC).
2.6. Measurements of glutamate and GABA in PVN tissues
The concentrations of glutamate and GABA were measured using HPLC with electrochemical detection by the ECD-300 (Eicom Corporation, Japan) as previously described.18 An o-phthalaldehyde (OPA) solution (40 mM) was prepared by adding 13.5 mg OPA and 10 µL 2-mercaptoethanol to 2.5 mL of 0.1 M K2CO3 buffer (pH 9.5) with 10% ethanol. The solution was then stored at −4°C and diluted in 0.1 M K2CO3 to yield a 4 mM OPA solution. The dialysate (12 µL) was mixed with 3 µL of the 4 mM OPA solution and allowed to react for 3 min at 25°C incubation. Then 10 µL of the reaction mixture was applied to HPLC with an Eicompak SC-5ODS column (Eicom, Japan). Detection was accomplished with an ECD-300 (Eicom Corporation, Japan) with +700 mV Ag/AgCl electrodes. The elution buffer was composed of 0.1 M phosphate buffer, 30% methanol, and 0.5 mM EDTA (pH 6.5). Signals were recorded and quantified by the eDAQ Powerchrom 280 software (eDAQ Pty Ltd, Australia).
2.7. Measurement of NE in PVN tissues
The concentration of NE was measured using HPLC with electrochemical detection (HTEC-500, Eicom Corporation, Japan) as previously described.18,19 The isocratic mobile phase was composed of 83% 0.1 M citrate–acetate buffer (pH 3.9), 17% methanol, 140 mg/l sodium octane sulfonate, and 5 mg/l EDTA-2Na. The mobile phase was pumped at 230 µL/min with a Lachrom system (Eicom-Hitachi, Japan) and the separation was performed with a column (Eicompak SC-5ODS) reverse phase. The electrochemical detector (Gasket GS-25) was composed of a glassy carbon working set at 750 mV with reference to an Ag/AgCl electrode, and signals were recorded and quantified by the eDAQ Powerchrom 280 software (eDAQ Pty Ltd, Australia).
2.8. Immunohistochemical studies
Transverse sections from brains were obtained from the region approximately 1.80 mm from the bregma. Immunohistochemical labelling was performed in floating sections as described previously20 to identify Fra-like (Fra-LI, a marker of chronic neuronal activation; Santa Cruz Biotechnology), tyrosine hydroxylase (TH; Abcam Inc.), GAD67 (Abcam Inc.), and neuronal nitric oxide synthase (nNOS; Santa Cruz Biotechnology). For each animal, the positive neurons within the bilateral borders of the PVN were manually counted in three consecutive sections and an average value was reported. Fra-LI-, TH-, GAD67-, or nNOS-positive neurons within a window superimposed over the dorsal parvocellular (dpPVN), ventrolateral parvocellular (vlpPVN), and magnocellular (mPVN) subregions of the PVN and were counted similarly for data analysis.
2.9. Analysis of nNOS mRNA expression by real-time RT-PCR
Total RNA was extracted from the PVN tissues, and nNOS mRNA expression was determined by real-time RT-PCR and normalized to GAPDH levels as described previously.21,22
2.10. Measurement of circulating catecholamine levels
Plasma NE and epinephrine (EPI) were measured using HPLC as described previously.21,22
2.11. ELISA studies
Plasma and tissue cytokine levels were measured using ELISA (Biosource International Inc.) techniques, as described before.13,14,23 Plasma angiotensin II was measured using an EIA kit (Cayman Chemical Company) as described previously.13
2.12. Electrophysiological recordings and anatomical measurements
Arterial pressure, heart rate, and renal sympathetic nerve activity (RSNA) were recorded. The general methods for recording and data analysis have been described previously.21,24,25 Maximum RSNA was detected using an intravenous bolus administration of sodium nitroprusside (SNP, 10 µg).24 At the end of the experiment, the background noise, defined as the signal-recorded postmortem, was subtracted from actual RSNA and subsequently expressed as percent of maximum (in response to SNP).21,25 A 1-mm micromanometer-tipped catheter (Millar Instruments) was inserted via the right carotid artery. LV end-diastolic pressure (LVEDP) was derived from the LV pressure tracing. At the end of the experiments, the heart and the lungs were removed. The LV and the right ventricles (RV) and lungs were weighed. Weights of RV and lungs were expressed as a function of body weight (BW).
2.13. Statistical analysis
All data are expressed as mean ± SEM. Data were analysed by analysis of variance (ANOVA). Multiple testing was corrected for by using Tukey's test. The echocardiography data were analysed by ANOVA allowing for repeated measurement. A probability value of P < 0.05 was considered statistically significant.
3. Results
3.1. Echocardiography
At 24 h of coronary artery ligation or sham operation, rats assigned to treatment with PTX, ETN, and VEH were assigned based on echocardiographically defined LV function (Table 1). The infarct sizes in this study ranged from 40–50% of the LV. LVEF was significantly reduced, and LVEDV and LVEDV/mass ratio were significantly increased in rats with ischaemic injury assigned to PTX, ETN, or VEH, when compared with sham-operated rats assigned to those same treatments. At 24 h, however, there were no differences in LVEF, LVEDV, LVEDV/mass ratio, or %IZ among rats with ischaemic injury assigned to PTX, ETN vs. VEH treatment. At 4 weeks, LVEDV and LVEDV/mass ratio were significantly higher than the 24 h baseline values in the PTX-, ETN-, and VEH-treated HF rats, and LVEF was significantly lower in the VEH-treated HF rats. At 4 weeks, LVEF was higher in the high dose of ICV PTX- or ETN-treated HF rats when compared with the ICV aCSF-treated HF rats. However, there were no significant differences in LVEDV, LVEDV/mass ratio, or %IZ between the ICV PTX-, ICV ETN-, and ICV aCSF-treated HF rats. There were no significant differences in LVEF, LVEDV, LVEDV/mass ratio, or %IZ between the ip PTX-, ip ETN-, and ip saline-treated HF rats.
Table 1.
Echocardiographic measurements (n = 14)
| Groups | Measurements at 24 h |
Measurements at 4 weeks |
||||||
|---|---|---|---|---|---|---|---|---|
| LVEDV (mL) | LVEDV/mass | LVEF | IZ (%) | LVEDV (mL) | LVEDV/mass | LVEF | IZ (%) | |
| HF + ICV 10 µg/h PTX | 0.74 ± 0.09* | 1.01 ± 0.06* | 0.35 ± 0.04* | 45.4 ± 4* | 1.14 ± 0.08*,‡ | 1.46 ± 0.11*,‡ | 0.39 ± 0.04* | 45.1 ± 5* |
| HF + ICV 5 µg/h ETN | 0.72 ± 0.08* | 1.06 ± 0.08* | 0.37 ± 0.05* | 45.7 ± 4* | 1.16 ± 0.09*,‡ | 1.49 ± 0.12*,‡ | 0.37 ± 0.05* | 45.8 ± 5* |
| HF + ICV 40 µg/h PTX | 0.76 ± 0.09* | 1.04 ± 0.07* | 0.36 ± 0.04* | 45.8 ± 4* | 1.06 ± 0.08*,‡ | 1.32 ± 0.11*,‡ | 0.50 ± 0.05*,† | 42.6 ± 4* |
| HF + ICV 10 µg/h ETN | 0.75 ± 0.09* | 1.05 ± 0.06* | 0.38 ± 0.05* | 46.1 ± 4* | 1.09 ± 0.09*,‡ | 1.36 ± 0.12*,‡ | 0.48 ± 0.04*,† | 43.5 ± 4* |
| HF + ICV aCSF | 0.73 ± 0.09* | 1.05 ± 0.08* | 0.36 ± 0.05* | 45.8 ± 4* | 1.26 ± 0.09*,‡ | 1.57 ± 0.12*,‡ | 0.34 ± 0.04* | 47.3 ± 5* |
| SHAM + ICV 10 µg/h PTX | 0.35 ± 0.04 | 0.57 ± 0.06 | 0.83 ± 0.06 | — | 0.34 ± 0.04 | 0.55 ± 0.07 | 0.84 ± 0.07 | — |
| SHAM + ICV 5 µg/h ETN | 0.34 ± 0.04 | 0.56 ± 0.06 | 0.84 ± 0.07 | — | 0.35 ± 0.05 | 0.54 ± 0.06 | 0.85 ± 0.07 | — |
| SHAM + ICV 40 µg/h PTX | 0.35 ± 0.05 | 0.53 ± 0.07 | 0.82 ± 0.05 | — | 0.33 ± 0.05 | 0.50 ± 0.05 | 0.84 ± 0.06 | — |
| SHAM + ICV 10 µg/h ETN | 0.36 ± 0.06 | 0.55 ± 0.07 | 0.81 ± 0.07 | — | 0.35 ± 0.04 | 0.52 ± 0.06 | 0.83 ± 0.07 | — |
| SHAM + ICV aCSF | 0.35 ± 0.06 | 0.56 ± 0.06 | 0.83 ± 0.06 | — | 0.36 ± 0.05 | 0.59 ± 0.07 | 0.82 ± 0.06 | — |
| HF + IP 40 µg/h PTX | 0.75 ± 0.07* | 1.06 ± 0.07* | 0.37 ± 0.06* | 45.9 ± 4* | 1.15 ± 0.08*‡ | 1.44 ± 0.13*,‡ | 0.36 ± 0.05* | 44.8 ± 4* |
| HF + IP 10 µg/h ETN | 0.76 ± 0.08* | 1.07 ± 0.09* | 0.36 ± 0.06* | 47.1 ± 4* | 1.18 ± 0.09*,‡ | 1.47 ± 0.12*,‡ | 0.35 ± 0.05* | 46.2 ± 5* |
| HF + ip saline | 0.74 ± 0.08* | 1.04 ± 0.08* | 0.35 ± 0.05* | 46.2 ± 5* | 1.22 ± 0.08*,‡ | 1.52 ± 0.14*,‡ | 0.32 ± 0.04* | 47.9 ± 5* |
| SHAM + ip saline | 0.36 ± 0.05 | 0.56 ± 0.07 | 0.82 ± 0.06 | — | 0.35 ± 0.06 | 0.56 ± 0.07 | 0.84 ± 0.08 | — |
SHAM, sham-operated control; HF, heart failure; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; IZ%, percent ischaemic zone; ICV, intracerebroventricular; PTX, pentoxifylline; ETN, etanercept. Values are mean ± SEM.
*P < 0.05 vs. SHAM.
†P < 0.05 HF + treated vs. HF + VEH.
‡P < 0.05, 4 weeks vs. 24 h value.
3.2. Fra-LI activity, an indicator of chronic neuronal activation, in the PVN
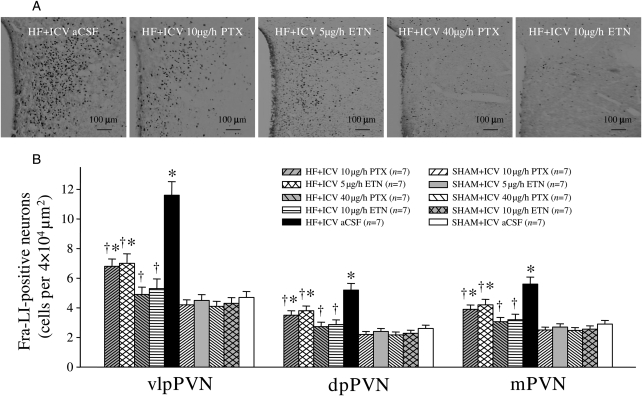
Fra-LI activity was higher in the PVN of VEH-treated HF rats when compared with VEH-treated SHAM rats. ICV treatment with PTX or ETN dose-dependently decreased the number of Fra-LI-positive PVN neurons of HF rats (Figure 1). However, ip treatment with the same doses of PTX or ETN did not alter the number of Fra-LI-positive PVN neurons.
Figure 1.
The effects of ICV PTX or ICV ETN on neuronal activity in the paraventricular nucleus (PVN) of rats with ischaemia-induced HF. Fra-LI activity (black dots), an indicator of chronic neuronal excitation, increased in the PVN in ICV aCSF-treated HF rats, compared with ICV aCSF-treated SHAM. Fra-LI activity was also lower in the ICV PTX- or ICV ETN-treated rats than in ICV aCSF-treated HF rats. *P < 0.05 vs. SHAM + treated or SHAM + ICV aCSF. †P < 0.05 HF + treated vs. HF + ICV aCSF.
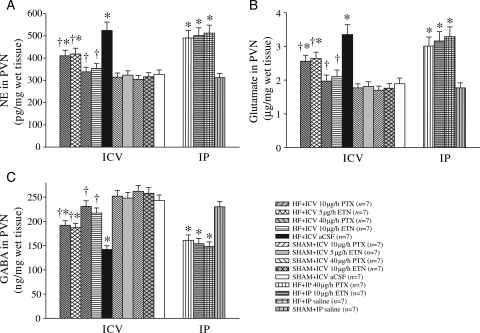
3.3. Neurotransmitters in the PVN
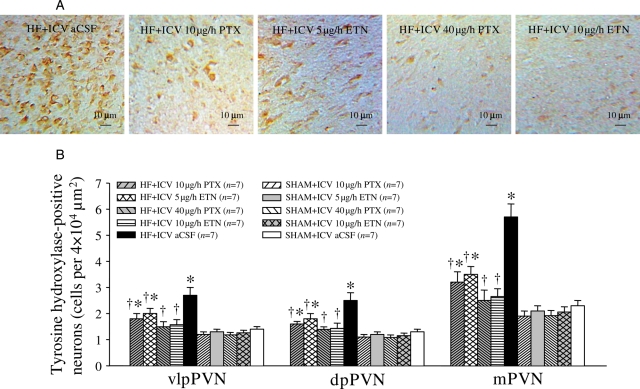
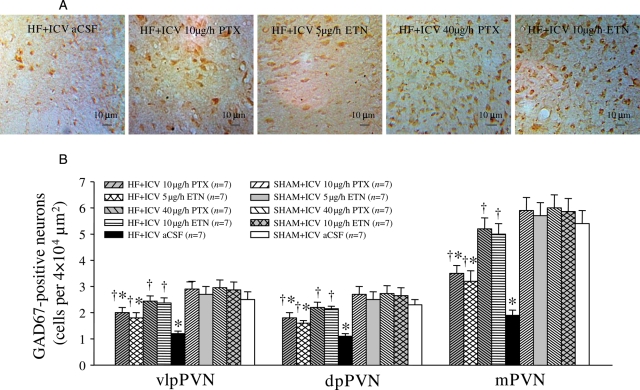
ICV aCSF-treated HF rats had higher levels of NE and glutamate, and lower levels of GABA in the PVN. NE and glutamate in the PVN were decreased, and GABA in the PVN was increased after treatment with ICV PTX or ICV ETN for 4 weeks (Figure 2). However, ip treatment with the same doses of PTX or ETN did not alter NE, glutamate, and GABA in the PVN of HF rats (Figure 2). Compared with SHAM rats, HF rats had higher levels of TH-immunoreactivity (Figure 3) and lower levels of GAD67 immunoreactivity (Figure 4) in the PVN. ICV treatment with PTX or ETN dose-dependently decreased the expression of TH (Figure 3) and increased GAD67 expression (Figure 4) in the PVN of HF rats. However, ip treatment with the same doses of PTX or ETN did not alter the expression of TH or GAD67 in the PVN of HF rats; expression of these proteins was similar to ICV vehicle-treated HF rats.
Figure 2.
PVN levels of NE, glutamate, and GABA in HF and sham operated (SHAM) rats treated for 4 weeks with ICV PTX, ICV ETN, or ICV aCSF. PVN levels of (A) NE, (B) glutamate, and (C) GABA were lower in HF rats treated with ICV PTX or ICV ETN. IP treatment with the same doses of PTX or ETN did not alter NE, glutamate, and GABA in the PVN of HF rats. *P < 0.05 vs. SHAM + treated or SHAM + ICV aCSF. †P < 0.05 HF + treated vs. HF+ICV aCSF.
Figure 3.
PVN levels of tyrosine hydroxylase (TH) immunoreactivity in HF and sham operated (SHAM) rats treated for 4 weeks with ICV PTX, ICV ETN, or ICV aCSF. PVN levels of TH immunoreactivity (brown) were lower in HF rats treated with ICV PTX or ICV ETN. *P < 0.05 vs. SHAM + treated or SHAM + ICV aCSF. †P < 0.05 HF + treated vs. HF + ICV aCSF.
Figure 4.
PVN levels of 67-kDa isoform of glutamate decarboxylase (GAD67) immunoreactivity in HF and sham-operated (SHAM) rats treated for 4 weeks with ICV PTX, ICV ETN, or ICV aCSF. PVN levels of GAD67 immunoreactivity (brown) were lower in HF rats treated with ICV PTX or ICV ETN. *P < 0.05 vs. SHAM + treated or SHAM + ICV aCSF. †P < 0.05 HF + treated vs. HF+ICV aCSF.
3.4. nNOS in the PVN
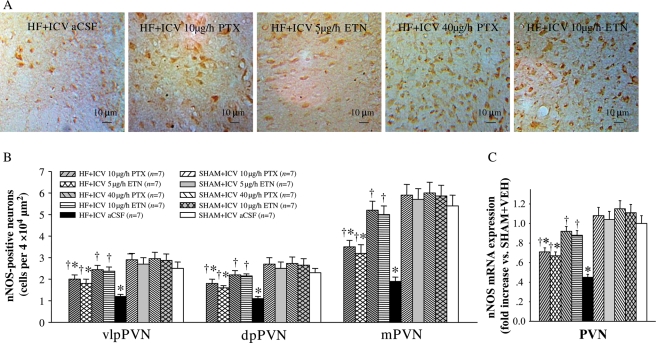
The mRNA expression of nNOS in the PVN was reduced in HF rats when compared with SHAM rats. ICV PTX or ICV ETN increased the mRNA expression of nNOS within the PVN of HF rats (Figure 5C). Immunohistochemistry studies revealed less nNOS-positive cells in the PVN in HF rats when compared with the SHAM groups; ICV treatment with PTX or ETN increased the number of nNOS-positive neurons in the PVN in HF rats (Figure 5A and B). However, ip treatment with the same doses of PTX or ETN had no effect on nNOS levels in the PVN.
Figure 5.
PVN levels of nNOS in HF and sham-operated (SHAM) rats treated for 4 weeks with ICV PTX, ICV ETN, or ICV aCSF. (A) Immunohistochemistry for nNOS-positive neurons (dark brown) in different groups. (B) Bar graph comparing nNOS-positive neurons in different groups. (C) nNOS mRNA expression in different groups. *P < 0.05 vs. SHAM + treated or SHAM + ICV aCSF. †P < 0.05 HF + treated vs. HF+ICV aCSF.
3.5. PVN levels of proinflammatory cytokines
PVN levels of TNF-α, IL-1β, and IL-6 were higher in HF when compared with SHAM rats. PVN levels of TNF-α, IL-1β, and IL-6 were lower in the ICV PTX or ICV ETN-treated HF rats, compared with ICV aCSF-treated HF rats (Table 2). IP treatment with the same doses of PTX or ETN had no effect on PICs levels in the PVN of HF rats (Table 2).
Table 2.
Proinflammatory cytokines in the PVN and plasma (n = 7)
| Measurements at 4 weeks | PVN (pg/mg protein) |
Plasma (pg/mL) |
||||||
|---|---|---|---|---|---|---|---|---|
| TNF-α | IL-1β | IL-6 | TNF-α | IL-1β | IL-6 | ANGII | ||
| HF + ICV 10 µg/h PTX | 5.1 ± 0.5*† | 33.7 ± 2.8*,† | 39.3 ± 3.6*,† | 12.9 ± 1.4† | 109.1 ± 9.6* | 90.7 ± 6.0* | 91.3 ± 8.6*,† | |
| HF + ICV 5 µg/h ETN | 5.5 ± 0.5*,† | 38.1 ± 3.4*,† | 43.4 ± 4.1*,† | 13.7 ± 1.5† | 115.3 ± 10.2* | 98.1 ± 8.8* | 95.1 ± 9.1*,† | |
| HF + ICV 40 µg/h PTX | 3.9 ± 0.4† | 21.8 ± 2.5† | 25.7 ± 2.3† | 12.5 ± 1.4† | 69.3 ± 6.1† | 44.2 ± 3.9† | 66.2 ± 5.6† | |
| HF + ICV 10 µg/h ETN | 4.1 ± 0.4† | 22.4 ± 2.6† | 26.9 ± 2.5† | 13.0 ± 1.5† | 67.3 ± 6.0† | 46.1 ± 4.0† | 68.7 ± 5.7† | |
| HF + ICV aCSF | 7.5 ± 0.6* | 54.2 ± 4.7* | 66.5 ± 6.4* | 37.7 ± 3.5* | 122.8 ± 10.9* | 102.1 ± 9.7* | 125.4 ± 10.2* | |
| SHAM + ICV 10 µg/h PTX | 3.4 ± 0.3 | 16.7 ± 1.4 | 18.6 ± 1.8 | 10.8 ± 1.2 | 55.7 ± 4.5 | 36.9 ± 3.7 | 56.2 ± 4.6 | |
| SHAM + ICV 5 µg/h ETN | 3.6 ± 0.5 | 19.0 ± 1.7 | 22.3 ± 2.0 | 11.5 ± 1.3 | 62.8 ± 5.6 | 40.5 ± 3.5 | 60.7 ± 5.1 | |
| SHAM + ICV 40 µg/h PTX | 3.3 ± 0.4 | 18.9 ± 2.2 | 22.6 ± 2.1 | 10.5 ± 1.4 | 60.3 ± 5.7 | 39.6 ± 3.7 | 57.4 ± 5.0 | |
| SHAM + ICV 10 µg/h ETN | 3.4 ± 0.5 | 19.6 ± 2.3 | 23.7 ± 2.2 | 11.0 ± 1.5 | 62.5 ± 5.8 | 40.9 ± 3.8 | 60.5 ± 5.2 | |
| SHAM + ICV aCSF | 3.8 ± 0.4 | 20.3 ± 2.4 | 24.3 ± 2.1 | 11.9 ± 1.4 | 64.2 ± 5.9 | 42.3 ± 3.8 | 63.9 ± 5.3 | |
| HF + IP 40 µg/h PTX | 7.0 ± 0.6* | 50.6 ± 4.5* | 60.1 ± 6.3* | 33.7 ± 3.4* | 110.8 ± 10.6* | 94.5 ± 9.6* | 116.2 ± 10.3* | |
| HF + IP 10 µg/h ETN | 7.2 ± 0.6* | 52.8 ± 4.6* | 62.7 ± 6.3* | 34.9 ± 3.5* | 113.4 ± 10.7* | 96.7 ± 9.8* | 119.8 ± 10.5* | |
| HF + ip saline | 7.4 ± 0.7* | 53.9 ± 4.7* | 64.3 ± 6.5* | 38.6 ± 3.6* | 120.9 ± 10.8* | 104.3 ± 9.8* | 127.3 ± 10.6* | |
| SHAM + ip saline | 3.7 ± 0.5 | 19.3 ± 2.5 | 23.6 ± 2.3 | 11.7 ± 1.4 | 63.7 ± 6.0 | 41.4 ± 3.9 | 61.5 ± 5.4 | |
ICV, intracerebroventricular; PTX, pentoxifylline; ETN, etanercept; HF, heart failure; SHAM, sham-operated control.
*P < 0.05 vs. SHAM + treated or SHAM + VEH.
†P < 0.05 HF + treated vs. HF + VEH.
3.6. Humoral indicators of HF
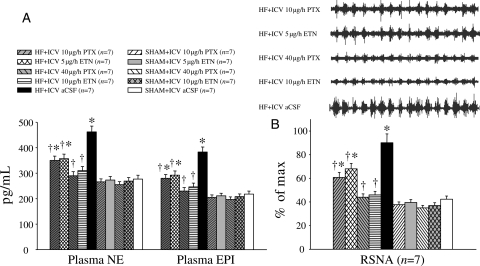
Humoral indicators of HF paralleled the PVN findings. Plasma levels of NE, EPI, ANG II, TNF-α, IL-1β, and IL-6 were all higher in HF than in SHAM rats. The plasma levels of TNF-α, IL-1β, IL-6, NE, EPI, and ANG II were lower in ICV PTX or ICV ETN-treated rats than in ICV aCSF-treated HF rats (Figure 6A, Table 2). However, the plasma levels were unaffected by ip treatment with the same doses of PTX or ETN (Table 2).
Figure 6.
Sympathetic activity. (A) Circulating norepinephrine (NE) and epinephrine levels were higher in HF rats compared with SHAM rats, but were lower in HF rats treated with ICV PTX or ICV ETN when compared with ICV aCSF-treated HF rats. (B) Renal sympathetic nerve activity (RSNA) of HF rats treated with ICV PTX or ICV ETN was lower than ICV aCSF-treated HF rats. *P < 0.05 vs. SHAM + treated or SHAM + ICV aCSF. †P < 0.05 HF + treated vs. HF + ICV aCSF.
3.7. Renal sympathetic nerve activity
At the end of the experiment, HF rats exhibited higher RSNA (% of max) when compared with SHAM rats; ICV treatment with PTX or ETN dose-dependently inhibited RSNA in HF rats (Figure 6B). IP treatment with the same doses of PTX or ETN did not affect RSNA; results were similar to those obtained from ICV vehicle-treated HF rats.
3.8. Functional/anatomical indicators of HF
Compared with VEH-treated SHAM rats, VEH-treated HF rats had significantly higher LVEDP, RV/BW, and lung/BW ratio. ICV PTX- or ICV ETN-treated HF rats had significantly lower LVEDP and lung/BW ratios than the ICV aCSF-treated HF rats (Table 3). ICV PTX or ICV ETN treatment appeared to improve survival [ICV 10 µg/h PTX-treated HF: 82.4% (14/17); ICV 40 µg/h PTX-treated HF: 88.2% (15/17); ICV 5 µg/h ETN-treated HF: 78.9% (15/19); ICV 10 µg/h ETN-treated HF: 84.2% (16/19); ICV aCSF-treated HF: 75.0% (15/20)] over the 4-week interval between the first and second echocardiograms, but some animals did not survive the second echocardiography session. IP treatment with the same doses of PTX or ETN did not affect LVEDP, RV/BW, or lung/BW ratio (Table 3).
Table 3.
Haemodynamic and anatomical measurements (n = 7)
| Measurements at 4 weeks | RV/BW (mg/g) | Lung/BW (mg/g) | HR (b.p.m.) | MAP (mmHg) | PP (mmHg) | LVEDP (mmHg) |
|---|---|---|---|---|---|---|
| HF + ICV 10 µg/h PTX | 1.12 ± 0.10* | 7.6 ± 0.4*,† | 339 ± 12 | 99 ± 6 | 30 ± 4 | 11.82 ± 1.31*,† |
| HF + ICV 5 µg/h ETN | 1.16 ± 0.12* | 7.9 ± 0.4*,† | 335 ± 11 | 97 ± 6 | 33 ± 3 | 16.34 ± 1.40*,† |
| HF + ICV 40 µg/h PTX | 0.75 ± 0.07† | 5.5 ± 0.4† | 336 ± 12 | 96 ± 6 | 31 ± 2 | 8.01 ± 1.32† |
| HF + ICV 10 µg/h ETN | 0.79 ± 0.08† | 5.7 ± 0.4† | 333 ± 11 | 98 ± 6 | 32 ± 3 | 7.54 ± 1.29† |
| HF + ICV aCSF | 1.22 ± 0.11* | 11.4 ± 0.6* | 344 ± 11 | 94 ± 5 | 34 ± 3 | 24.66 ± 1.71* |
| SHAM + ICV 10 µg/h PTX | 0.63 ± 0.05 | 4.5 ± 0.3 | 329 ± 10 | 107 ± 8 | 36 ± 4 | 6.12 ± 1.56 |
| SHAM + ICV 5 µg/h ETN | 0.65 ± 0.05 | 4.9 ± 0.4 | 324 ± 10 | 99 ± 8 | 35 ± 5 | 5.99 ± 1.58 |
| SHAM + ICV 40 µg/h PTX | 0.61 ± 0.05 | 4.4 ± 0.3 | 328 ± 11 | 101 ± 7 | 36 ± 4 | 6.01 ± 1.42 |
| SHAM + ICV 10 µg/h ETN | 0.64 ± 0.06 | 4.6 ± 0.3 | 330 ± 12 | 105 ± 8 | 35 ± 4 | 6.45 ± 1.57 |
| SHAM + ICV aCSF | 0.68 ± 0.05 | 5.1 ± 0.4 | 331 ± 11 | 109 ± 8 | 37 ± 5 | 7.03 ± 1.52 |
| HF + IP 40 µg/h PTX | 1.14 ± 0.11* | 10.4 ± 0.6* | 337 ± 12 | 98 ± 6 | 33 ± 4 | 21.57 ± 1.90* |
| HF + IP 10 µg/h ETN | 1.17 ± 0.12* | 10.7 ± 0.6* | 340 ± 11 | 97 ± 6 | 34 ± 5 | 22.09 ± 1.94* |
| HF + ip saline | 1.20 ± 0.11* | 11.1 ± 0.6* | 344 ± 12 | 95 ± 5 | 35 ± 5 | 23.15 ± 1.92* |
| SHAM + ip saline | 0.66 ± 0.05 | 5.0 ± 0.4 | 332 ± 11 | 106 ± 8 | 36 ± 5 | 6.74 ± 1.60 |
SHAM, sham-operated control; HF, heart failure; BW, body weight; RV, right ventricular; HR, heart rate; MAP, mean arterial pressure; PP, pulse pressure; LVEDP, left ventricular end-diastolic pressure; ICV, intracerebroventricular; PTX, pentoxifylline; ETN, etanercept. Values are mean ± SEM.
*P < 0.05 vs. SHAM + treated or SHAM + VEH.
†P < 0.05 HF + treated vs. HF + VEH.
4. Discussion
The novel finding of the present study is that brain cytokines induce an imbalance between excitatory and inhibitory neurotransmitters and their rate-limiting enzymes in the PVN in HF rats, which contributes to sympathoexcitation. Treatment with central TNF-blockers attenuated this imbalance and decreased the exaggerated sympathetic activity in HF rats. Similar doses of these blockers given peripherally did not restore the imbalance in the neurotransmitters in the PVN of HF animals, suggesting that central nervous system cytokines modulate neurotransmitters in the PVN and contribute to sympathoexcitation in HF.
A sustained increase in sympathetic nerve activity is the hallmark of congestive HF.26 The PVN is an important site that regulates fluid balance and vasopressin release, and mediates integration of sympathetic outflow to major organs, including the heart. The tonic activity of the PVN is related to an interplay of a number of neurotransmitters and neuromodulators in these sympathetic neurons. The sympathetic outflow from the PVN depends upon the balance of these excitatory and inhibitory neuromodulators, including glutamate, GABA, angiotensin II, and nitric oxide.27 In HF, tonic sympathetic activity is elevated, resulting in exaggerated RSNA and worsening of the disease. In this study, we observed an increase in the expression of Fra-LI activity in PVN neurons and elevated RSNA in HF rats. These increased activities were significantly reduced in the HF rats treated ICV with cytokine blockers, suggesting a role for cytokines in chronic neuronal excitation in HF. Moreover, in HF, endogenous GABA-mediated inhibition is decreased, because of the increased neuronal activity of the PVN. Interestingly, in the present study, we observed that treatment with cytokine blockers decreased the levels of TNF-α, IL-1β, and IL-6, and attenuated the GABA disinhibition in the PVN which is otherwise observed in HF.
There is also increasing evidence that the gaseous neurotransmitter, NO, acts to inhibit sympathetic outflow from these sympathetic neurons. Moreover, the sympatho-inhibitory effect of NO donors in the PVN was eliminated by blockade of the GABA system, while the sympathoexcitatory effect of NO inhibitors was eliminated by activation of the GABA system, suggesting that the inhibitory effects of NO in the PVN on RSNA were mediated through GABA.28 Previous studies have demonstrated that the effects of NO in the PVN are site-specific and are associated with local changes in specific amino acids, such as glutamate. In this context, NO is considered to cause GABA release, which is later followed by compensatory increases in glutamate production.29,30 This study, combined with previous work from our laboratory,22 demonstrates that treatment with cytokine blockers in HF can increase nNOS levels in the PVN. Here, we further show that ICV administration of cytokine blockers can improve GABA levels, while decreasing the glutamate levels in the PVN of HF rats, probably via a NO mechanism. Using an immunohistochemical approach, we also identified the neurons expressing GAD67, a marker to recognize GABAergic neurons in the PVN. Our results show that the expression of GAD67 in PVN neurons of HF rats was lower when compared with SHAM rats, and this reduction was attenuated in those treated with cytokine blockers.
In addition to decreased GABA and increased glutamate in the PVN, our findings suggest that a number of neurohumoral factors are activated in HF, including cytokines, NE, EPI, and ANGII. The NE levels in the PVN were also increased in HF. Increased ANGII molecules, similar to cytokines, from the periphery can enter the brain through circumventricular organs, and upregulate AT-1 receptors in the PVN and exhibit a negative feedback on NO inducing sympathoexcitation.31 Treatment with PTX or ETN not only reduced cytokines and NE levels in the PVN, but also reduced plasma TNF-α, NE, and EPI. However, the increased plasma IL-1β and IL-6 were not completely attenuated by either PTX or ETN. Microinjections of glutamate into the PVN have been shown to increase plasma NE and EPI levels.32 It is possible that the increase in the neurotransmitter glutamate plays a role in activating tyrosine hydroxylase in the PVN neurons, thereby contributing to increased catecholamine synthesis and release from neurons. However, whether these alterations in neurotransmitter levels actually result in alterations in rates of neuronal firing was not examined here. It is worth noting that the inhibition of cytokines in the PVN reduced plasma catecholamines, cytokines, and ANG II. In addition, inhibition of cytokines in the PVN also reduced pulmonary congestion as seen by reduced wet lung/body ratio and also LVEDP in HF. Most of the treatments in HF patients include ACE-blockers or angiotensin II type 1 receptor (AT1-R) blockers that are aimed at reducing afterload and intravascular volume. Although the clinical trials33 targeting cytokines in HF were disappointing, it is clear to date that HF is a complex mechanism and involves the interplay of several neuromodulators and neurotransmitters in the PVN, resulting in exaggerated sympathoexcitation. From this and previous studies targeting cytokines in HF, it is plausible to suggest that anti-cytokine agents can be used in conjunction with traditional HF therapies.
In this study, we demonstrate that cytokine blockers, when given in the brain, modulate neurotransmitters in the PVN in HF rats in a dose-dependent manner. Similar doses of cytokine blockers when given peripherally did not change neurotransmitters in the PVN and produced results similar to ICV aCSF-treated HF animals, suggesting that central cytokines modulate neurotransmitters in the PVN and contribute to exaggerated sympathoexcitation in HF. Recent findings indicate that angiotensin levels are increased in the brain and circulation of HF rats.14 Blockade of RAS modulates neurotransmitters in the PVN and decreases sympathetic activity, suggesting a central nervous system role in the sympathoexcitation in HF.34,35 However, the role of central nervous system cytokines in the modulation of neurotransmitters in the PVN of HF animals has not been explored. Here, we demonstrate that centrally administered cytokine blockers decrease ANGII levels in the circulation and modulate neurotransmitters in the PVN to contribute to sympathoexcitation in HF.
In summary, the present study demonstrates that treatment of HF rats with ICV PTX or ETN attenuates the HF-induced increases in pro-inflammatory cytokines, glutamate, and NE, and also attenuates the HF-induced decreases in GABA and nNOS, in the PVN. Elevated excitatory neurotransmitters and decreased inhibitory neurotransmitters in the PVN contribute to neurohumoral excitation in HF. PTX or ETN could decrease the sympathoexcitation in HF by decreasing excitatory neurotransmitters and increasing inhibitory neurotransmitters in the PVN.
Funding
Supported by U.S. National Institutes of Health Grant RO1-HL-080544-01 (PI: J.F.), National Natural Science Foundation of China (30770867), and Shantou University Medical College Grant.
Conflict of interest: none declared.
References
- 1.Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- 2.Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology. 1980;31:410–417. doi: 10.1159/000123111. [DOI] [PubMed] [Google Scholar]
- 3.Boudaba C, Szabo K, Tasker JG. Physiological mapping of local inhibitory inputs to the hypothalamic paraventricular nucleus. J Neurosci. 1996;16:7151–7160. doi: 10.1523/JNEUROSCI.16-22-07151.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hermes ML, Coderre EM, Buijs RM, Renaud LP. GABA and glutamate mediate rapid neurotransmission from suprachiasmatic nucleus to hypothalamic paraventricular nucleus in rat. J Physiol. 1996;496:749–757. doi: 10.1113/jphysiol.1996.sp021724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tasker JG, Boudaba C, Schrader LA. Local glutamatergic and GABAergic synaptic circuits and metabotropic glutamate receptors in the hypothalamic paraventricular and supraoptic nuclei. Adv Exp Med Biol. 1998;449:117–121. doi: 10.1007/978-1-4615-4871-3_11. [DOI] [PubMed] [Google Scholar]
- 6.Antonaccio MJ, Kerwin L, Taylor DG. Reductions in blood pressure, heart rate and renal sympathetic nerve discharge in cats after the central administration of muscimol, a GABA agonist. Neuropharmacology. 1978;17:783–791. doi: 10.1016/0028-3908(78)90065-5. [DOI] [PubMed] [Google Scholar]
- 7.Brennan TJ, Haywood JR, Ticku MK. GABA receptor binding and hemodynamic responses to ICV GABA in adult spontaneously hypertensive rats. Life Sci. 1983;33:701–709. doi: 10.1016/0024-3205(83)90774-9. [DOI] [PubMed] [Google Scholar]
- 8.Arabia AM, Catapano L, Storini C, Perego C, De Luigi A, Head GA, et al. Impaired central stress-induced release of noradrenaline in rats with heart failure: a microdialysis study. Neuroscience. 2002;114:591–599. doi: 10.1016/s0306-4522(02)00337-8. [DOI] [PubMed] [Google Scholar]
- 9.Basu S, Sinha SK, Shao Q, Ganguly PK, Dhalla NS. Neuropeptide Y modulation of sympathetic activity in myocardial infarction. J Am Coll Cardiol. 1996;27:1796–1803. doi: 10.1016/0735-1097(96)00063-0. [DOI] [PubMed] [Google Scholar]
- 10.Chen QH, Haywood JR, Toney GM. Sympathoexcitation by PVN injected bicuculline requires activation of excitatory amino acid receptors. Hypertension. 2003;42:725–731. doi: 10.1161/01.HYP.0000085197.20043.44. [DOI] [PubMed] [Google Scholar]
- 11.Cole RL, Sawchenko PE. Neurotransmitter regulation of cellular activation and neuropeptide gene expression in the paraventricular nucleus of the hypothalamus. J Neurosci. 2002;22:959–969. doi: 10.1523/JNEUROSCI.22-03-00959.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang K, Li YF, Patel KP. Reduced endogenous GABA-mediated inhibition in the PVN on renal nerve discharge in rats with heart failure. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1006–R1015. doi: 10.1152/ajpregu.00241.2001. [DOI] [PubMed] [Google Scholar]
- 13.Kang YM, Ma Y, Elks C, Zheng JP, Yang ZM, Francis J. Cross-talk between cytokines and renin-angiotensin in hypothalamic paraventricular nucleus in heart failure: role of nuclear factor-κB. Cardiovasc Res. 2008;79:671–678. doi: 10.1093/cvr/cvn119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang YM, Zhang ZH, Xue B, Weiss RM, Felder RB. Inhibition of brain proinflammatory cytokine synthesis reduces hypothalamicexcitation in rats with ischemia-induced heart failure. Am J Physiol Heart Circ Physiol. 2008;295:H227–H236. doi: 10.1152/ajpheart.01157.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunn AJ. Cytokine activation of the HPA axis. Ann N Y Acad Sci. 2000;917:608–617. doi: 10.1111/j.1749-6632.2000.tb05426.x. [DOI] [PubMed] [Google Scholar]
- 16.Kang YM, Zhang ZH, Johnson RF, Yu Y, Beltz T, Johnson AK, et al. Novel effect of mineralocorticoid receptor antagonism to reduce proinflammatory cytokines and hypothalamic activation in rats with ischemia-induced heart failure. Circ Res. 2006;99:758–766. doi: 10.1161/01.RES.0000244092.95152.86. [DOI] [PubMed] [Google Scholar]
- 17.MohanKumar SM, MohanKumar PS, Quadri SK. Specificity of interleukin-1 induced changes in monoamine concentrations in hypothalamic nuclei: blockade by interleukin-1 receptor antagonist. Brain Res Bull. 1998;47:29–34. doi: 10.1016/s0361-9230(98)00037-9. [DOI] [PubMed] [Google Scholar]
- 18.Yang LM, Hu B, Xia YH, Zhang BL, Zhao H. Lateral habenula lesions improve the behavioral response in depressed rats via increasing the serotonin level in dorsal raphe nucleus. Behav Brain Res. 2008;188:84–90. doi: 10.1016/j.bbr.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 19.Barber M, Kasturi BS, Austin ME, Patel KP, MohanKumar SM, MohanKumar PS. Diabetes-induced neuroendocrine changes in rats: role of brain monoamines, insulin and leptin. Brain Res. 2003;964:128–135. doi: 10.1016/s0006-8993(02)04091-x. [DOI] [PubMed] [Google Scholar]
- 20.Aston-Jones G, Zhu Y, Card JP. Numerous GABAergic afferents to locus ceruleus in the pericerulear dendritic zone: possible interneuronal pool. J Neurosci. 2004;24:2313–2321. doi: 10.1523/JNEUROSCI.5339-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guggilam A, Haque M, Kerut EK, McIlwain E, Lucchesi P, Seghal I, et al. TNF-alpha blockade decreases oxidative stress in the paraventricular nucleus and attenuates sympathoexcitation in heart failure rats. Am J Physiol Heart Circ Physiol. 2007;293:H599–H609. doi: 10.1152/ajpheart.00286.2007. [DOI] [PubMed] [Google Scholar]
- 22.Guggilam A, Patel KP, Haque M, Ebenezer PJ, Kapusta DR, Francis J. Cytokine blockade attenuates sympathoexcitation in heart failure: cross-talk between nNOS, AT-1R and cytokines in the hypothalamic paraventricular nucleus. Eur J Heart Fail. 2008;10:625–634. doi: 10.1016/j.ejheart.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang YM, Ma Y, Zheng JP, Elks C, Sriramula S, Yang ZM, et al. Brain nuclear factor-kappa B activation contributes to neurohumoral excitation in angiotensin II-induced hypertension. Cardiovasc Res. 2009;82:503–512. doi: 10.1093/cvr/cvp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagura S, Sakagami T, Kakiichi A, Yoshimoto M, Miki K. Acute shifts in baroreflex control of renal sympathetic nerve activity induced by REM sleep and grooming in rats. J Physiol. 2004;558:975–983. doi: 10.1113/jphysiol.2004.064527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu JL, Irvine S, Reid IA, Patel KP, Zucker IH. Chronic exercise reduces sympathetic nerve activity in rabbits with pacing-induced heart failure: a role for angiotensin II. Circulation. 2000;102:1854–1862. doi: 10.1161/01.cir.102.15.1854. [DOI] [PubMed] [Google Scholar]
- 26.Zucker IH, Wang W, Brändle M, Schultz HD, Patel KP. Neural regulation of sympathetic nerve activity in heart failure. Prog Cardiovasc Dis. 1995;37:397–414. doi: 10.1016/s0033-0620(05)80020-9. [DOI] [PubMed] [Google Scholar]
- 27.Dampney RA, Horiuchi J, Killinger S, Sheriff MJ, Tan PS, McDowall LM. Long-term regulation of arterial blood pressure by hypothalamic nuclei: some critical questions. Clin Exp Pharmacol Physiol. 2005;32:419–425. doi: 10.1111/j.1440-1681.2005.04205.x. [DOI] [PubMed] [Google Scholar]
- 28.Zhang K, Patel KP. Effect of nitric oxide within the paraventricular nucleus on renal sympathetic nerve discharge: role of GABA. Am J Physiol. 1998;275:R728–R734. doi: 10.1152/ajpregu.1998.275.3.R728. [DOI] [PubMed] [Google Scholar]
- 29.Horn T, Smith PM, McLaughlin BE, Bauce L, Marks GS, Pittman QJ, et al. Nitric oxide actions in paraventricular nucleus: cardiovascular and neurochemical implications. Am J Physiol. 1994;266:R306–R313. doi: 10.1152/ajpregu.1994.266.1.R306. [DOI] [PubMed] [Google Scholar]
- 30.Zheng H, Li YF, Cornish KG, Zucker IH, Patel KP. Exercise training improves endogenous nitric oxide mechanisms within the paraventricular nucleus in rats with heart failure. Am J Physiol Heart Circ Physiol. 2005;288:H2332–H2341. doi: 10.1152/ajpheart.00473.2004. [DOI] [PubMed] [Google Scholar]
- 31.Li YF, Patel KP. Paraventricular nucleus of the hypothalamus and elevated sympathetic activity in heart failure: the altered inhibitory mechanisms. Acta Physiol Scand. 2003;177:17–26. doi: 10.1046/j.1365-201X.2003.01043.x. [DOI] [PubMed] [Google Scholar]
- 32.Martin DS, Haywood JR. Sympathetic nervous system activation by glutamate injections into the paraventricular nucleus. Brain Res. 1992;577:261–267. doi: 10.1016/0006-8993(92)90282-e. [DOI] [PubMed] [Google Scholar]
- 33.Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL) Circulation. 2004;109:1594–1602. doi: 10.1161/01.CIR.0000124490.27666.B2. [DOI] [PubMed] [Google Scholar]
- 34.Patel KP. Role of paraventricular nucleus in mediating sympathetic outflow in heart failure. Heart Fail Rev. 2000;5:73–86. doi: 10.1023/A:1009850224802. [DOI] [PubMed] [Google Scholar]
- 35.Kleiber AC, Zheng H, Schultz HD, Peuler JD, Patel KP. Exercise training normalizes enhanced glutamate-mediated sympathetic activation from the PVN in heart failure. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1863–R1872. doi: 10.1152/ajpregu.00757.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]