Abstract
Aims
The pathological proliferation of cardiac fibroblasts (CFs) in response to heart injury results in fibrosis, which correlates with arrhythmia generation and heart failure. Here we systematically examined the effect of fibroblast-derived paracrine factors on electrical propagation in cardiomyocytes.
Methods and results
Neonatal rat cardiac monolayers were exposed for 24 h to media conditioned by CFs. Optical mapping, sharp microelectrode recordings, quantitative RT–PCR, and immunostaining were used to assess the changes in the propagation and shape of the action potential and underlying changes in gene and protein expression. The fibroblast paracrine factors produced a 52% reduction in cardiac conduction velocity, a 217% prolongation of action potential duration, a 64% decrease of maximum capture rate, a 21% increase in membrane resting potential, and an 80% decrease of action potential upstroke velocity. These effects were dose dependent and partially reversible with removal of the conditioned media. No fibroblast proliferation, cardiomyocyte apoptosis, or decreased connexin-43 expression, phosphorylation, and function were found in conditioned cardiac cultures. In contrast, the expression of the fast sodium, inward rectifying potassium, and transient outward potassium channels were, respectively, reduced 3.8-, 6.6-fold, and to undetectable levels. The expression of β-myosin heavy chain increased 17.4-fold. No electrophysiological changes were observed from media conditioned by CFs in the presence of cardiomyocytes.
Conclusion
Paracrine factors from neonatal CFs alone produced significant electrophysiological changes in neonatal rat cardiomyocytes resembling those found in several cardiac pathologies.
Keywords: Paracrine action, Ion channels, Cardiac fibroblasts, Electrophysiology
1. Introduction
In the healthy heart, up to two-thirds of the total cell population consists of non-myocytes, the majority being cardiac fibroblasts (CFs) that perform essential functions for the maintenance of myocardial structure and function.1–3 However, CFs are also activated in the wound healing response in several cardiac pathologies, including ischaemic heart disease, inflammation, hypertrophy, and myocardial infarction, where they can lead to tissue remodelling and fibrosis.1,3,4 The presence of fibrotic regions within diseased heart tissue has been shown to correlate with heart failure and arrhythmias.5,6 Arrhythmogenic properties are primarily thought to be a result of increased fibroblast number and extracellular matrix deposition that form direct physical barriers to electrical propagation.5,7 However, the potential indirect arrhythmogenic or anti-arrhythmic roles of CFs exerted through the secretion of different soluble factors are yet to be explored.
It is well known that CFs produce factors that contribute to pathological cardiac hypertrophy characterized by increased cardiomyocyte size and reorganization of contractile proteins.4,8 In contrast, less is understood about the potential roles of paracrine factors secreted by CFs in cardiomyocyte electrophysiology and, specifically, in cardiac action potential propagation. The role of non-cardiac paracrine factors in altering cardiomyocyte function is difficult to systematically assess in situ because of confounding factors, including the presence of multiple cell types and their paracrine factors, the ever changing concentrations of neurohumoral factors, and the ‘washing’ effect of surrounding capillaries.
Therefore, we assessed the role of paracrine factors from CFs in cardiac electrophysiological function in vitro using well-controlled, serum-free conditioned media studies, in which media conditioned by CFs for 24 h was applied to cardiomyocytes for 24 h, followed by optical and intracellular recordings of action potential propagation. In response to CF conditioning, we observed a remarkable slowing of conduction velocity (CV), increase of the action potential duration (APD), and depolarization of the resting membrane potential (RMP) in cardiomyocytes. These effects were dose dependent and caused by the down-regulated expression of specific membrane ion channels but not by a change in gap junction expression or function. Importantly, controlled co-culture studies between cardiomyocytes and fibroblasts suggest the existence of a cross-talk mechanism where cardiomyocytes, when in the presence of fibroblasts, secrete factors that counteract the described fibroblast paracrine actions.
2. Methods
A detailed description of materials and methods used in this study is included in the Supplemental material online. This study conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication NO. 85-23, revised 1996) and the Institutional Animal Care and Use Committee protocol #A277-06-08.
2.1. Cell cultures
2.1.1. Cardiomyocytes
Ventricular cardiomyocytes were dissociated from 2-day-old neonatal Sprague–Dawley rats, preplated in two steps and plated onto fibronectin-coated coverslips at a density of 2300 cells/mm2 to yield confluent cardiac monolayers, as described previously.9 Forty-eight hours after seeding, culture media were switched to serum-free (control) media,10 which was used for the remainder of the culture time.
2.1.2. Cardiac fibroblasts
Neonatal rat CFs were recovered from the preplating steps of the cardiomyocyte isolation and cultured in maintenance media. Upon reaching confluence, the fibroblasts were split and passaged at a 1:2 ratio and used at passage one.10
2.2. Conditioned media studies
Fibroblast cultures were washed thoroughly with phosphate-buffered saline and allowed to condition fresh serum-free (control) media for 24 h. The resultant fibroblast conditioned media were filtered through a 0.22 µm filter to remove any cellular debris and added to 6-day-old confluent cardiac monolayers previously cultured in control media. After 24 h, conditioned and control (cultured exclusively in control media) cardiac monolayers were compared for electrophysiological properties as well as gene and protein expression.
To assess whether the effects of conditioning were specific to CFs, media conditioned for 24 h by an innocuous cell type, human embryonic kidney 293 (HEK-293) cells, were used to condition cardiac monolayers for 24 h.
Cardiac monolayers were also conditioned for 48 h (by adding fresh CF-conditioned medium every 24 h) to assess whether the observed effects were transient or sustained after prolonged exposure to conditioned media. To determine whether the observed effects could be reversed after removal of the CF-conditioned media, cardiac cultures that had been conditioned for 24 h were incubated in control medium for an additional 24 h.
To examine whether the observed conditioning effects were dose dependent, CF-conditioned media were diluted with control media to 50 or 25% of the original concentration and applied to cardiac monolayers for 24 h. Cardiac monolayers were also exposed for 24 h to CF-conditioned medium that was concentrated 10-fold using a centrifugal filtering device (3,000MW cutoff, Millipore) and then diluted to 1× with fresh control media to test whether the depletion of some factors from the control medium during conditioning by CFs (instead of, or in addition to, the secreted paracrine factors) contributed to the observed effects on cardiomyocytes.
To assess whether CF paracrine action was exerted by secreted proteins, inactivated CF-conditioned media were applied to cardiac monolayers for 24 h. Protein inactivation involved treating CF-conditioned media with 25 µg/mL of trypsin for 2 h at 37°C followed by heating at 90°C to destroy the remaining trypsin activity.11
Finally, in controlled co-culture studies, we assessed whether CF-conditioned media had the same effect on cardiomyocytes when conditioning was performed in the presence of cardiomyocytes. Six-day-old cardiac monolayers were placed into the centre of 60 mm Petri dishes containing a surrounding confluent layer of CFs for 24 h, allowing for the sharing of media but no physical contact between the CFs and cardiac monolayers. Fibroblasts were present at a density that provided the same concentration of conditioned factors (by using the same number of cells per volume of media) as in regular CF-conditioning experiments. Dishes with cardiac monolayers but without surrounding fibroblasts incubated for 24 h in either control or CF-conditioned media served as controls.
2.3. Optical mapping of electrical propagation
A 504-channel optical mapping system and voltage-sensitive dye (di-4-ANNEPS) were used to record action potential propagation in electrically stimulated cardiac monolayers, as described previously.9,12
2.4. Sharp microelectrode recordings
Sharp microelectrodes with tip resistances of 50–80 MΩ were used to record propagated action potentials in cardiac monolayers during 2 Hz pacing and to measure the RMP, maximum depolarization slope of the action potential, and APD at 80% of repolarization.
2.5. Immunocytochemistry
2.6. Fluorescence recovery after photobleaching
Fluorescence recovery after photobleaching (FRAP) was used to assess functional connectivity between cardiomyocytes. Briefly, the recovery of fluorescence in a photobleached cell, through gap junctional coupling to neighbouring cells, was assessed using confocal microscopy, as described previously.13,14
2.7. Apoptosis detection
The presence of apoptotic cells was quantified using a commercially available TUNEL fluorescence kit (Chemicon), as per manufacturer's instructions.
2.8. Quantitative reverse transcriptase–polymerase chain reaction
Total RNA was isolated from cardiac monolayers using an RNeasy Mini Kit (Qiagen). Reverse transcriptase–polymerase chain reaction (RT–PCR) primers and probes were custom designed (see Supplemental material online, Table S1) and used to perform a one-step RT–PCR for each sample and gene in triplicate. The TaqMan PCR data were quantified using the 2−ΔΔCt method,15 by comparing the signal of the target transcript of the treated group with that of the control group, both relative to an internal control, 18S.
2.9. Statistics
Data were expressed as a mean ± SD and analysed by unpaired t-test or ANOVA, using Excel, S-Plus, and Graphpad software. Differences were considered statistically significant when P < 0.05.
3. Results
3.1. CF-conditioned media adversely affects electrical propagation in cardiac monolayers
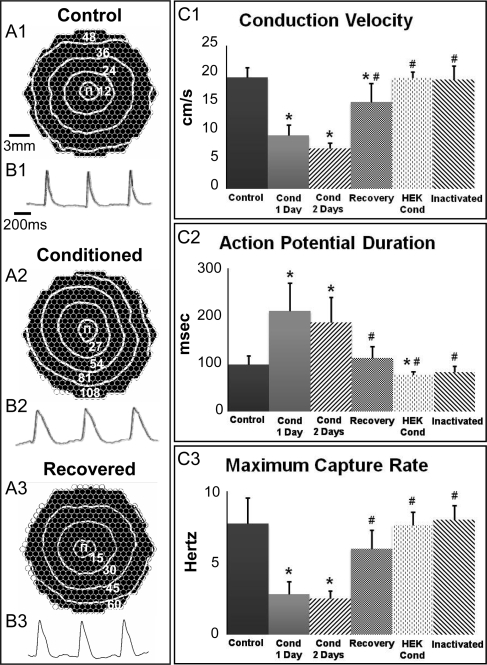
Immediately upon removal from the incubator, ∼75% of cardiac monolayers exposed for 24 h to CF-conditioned media exhibited spontaneous beating (0.8–3.2 Hz rate), while unconditioned or acutely conditioned (for 1 h) monolayers remained quiescent. Furthermore, cardiac monolayers conditioned for 24 h (but not 1 h) exhibited apparent conduction slowing (Figure 1A1 and A2) and APD prolongation (Figure 1B1 and B2). Specifically, CV (Figure 1C1) and maximum capture rate (MCR, Figure 1C3) were, respectively, decreased to 48% (from 19.5 ± 1.8 to 9.4 ± 1.8 cm/s, P < 0.0001) and 36% (from 7.7 ± 1.8 to 2.8 ± 0.9 Hz, P < 0.0001) of the control values, while APD (Figure 1C2) increased 217% (from 99.1 ± 17.6 to 211 ± 58.5 ms, P < 0.0001) of that measured in unconditioned controls. Prolonging the exposure to CF paracrine factors from 24 to 48 h induced no additional changes in measured electrophysiological properties (Figure 1C1–3). The addition of fresh control medium after 24 h of conditioning resulted in complete reversal of APD and MCR changes and partial reversal of the CV to a level 78.2% of that measured in control cultures (Figure 1C1–3). The addition of the excitation–contraction uncoupler blebbistatin (10 µM16) to the conditioned media had no measurable effect on the observed electrophysiological changes (not shown), suggesting that these changes were not mechanically mediated.
Figure 1.
Effect of CF-conditioned media on electrical propagation in cardiac monolayers. (A) Representative isochrone maps of control, CF-conditioned, and recovered cardiac monolayers stimulated by a point electrode (Π) at 2 Hz pacing rate. Recovered monolayers were exposed to CF-conditioned media for 24 h and then switched to control media for 24 h to recover. The isochrone lines are labelled in milliseconds. CVs were 19 cm/s (control), 8 cm/s (conditioned), and 15 cm/s (recovered). Circles represent 504 optical recording sites. (B) Optical action potential traces from respective monolayers in (A). (C) Parameters of electrical propagation. Cond, conditioned. Bars from left to right, respectively, correspond to n = 53, 53, 10, 10, 8, and 3 monolayers. Asterisks denote significantly different results from control; hash symbols indicate significantly different results from 1 and 2 day conditioned cultures.
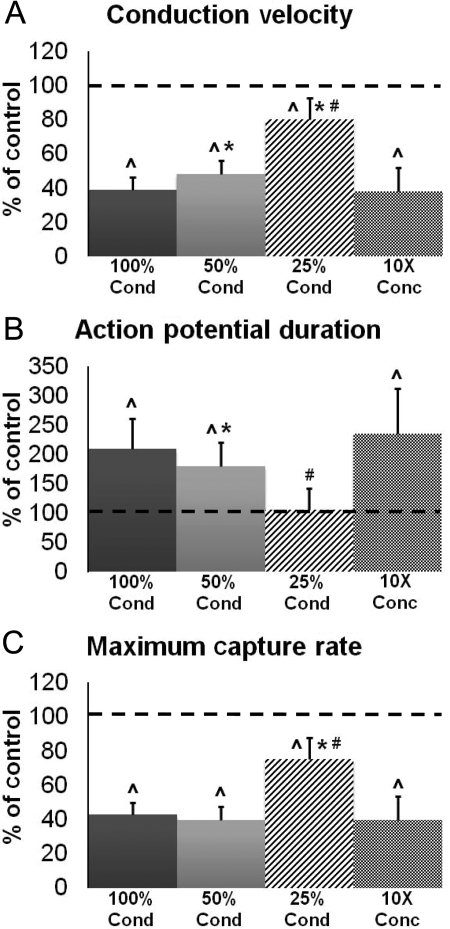
Figure 2.
Effect of CF-conditioned media dilution on electrical propagation in cardiac monolayers. Conditioned media (100% Cond) were diluted twice (50% Cond) and four times (25% Cond) by mixing with control media. CF paracrine factors in conditioned media were also concentrated 10× and diluted back to 1× using control media (10× Conc group). Electrophysiological parameters in (A–C) are reported as per cent relative to unconditioned control (dashed line). Bars from left to right, respectively, correspond to n = 10, 10, 10, and 7 monolayers. Hat symbols denote significantly different results from control; asterisks denote significantly different results from 100% Cond; hash symbols indicate significantly different results from 50% Cond.
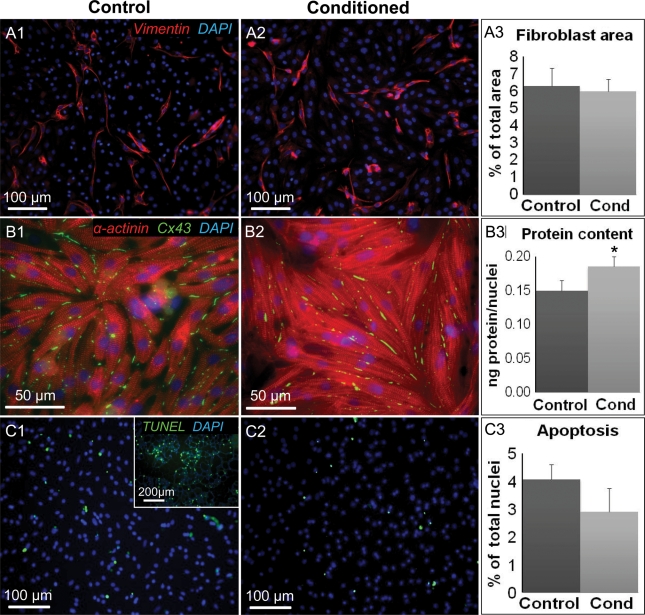
Figure 3.
Effect of CF-conditioned media on cellular content, size, and apoptosis in cardiac monolayers. (A) Representative stainings for vimentin-positive fibroblasts in control (A1) and conditioned (A2) monolayers. (A3) Fibroblast percent area derived from the total of n = 4 monolayers per group. (B) Representative stainings showing cell size in control (B1) and conditioned (B2) monolayers. (B3) Total protein divided by number of nuclei was used as an index of cell size; n = 4 monolayers per group. (C) Representative stainings of apoptotic cells in control (C1) and conditioned (C2) monolayers. The inset in C1 is a positive control supplied by the manufacturer. (C3) Per cent of apoptotic nuclei derived from the total of n = 4 monolayers per group. Asterisks indicate significantly different results from control.
3.2. Active paracrine factors in CF-conditioned media are proteins
The effect of the CF-conditioned media was abolished by proteolytic cleavage with trypsin and protein denaturation with boiling11 (Figure 1C1–3), which along with the lack of acute conditioning effects, indicated that the relevant paracrine factors in the CF-conditioned media were peptides or proteins. Furthermore, the electrophysiological properties of cardiac monolayers exposed to media conditioned by HEK-293 cells remained comparable to control (Figure 1C1–3), suggesting the specificity of the observed effects to CF-conditioning.
3.3. CF-conditioned media effects are dose dependent and not the result of fibroblast-induced media depletion
The observed cardiac electrophysiological effects were progressively lessened by diluting pure CF-conditioned medium two- and four-fold with control medium (Figure 2). Specifically, the four-fold dilution completely neutralized the paracrine effect on cardiac APD (Figure 2B), while the effects on CV and MCR were only partially neutralized to 80.3 and 75.4%, respectively, of those in the unconditioned monolayers (Figure 2A and C). In addition, media that were concentrated 10-fold and then diluted to 1× with fresh control medium exerted a similar electrophysiological effect on cardiac monolayers as the CF-conditioned medium (compare 100% Cond and 10× Conc groups in Figure 2A–C). This result suggests that CF-secreted paracrine factors, rather than the depletion of the control medium by CFs during conditioning, were the cause of the observed electrophysiological changes.
3.4. CF-conditioned media do not increase cell apoptosis or fibroblast proliferation in cardiac monolayers
We further investigated whether the observed paracrine effects originated from non-electrophysiological changes in cardiac monolayers. In particular, no increase in the number of fibroblasts (average of 6% of total monolayer area based on vimentin staining, Figure 3A1–3) or apoptotic cells (3–4% of all cells, based on TUNEL staining, Figure 3C1–3) were found in CF-conditioned vs. control monolayers. Simultaneously, the cardiac cell size, assessed as the amount of total protein per nucleus, was slightly but significantly increased (by 20%) due to the CF-conditioning (Figure 3B1–3), consistent with other studies.17,18
3.5. CF-conditioned media do not change intercellular connectivity in cardiac monolayers
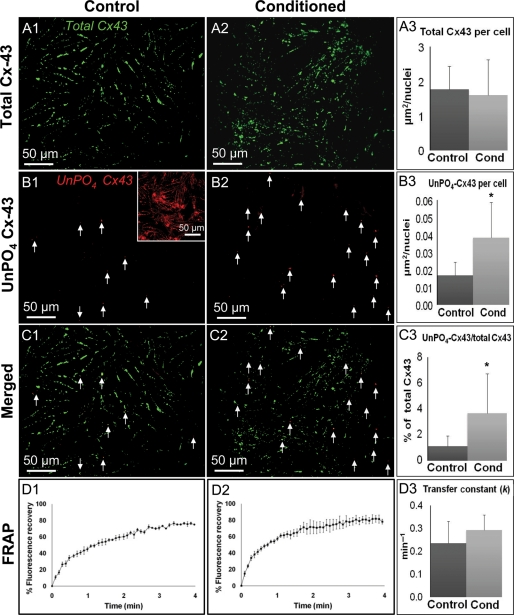
To assess whether the decrease in CV in conditioned cardiac monolayers was caused by a decrease in intercellular coupling, we quantified the amount of total (unphosphorylated + phosphorylated) (Figure 4A1–3) and unphosphorylated only (UnPO4, Figure 4B1–3) connexin-43 (Cx43) using immunostaining with phosphorylation-sensitive antibodies. The total area of positive staining for total Cx43 per cell nucleus was found to be comparable in conditioned (1.77 ± 0.66 µm2/nucleus) and control (1.60 ± 1 µm2/nucleus) monolayers (Figure 4A3). Conversely, the total area of positive staining for UnPO4 Cx43 per cell nucleus was found to be significantly larger in conditioned monolayers (0.04 ± 0.02 µm2/nucleus) than control monolayers (0.02 ± 0.01 µm2/nucleus) (Figure 4B3). However, the amounts of UnPO4 Cx43 in both conditioned and control cultures were virtually negligible relative to the total amount of Cx43 (Figure 4C1–3). Furthermore, the FRAP assessment showed that control and CF-conditioned cardiomyocytes recovered fluorescence via gap junctional coupling to neighbouring cells over nearly identical time courses (Figure 4D1–2). The derived transfer constants13 were comparable for both groups (0.24 ± 0.10 vs. 0.29 ± 0.07 min−1 in control vs. conditioned monolayers, P = 0.39, Figure 4D3), indicating that CF-conditioned media did not affect functional connectivity between the cells.
Figure 4.
Effects of CF-conditioned media on the presence and phosphorylation of Cx43 in cardiac monolayers. (A) Representative stainings of total Cx43 in control (A1) and conditioned (A2) monolayers. (A3) Total Cx43 area per nucleus quantified from n = 5 monolayers per group. (B) Representative stainings of UnPO4 Cx43 in control (B1) and conditioned (B2) monolayers. Inset, positive control in hypoxic cardiomyocytes. Arrows point to positive staining (red). (B3) UnPO4 Cx-43 area per nucleus quantified from n = 5 monolayers per group. (C) Merged images of control (C1) and conditioned (C2) monolayers. (C3) The ratio of UnPO4-Cx43/total Cx43. (D) Time course of FRAP in control (D1) and conditioned (D2) cells averaged from n = 4 cells per group. (D3) The resulting transfer constants (k). Asterisks denote significantly different results from control.
3.6. CF-conditioned media significantly alter expression of cardiac genes encoding ion channels and contractile proteins
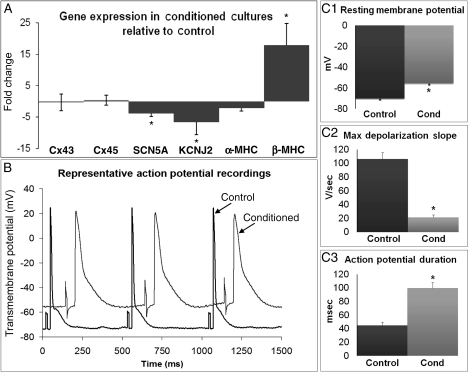
We also quantified the changes in expression of major rat ion channel genes that could possibly produce the changes in electrophysiology observed by optical mapping. As shown in Figure 5A, the expression of the genes encoding the cardiac sodium channel (SCN5A) and the inwardly rectifying potassium channel (KCNJ2) in the conditioned cardiomyocytes were, respectively, decreased by 3.8- and 6.6-fold relative to control. Furthermore, the expression of the transient outward potassium channel gene (KCND3) in CF-conditioned cultures was downregulated to undetectable levels. To verify immunostaining results, we also quantified the expression of genes coding for the major neonatal rat ventricular gap junction proteins (Cx43 and Cx45) and found no difference between conditioned and control monolayers. Finally, qRT-PCR assessment revealed that in CF-conditioned monolayers, α-MHC expression was unchanged (2.2-fold decrease, P = 0.08) while β-MHC expression increased by 17.4-fold compared to control cultures.
Figure 5.
Effects of CF-conditioned media on cardiac gene expression and single cell electrophysiology. (A) Gene expression in conditioned relative to control cultures. Cx, connexin; SCN5A, cardiac sodium channel NaV1.5 α-subunit; KCNJ2, inwardly rectifying potassium channel (Kir2.1); MHC, myosin heavy chain; KCND3, transient outward potassium channel (Kv4.3) was downregulated to an undetectable level by conditioning. (B) Representative cardiac action potentials traces from 2 Hz paced conditioned and control monolayers. (C1–3) Action potential parameters obtained from n = 18 (control) and 39 (conditioned) cells. Asterisks denote significantly different results from control.
3.7. CF-conditioned media cause depolarization, slower rise, and prolongation of action potentials in cardiomyocytes
On the basis of the altered ion channel gene expression and optical mapping studies, we expected to find altered properties of propagated cardiac action potentials (Figure 5B). Sharp microelectrode recordings in CF-conditioned monolayers when compared with control cardiac monolayers revealed significant RMP depolarization (−55.6 ± 1.3 vs. −70.1 ± 1.2 mV, Figure 5C1), slowing of action potential upstroke velocity (21.5 ± 3.6 vs. 105.9 ± 9.5 V/s, Figure 5C2) and, consistent with optical mapping, prolongation of the APD (99.6 ± 8.3 vs. 44.7 ± 4.5 ms, Figure 5C3).
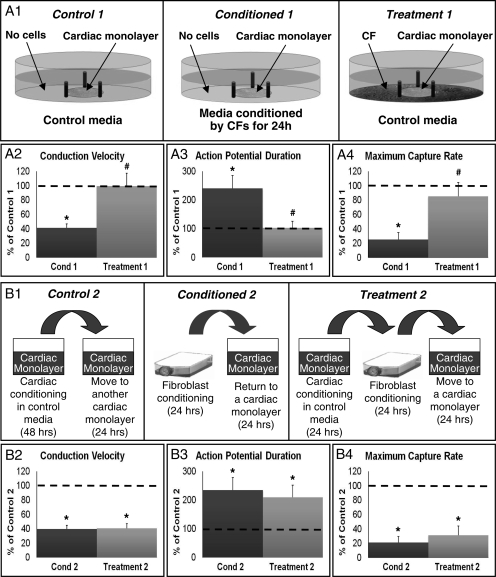
3.8. CF-conditioning in the presence of cardiomyocytes has no effect on cardiac electrical propagation
To explore whether CF-conditioning is altered in the presence of cardiomyocytes, the shared media, non-contact co-cultures consisting of cardiac monolayers surrounded by CFs were studied (Figure 6A1). Interestingly, the presence of cardiomyocytes during CF-conditioning prevented the detrimental CF paracrine action, yielding no change in cardiac electrophysiological properties compared with those of control cultures (Figure 6A2–4). These results suggest that cardiomyocytes secrete factors that either prevent or neutralize the secretion of damaging CF paracrine factors.
Figure 6.
Effects of CF-conditioning in the presence of cardiomyocytes or cardiac conditioned media. (A1) Experimental setup. Control 1, a cardiac monolayer exposed to control medium for 24 h. Conditioned 1 (Cond 1), a cardiac monolayer exposed to CF-conditioned media for 24 h. Treatment 1, a cardiac monolayer surrounded by cardiac fibroblasts (without direct contact) and exposed to control media for 24 h. (A2–A4) Electrophysiological parameters in Cond 1 and Treatment 1, shown relative to Control 1 (dashed line) group. n = 12 monolayers per group. (B1) Experimental setup. Control 2, a cardiac monolayer exposed for 24 h to media conditioned by another cardiac monolayer for 48 h. Conditioned 2 (Cond 2), the same as Condition 1 in A1. Treatment 2, a cardiac monolayer exposed for 24 h to media conditioned for 24 h by CFs after being conditioned for 24 h by a cardiac monolayer. (B2–B4) Electrophysiological parameters in Cond 2 and Treatment 2, shown relative to Control 2 (dashed line) group. n = 12 monolayers per group. Asterisks denote significantly different results from control, while hash symbols denote significantly different from conditioned.
In order to assess whether these protective cardiac factors are expressed by cardiomyocytes in a constitutive fashion or only in the presence of CFs, we applied media conditioned by cardiomyocytes for 24 h to pure CF cultures; after 24 h of CF-conditioning we added this medium to separate cardiac monolayers (Figure 6B1). Interestingly, CF-conditioning of medium previously conditioned by cardiomyocytes still exhibited the same adverse effects on the electrophysiological function of cardiac monolayers (Figure 6B2–4). These results suggest that in order to secrete self-protecting factors, cardiomyocytes have to be in the presence of a significant number of fibroblasts.
4. Discussion
The prevailing paradigm for explaining arrhythmogenesis in fibrotic hearts is that structural discontinuities due to fibrous, collagen-rich tissue5–7 create a non-uniform substrate of slowed conduction that facilitates arrhythmia induction and maintenance. In addition, the cardiac electrophysiological substrate is directly affected by the presence of CFs, which can create conduction obstacles if unconnected to surrounding cardiomyocytes, or as suggested in recent in vitro studies,19,20 act to form electrotonic sinks and short-range conductors if electrically coupled to cardiomyocytes.21 Our study proposes a novel, indirect mechanism by which fibroblast-rich areas in the diseased heart could induce localized conduction slowing in neighbouring cardiomyocytes and potentially lead to arrhythmia generation. Using well-controlled conditioned media studies and functional electrophysiological analyses, we have shown that media conditioned for only 24 h by CFs can exert adverse effects on the electrophysiological properties of neonatal rat cardiomyocytes, most notably significant CV slowing, depolarization of the RMP and APD prolongation. These effects appear to be dose dependent, stable for at least 48 h, partially recoverable 24 h after removal of the conditioned media, and induced by unknown protein factors secreted by CFs (but not another innocuous cell type). In addition, these effects were prevented if fibroblast conditioning occurred in the presence of cardiomyocytes, but not cardiac conditioned media, suggesting the existence of a physiological cross-talk mechanism that prevented these detrimental effects given the normal balance of myocytes and fibroblasts. The observed effects in cardiac monolayers were mainly caused by the downregulation of specific cardiac ion channel genes (SCN5A, KCNJ2, and KCDN3) rather than decreased intercellular coupling, increased apoptosis, or fibroblast proliferation. Finally, these electrophysiological changes were associated with an increase in cardiac cell size and an upregulation of β-MHC gene expression (i.e. hallmarks of pathological hypertrophy in rats).
Cardiac fibroblasts have previously been shown to produce soluble factors that affect cardiomyocyte size and function. Specifically, similar to our study, the study by Harada showed that CF-conditioned media increased protein expression in cardiomyocytes.17,18 Guo et al.22 reported APD prolongation and Kv4.2 downregulaton in CF-conditioned cardiomyocytes, similar to the downregulation of the Kv4.3 subunit gene (KCND3) and the APD prolongation observed in our study. In the study by Suzuki,17 CF-conditioned media increased the incidence of cardiomyocyte spontaneous beating, a finding which in our study was likely caused by the significant membrane depolarization of conditioned cells23,24 (Figure 5B and C1). Although no electrophysiological differences were found between spontaneously beating and quiescent CF-conditioned monolayers, further studies are needed to discern if the intracellular calcium oscillations in spontaneously beating CF-conditioned cells directly alter the expression of different genes and proteins.
4.1. The effects of CF-conditioned media on the expression of genes and proteins related to cardiac electrophysiological and contractile functions
On the basis of the qRT-PCR analysis (Figure 5A), CF paracrine factors altered the expression of several cardiac ion channel genes. Specifically, we observed a 3.8-fold downregulation of SCN5A, the gene encoding the α-subunit of the cardiac sodium channel (NaV1.5), and a 6.6-fold downregulation of KCNJ2, the gene encoding the inwardly rectifying potassium channel (Kir2.1). As expected from the downregulation of the Kir2.1 channel and the resulting current (Ik1),23,25 the RMP in conditioned cardiomyocytes was significantly depolarized when compared with that of unconditioned cells (Figure 5B and C1). The combination of decreased cell excitability (due to downregulation of SCN5A gene expression) and reduced sodium current availability (due to sodium channel inactivation at depolarized resting potentials) synergistically produced a slow action potential upstroke and significant conduction slowing, despite unaltered gap junction gene (Figure 5A) and protein (Figure 4A–C) expression and function (Figure 4D). Furthermore, KCND3, the gene encoding the KV4.3 transient outward potassium channel [i.e. the major repolarizing current (Ito) in rats], was downregulated to undetectable levels in the conditioned cardiomyocytes, probably contributing the observed APD prolongation and MCR decrease (Figure 1C2–3).26 Although the observed decrease in Ito and/or Ik1 gene expression could account for the extent of the observed APD prolongation,23,25,26 changes in the expression of related interacting proteins (e.g. KChIP227) and other ion channels (e.g. L-type Ca current, Na–Ca exchanger) cannot be excluded as potential contributors.
We also examined the expression of genes related to cardiac contractile function. Significant upregulation of β-MHC, as found in CF-conditioned cardiac cultures, is considered a hallmark of pathological hypertrophy and heart failure in rodents.28 In particular, when upregulated in the place of the α-MHC isoform in rodents, β-MHC yields reduced shortening velocity of the cardiac myofibers29 and decreased contractility.30 Recently, upregulation of β-MHC in a mouse model of cardiac hypertrophy has been shown to occur predominately in cardiomyocytes neighbouring the localized regions of fibrosis.29 One of the potential explanations for this finding based on our results could be that locally secreted paracrine factors from fibrotic regions induced upregulation of β-MHC in surrounding cardiomyocytes.
4.2. A balanced paracrine cross-talk may exist between neonatal rat cardiomyocytes and CFs
Interestingly, CF-conditioning in the presence of cardiomyocytes exerted no adverse effects on cardiac electrophysiological function. On the basis of this finding, we can speculate the existence of a balanced paracrine cross-talk between the neonatal rat cardiomyocytes and CFs as follows. In response to a damaging substance X produced by CFs, the cardiomyocytes produce protective substance Y, which in turn acts on CFs to either block their production of X or bind with X to form an inactive complex. On the basis of the results that cardiac conditioned media alone do not prevent CF secretion of damaging factors and that the cardiomyocyte self-protection does not take place without the presence of CFs, we further speculate that cardiomyocytes must be both (i) ‘activated’ by the CF-conditioned media and (ii) able to act on neighbouring CFs for this protective cross-talk action to occur.
Importantly, if this hypothetical in vitro scenario is extrapolated to native cardiac tissue, a physiological cellular mixture of cardiomyocytes and CFs in the healthy heart would be expected to be balanced, such that the existence of an efficient cross-talk between cardiomyocytes and CFs would prevent the described CF paracrine actions on cardiomyocytes. However, if the number of fibroblasts was locally increased and/or the number of cardiomyocytes was locally decreased, such as in fibrosis resulting from infarction, cardiomyopathies, myocarditis, etc., the protective balance would be compromised and paracrine action from CFs could negatively affect the function of surrounding cardiomyocytes. As a consequence, locally altered electrophysiological properties of cardiomyocytes would increase the susceptibility of the heart to lethal arrhythmias.
4.3. Clinical implications
Any potential clinical implications of this study can only be speculated as the neonatal age of the studied cells and the use of an in vitro culture setting may render our findings not applicable to the adult heart in vivo. Nevertheless, the observed CV slowing and APD prolongation due to the downregulation of specific ion currents (Ito, IK1, and INa) as well as the increased β-MHC gene expression (with a trend towards decreased α-MHC expression) found in this study mirror some of the functional changes observed in acquired arrhythmias,31,32 acute and healed myocardial infarction,32 and chronic heart failure.32–34 In contrast, significantly decreased expression and phosphorylation of Cx43, also present in some of these diseases,35 have not been observed in the current study. It is important to note that cardiomyocytes in different pathologies often experience ischaemia, changes in oxidative stress, mechanical strain, or systemic renin–angiotensin activation, etc. Our studies, however, suggest that independent of these factors, increased fibroblast paracrine action alone might be sufficient to significantly alter cardiomyocyte electrical function and, thus, might eventually have a prospect as a potential pharmacological target.
Identifying the related paracrine factors and signalling mechanisms involved in the hypothesized cross-talk scenario is the next step in our studies. While media conditioned by fibroblasts were previously shown to contain VEGF, GFO/KC, MCP-1, leptin, MIP-1a, IL-6, IL-10, IL-12p70, IL-17, TNF-α, TGF-β, and RANTES,36 a comprehensive proteomic and functional analysis will be needed to identify specific paracrine factors that exerted the observed adverse effects on cardiomyocyte function. Furthermore, the use of DNA microarrays to investigate gene expression in fibroblasts and cardiomyocytes when cultured alone and in the non-contact co-cultures may offer further clues to the signalling pathways involved in the described cardiac–fibroblast paracrine interactions.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by the American Heart Association [0515377U to D.P. and 0530256N to N.B.] and the National Heart, Lung, and Blood Institute [HL083342].
Acknowledgements
The authors acknowledge James Scull for assistance with the optical mapping setup, Nima Badie for assistance with data analysis, Dr Howard Rockman for critical reading of the manuscript, and Dr Hyung-Suk Kim, Director of the Gene Expression Core Facility at the University of North Carolina, for assistance with the design of the qRT-PCR primers.
Conflict of interest: none declared.
References
- 1.Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res. 2005;65:40–51. doi: 10.1016/j.cardiores.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 2.Nag A. Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution. Cytobios. 1980;28:41–61. [PubMed] [Google Scholar]
- 3.Banerjee I, Yekkala K, Borg TK, Baudino TA. Dynamic interactions between myocytes, fibroblasts, and extracellular matrix. Ann N Y Acad Sci. 2006;1080:76–84. doi: 10.1196/annals.1380.007. [DOI] [PubMed] [Google Scholar]
- 4.Brown R, Ambler S, Mitchell M, Long C. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol. 2005;45:657–687. doi: 10.1146/annurev.pharmtox.45.120403.095802. [DOI] [PubMed] [Google Scholar]
- 5.de Bakker J, van Capelle F, Janse M, Tasseron S, Vermeulen J, de Jonge N, et al. Slow conduction in the infarcted human heart. ‘Zigzag’ course of activation. Circulation. 1993;88:915–926. doi: 10.1161/01.cir.88.3.915. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka K, Zlochiver S, Vikstrom KL, Yamazaki M, Moreno J, Klos M, et al. Spatial distribution of fibrosis governs fibrillation wave dynamics in the posterior left atrium during heart failure. Circ Res. 2007;101:839–847. doi: 10.1161/CIRCRESAHA.107.153858. [DOI] [PubMed] [Google Scholar]
- 7.Spach M, Boineau J. Microfibrosis produces electrical load variation due to loss of side-to-side connections: a major mechanism of structural heart disease arrhythmias. Pacing Clin Electrophysiol. 1997;20:397–413. doi: 10.1111/j.1540-8159.1997.tb06199.x. [DOI] [PubMed] [Google Scholar]
- 8.Gray M, Long C, Kalinyak J, Li H, Karliner J. Angiotensin II stimulates cardiac myocyte hypertrophy via paracrine release of TGF-beta1 and endothelin-1 from fibroblasts. Cardiovasc Res. 1998;40:352–363. doi: 10.1016/s0008-6363(98)00121-7. [DOI] [PubMed] [Google Scholar]
- 9.Bursac N, Parker KK, Iravanian S, Tung L. Cardiomyocyte cultures with controlled macroscopic anisotropy: a model for functional electrophysiological studies of cardiac muscle. Circ Res. 2002;91:e45–e54. doi: 10.1161/01.res.0000047530.88338.eb. [DOI] [PubMed] [Google Scholar]
- 10.Pedrotty DM, Klinger RY, Badie N, Hinds S, Kardashian A, Bursac N. Structural coupling of cardiomyocytes and noncardiomyocytes: quantitative comparisons using a novel micropatterned cell pair assay. Am J Physiol Heart Circ Physiol. 2008;295:H390–H400. doi: 10.1152/ajpheart.91531.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tung P, Fritz I. Sertoli cells in culture secrete paracrine factor(s) that inhibit peritubular myoid cell proliferation: identification of heparinoids as likely candidates. J Cell Physiol. 1991;147:470–478. doi: 10.1002/jcp.1041470313. [DOI] [PubMed] [Google Scholar]
- 12.Klinger R, Bursac N. Cardiac cell therapy in vitro: reproducible assays for comparing the efficacy of different donor cells. IEEE Eng Med Biol Mag. 2008;27:72–80. doi: 10.1109/MEMB.2007.913849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbaci M, Barberi-Heyob M, Stines J, Blondel W, Dumas D, Guillemin F, et al. Gap junctional intercellular communication capacity by gap-FRAP technique: a comparative study. Biotechnol J. 2007;2:50–61. doi: 10.1002/biot.200600092. [DOI] [PubMed] [Google Scholar]
- 14.Wade M, Trosko J, Schindler M. Fluorescence photobleaching assay of gap junction-mediated communication between human cells. Science. 1986;232:525–528. doi: 10.1126/science.3961495. [DOI] [PubMed] [Google Scholar]
- 15.Livak K, Schmittgen T. Analysis of relative gene expression data using real-time quantitative PCR and the 2-delta deltaCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Fedorov VV, Lozinsky IT, Sosunov EA, Anyukhovsky EP, Rosen MR, Balke CW, et al. Application of blebbistatin as an excitation-contraction uncoupler for electrophysiologic study of rat and rabbit hearts. Heart Rhythm. 2007;4:619–626. doi: 10.1016/j.hrthm.2006.12.047. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki T, Tsuruda A, Katoh S, Kubodera A, Mitsui Y. Purification of endothelin from a conditioned medium of cardiac fibroblastic cells using beating rate assay of myocytes cultured in a serum-free medium. J Mol Cell Cardiol. 1997;29:2087–2093. doi: 10.1006/jmcc.1997.0443. [DOI] [PubMed] [Google Scholar]
- 18.Harada M, Itoh K, Nakagawa O, Ogawa Y, Miyamoto Y, Kuwahara K, et al. Significance of ventricular myocytes and nonmyocytes interaction during cardiocyte hypertrophy: evidence for endothelin-1 as a paracrine hypertrophic factor from cardiac nonmyocytes. Circulation. 1997;96:3737–3744. doi: 10.1161/01.cir.96.10.3737. [DOI] [PubMed] [Google Scholar]
- 19.Gaudesius G, Miragoli M, Thomas SP, Rohr S. Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac origin. Circ Res. 2003;93:421–428. doi: 10.1161/01.RES.0000089258.40661.0C. [DOI] [PubMed] [Google Scholar]
- 20.Chilton L, Giles WR, Smith GL. Evidence of intercellular coupling between co-cultured adult rabbit ventricular myocytes and myofibroblasts. J Physiol. 2007;583:225–236. doi: 10.1113/jphysiol.2007.135038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohl P, Camelliti P, Burton FL, Smith GL. Electrical coupling of fibroblasts and myocytes: relevance for cardiac propagation. J Electrocardiol. 2005;38(Suppl.):45–50. doi: 10.1016/j.jelectrocard.2005.06.096. [DOI] [PubMed] [Google Scholar]
- 22.Guo W, Kamiya K, Yasui K, Kodama I, Toyama J. Paracrine hypertrophic factors from cardiac non-myocyte cells downregulate the transient outward current density and Kv4.2 K+ channel expression in cultured rat cardiomyocytes. Cardiovasc Res. 1999;41:157–165. doi: 10.1016/s0008-6363(98)00157-6. [DOI] [PubMed] [Google Scholar]
- 23.Miake J, Marban E, Nuss H. Functional role of inward rectifier current in heart probed by Kir2.1 overexpression and dominant-negative suppression. J Clin Invest. 2003;111:1529–1536. doi: 10.1172/JCI17959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miragoli M, Salvarani N, Rohr S. Myofibroblasts induce ectopic activity in cardiac tissue. Circ Res. 2007;101:755–758. doi: 10.1161/CIRCRESAHA.107.160549. [DOI] [PubMed] [Google Scholar]
- 25.Sekar R, Kizana E, Cho H, Molitoris J, Hesketh G, Eaton B, et al. IK1 heterogeneity affects genesis and stability of spiral waves in cardiac myocyte monolayers. Circ Res. 2009;104:355–364. doi: 10.1161/CIRCRESAHA.108.178335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bursac N, Papadaki M, White JA, Eisenberg SR, Vunjak-Novakovic G, Freed LE. Cultivation in rotating bioreactors promotes maintenance of cardiac myocyte electrophysiology and molecular properties. Tissue Eng. 2003;9:1243–1253. doi: 10.1089/10763270360728152. [DOI] [PubMed] [Google Scholar]
- 27.Deschenes I, Armoundas AA, Jones SP, Tomaselli GF. Post-transcriptional gene silencing of KChIP2 and Navbeta1 in neonatal rat cardiac myocytes reveals a functional association between Na and Ito currents. J Mol Cell Cardiol. 2008;45:336–346. doi: 10.1016/j.yjmcc.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harada K, Sugaya T, Murakami K, Yazaki Y, Komuro I. Angiotensin II type 1A receptor knockout mice display less left ventricular remodeling and improved survival after myocardial infarction. Circulation. 1999;100:2093–2099. doi: 10.1161/01.cir.100.20.2093. [DOI] [PubMed] [Google Scholar]
- 29.Pandya K, Kim H-S, Smithies O. Fibrosis, not cell size, delineates beta-myosin heavy chain reexpression during cardiac hypertrophy and normal aging in vivo. Proc Natl Acad Sci USA. 2006;45:16864–16869. doi: 10.1073/pnas.0607700103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herron T, Vandenboom R, Fomicheva E, Mundada L, Edwards T, Metzger J. Calcium-independent negative inotropy by beta-myosin heavy chain gene transfer in cardiac myocytes. Circ Res. 2007;100:1182–1190. doi: 10.1161/01.RES.0000264102.00706.4e. [DOI] [PubMed] [Google Scholar]
- 31.Marban E. Cardiac channelopathies. Nature. 2002;415:213–218. doi: 10.1038/415213a. [DOI] [PubMed] [Google Scholar]
- 32.Nattel S, Maguy A, Le Bouter S, Yeh Y-H. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev. 2007;87:425–456. doi: 10.1152/physrev.00014.2006. [DOI] [PubMed] [Google Scholar]
- 33.Nass RD, Aiba T, Tomaselli GF, Akar FG. Mechanisms of disease: ion channel remodeling in the failing ventricle. Nat Clin Pract Cardiovasc Med. 2008;5:196–207. doi: 10.1038/ncpcardio1130. [DOI] [PubMed] [Google Scholar]
- 34.Miyata S, Minobe W, Bristow M, Leinwand L. Myosin heavy chain isoform expression in the failing and nonfailing human heart. Circ Res. 2000;86:386–390. doi: 10.1161/01.res.86.4.386. [DOI] [PubMed] [Google Scholar]
- 35.Severs NJ, Bruce AF, Dupont E, Rothery S. Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc Res. 2008;80:9–19. doi: 10.1093/cvr/cvn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LaFramboise W, Scalise D, Stoody P, Graner S, Guthrie R, Magovern J, et al. Cardiac fibroblasts influence cardiomyocyte phenotype in vitro. Am J Physiol Cell Physiol. 2007;292:C1799–C1808. doi: 10.1152/ajpcell.00166.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.