Abstract
The therapeutic use of ultrasound contrast agents (UCAs) is an emerging methodology with high potential for enhanced directed therapeutic gene, bioactive gas, drug, and stem cell delivery. Ultrasound-targeted microbubble destruction has already demonstrated feasibility for plasmid DNA delivery. Similarly, therapeutic ultrasound for thrombolysis treatment has been taken into the clinical setting, and the addition of UCAs for therapeutic delivery or enhanced effect through cavitation is a natural progression to this investigation. However, as with any new technique, safety needs to be first demonstrated before translation into clinical practice. This review article will focus on the development of UCAs for cardiac and vascular therapeutics as well as the limitations/concerns for the use of therapeutic ultrasound in clinical medicine in order to lay a foundation for investigators planning to enter this exciting field or for those who want to broaden their understanding.
Keywords: Ultrasound, Ultrasound contrast agents, Targeted delivery, Drug and gene delivery
Ultrasound is best known as a diagnostic imaging tool in clinical medicine. However, the use of ultrasound for therapy actually predated the development of ultrasound for diagnosis. In the 1930s, ultrasound was first introduced in Europe as a modality to generate heat for deep tissue warming (thermal bioeffect).1 Current interest in the use of ultrasound for therapy, however, centres on its ‘non-thermal bioeffects’. Ultrasound induces an oscillating convective motion to partially absorbing liquids which may enhance the diffusion of drugs in vivo.2 This technique, termed phonophoresis, has since been used to drive high molecular weight peptides and proteins through intact skin.3–5
The other important ultrasound non-thermal bioeffect is termed ‘cavitation’. This is the biophysical interaction between the ultrasonic field in a liquid and a gaseous inclusion (or bubble).6 There are two types of cavitation: (i) gas body cavitation (or stable cavitation) and (ii) inertial cavitation (or transient cavitation).6 In gas body cavitation, the bubble undergoes periodic and regular changes in volume in response to the applied acoustic pressure. This results in mechanical vibration within the tissue, creating eddies of current flow around the oscillating bubble. In contrast, in the case of inertial cavitation, although the bubble also undergoes periodic changes, these changes are qualitatively different in volume—rapidly increasing in size, becoming unstable, and then imploding violently. This can then result in acoustic microstreaming, hydrodynamic flow around bubbles, and bubble–cell collisions.6 In addition, high temperatures can be generated briefly during bubble collapse such that free radical and sonochemical formation is possible.1 Fortunately, cavitation is rare in vivo due to the lack of naturally occurring gas inclusions in living biological tissues.
1. Ultrasound contrast agents
In the late 1960s, it was observed that agitated saline gave strong echoes during echocardiography.7 These strong echoes were produced because of acoustic mismatch between free air microbubbles in the agitated saline solution and the surrounding blood. Although efficient reflectors of ultrasound, microbubbles produced by agitation were large and unstable, and hence, were unsuitable as a viable clinical contrast agent. It was the development of coated microbubbles (which have the advantage of being stable in vivo as the shells serve to protect the diffusion of gas into the bloodstream allowing transpulmonary passage)8 that essentially ‘jump-started’ the field of contrast echocardiography. Second-generation contrast agents, which contain perfluorocarbon gas rather than air (resulting in longer life span in the circulation), are now routinely used in clinical echocardiography. To date, these agents are being used not only for left ventricular opacification, but are also being developed to explore new functional imaging methods, including imaging of capillary flow9 and myocardial microperfusion.10,11 Myocardial contrast echocardiography (MCE) allows for the real-time assessment of myocardial perfusion by imaging the coronary microcirculation.11–13 Applications include defining the presence and size of the area at risk during acute coronary occlusions,14,15 evaluating the success of tissue reperfusion and residual infarct size,16,17 risk stratification for patients in the emergency department who present with chest pain and non-diagnostic electrocardiograms,18,19 and the assessment of myocardial viability and hibernating myocardium in patients with heart failure to guide further therapy.20,21 To date, however, there are still no ultrasound contrast agents (UCAs) approved by the Food and Drug Administration (FDA) for MCE, and most of the clinical experience reported have been obtained in a research setting. Finally, UCAs have opened up the possibility for molecular-targeted imaging using labelled microbubbles with better spatial and temporal resolution compared with computerized tomography or magnetic resonance imaging.22–26
2. Ultrasound and UCAs
In 1990, Holland and Apfel27 showed that UCAs can significantly lower the acoustic cavitation production threshold. This work suggested that UCAs can be used to induce cavitation for therapeutic applications with much lower ultrasound energy. Cavitation effects can then be harnessed to cause a local controlled ‘shockwave’ to improve cellular uptake of the drug or gene. The reduction in ultrasound energy needed to produce this effect may allow for a reduction in ultrasound exposure time and obviate the potential heating effect that could harm surrounding normal tissues.
The earliest study that reported the use of UCAs for therapeutic application was by Tachibana and Tachibana.28 In this study, they demonstrated that albumin microbubbles (Albunex) accelerated urokinase-mediated thrombolysis, shortening lysis time to one-fifth. It is hypothesized that the contrast agent adheres to the clot with resultant shearing effect during bubble destruction. This mechanically erodes the clot allowing more fibrin to be exposed to the lytic agent.
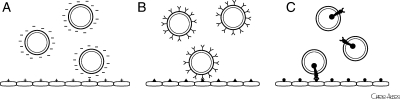
Since then, many investigators have reported on the syngeristic effect of ultrasound and UCAs for directed drug and gene delivery.29–39 Efficacy further improves by promoting retention of the delivery agent to the diseased site using targeting techniques. Targeting can achieved by (i) taking advantage of the inherent chemical or electrostatic properties of the microbubble shell, (ii) attachment of specific antibodies or other ligands directed to disease antigens to the microbubble surface,40 or (iii) incorporating therapeutics with ‘homing’ capabilities for their target site, such as tissue-plasminogen activator (tPA) (Figure 1).41,42 tPA contains fibrin-binding domains and our group has demonstrated retention of both fibrin-binding property and proteolytic activity upon incorporation of this drug into an UCA.41,42
Figure 1.
Targeting strategies for UCAs to diseased sites. (A) Inherent chemical or electrostatic properties of the microbubble shell to allow non-covalent binding to the diseased tissue, (B) attachment of specific antibodies or other ligands on the microbubble surface directed to disease antigens, and (C) incorporation of therapeutics with ‘homing’ capabilities for their target site.
Efficient targeting of UCAs depends on several important factors including (i) selective binding and localization of the ligand to the desired target tissue, (ii) stable and prolonged attachment to the target site with a long-circulating half-life, (iii) reproducible coupling chemistry of the ligand to the UCA, (iv) minimal toxicity and immunogenicity, (v) cost-efficiency, and (vi) ease of preparation and administration. An antibody-based strategy for targeting has several advantages, including excellent bond dissociation constants and high affinity and specificity to the target antigens.43 However, this strategy also has potential problems including relatively low bond formation rates that could prevent adequate attachment of the vector in high-shear vessels.44 Temporary interruption of flow has been used to obviate this problem in animal studies;32 however, this has limited clinical application. Immune response up-regulation and the ability to take non-FDA approved antibodies or peptides into the clinical setting are other concerns. Nevertheless, we and others43,45,46 have demonstrated efficient targeting to vascular surface antigens, such as adhesion molecules, in animal models of atherosclerosis, validating the feasibility of an antibody-based targeting strategy for directed drug and gene delivery. One exciting research focus is the use of non-destructive ultrasound energy (radiation force) to direct the drug- or gene-loaded UCA towards the vessel wall in order to improve contact between the ligand and the delivery agent at the diseased site.47,48
Recently, it has been demonstrated that microbubbles can be intentionally ruptured by ultrasound [referred to as ultrasound-targeted microbubble destruction (UTMD)],49,50 which may then be exploited to enhance drug and gene delivery into cells. UTMD not only leads to directed release of the therapeutic at the desired site, but also produces microjets or microstreaming during bubble collapse6 that could promote diffusion of the therapeutic into the cell.51 The resultant shear stress on the cellular surface then results in increased membrane permeability by formation of transient non-lethal perforations on the cell membrane (sonoporation),52 or an increase in membrane fluidity.53 Ingress of therapeutics may also result from endocytosis of the microbubble, fusion of the microbubble membrane with the cell membrane, or a combination of these mechanisms.53 The underlying mechanism likely differs for each drug/gene-microbubble complex used and may also be variable depending on the ultrasound energy applied. Targeting to position the microbubble close to the diseased tissue is critical, as particle destruction and drug/gene release occurring in the centre of the vessel wall, for instance, will only result in the therapeutic being released to the bloodstream with ensuing reduced efficacy. In this regard, the use of UCAs affords another advantage, in that delivery to the diseased site can be confirmed by highlighting of pathological tissues before the application of ultrasound energy for triggered drug release.
3. UCAs for delivery of cardiovascular therapeutics
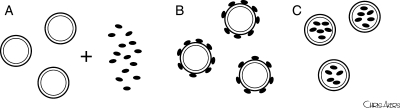
There are three methods to use UCAs for therapeutic delivery. The two more common applications of this technique are: (i) to co-administer the microbubble and the bioactive substance together (Figure 2A) and (ii) to incubate the microbubble and the therapeutic together for a certain amount of time, before administering the complex to the target site (Figure 2B). Drug- or gene-bearing non-echogenic vehicles/vectors can also be made and then co-administered or incubated with an acoustically active microbubble. These techniques allow attachment of the therapeutic to the microbubble shell, either by electrostatic or weak non-covalent interactions. The microbubble can then be targeted to the diseased site by ligand conjugation or other techniques as described above. The third methodology to use UCAs as therapeutic delivery agents is (iii) to manufacture the microbubbles de novo, incorporating the gene or drug into the shell or lumen (Figure 2C). The first two techniques will be reviewed together in the following section, whereas the third methodology will be discussed separately.
Figure 2.
Strategies for therapeutic delivery using UCAs. (A) Co-administration of the microbubble and the bioactive substance together, (B) incubation of the microbubble and the therapeutic together to form a therapeutic-bubble complex prior to delivery to the target site, and (C) de novo manufacture of the microbubble to incorporate the therapeutic into the microbubble shell or lumen.
3.1. Co-administration of microbubbles and therapeutics
3.1.1. Gene therapy
In 1997, Bao et al.29 described the use of ultrasound and albumin-coated microbubbles to enhance transfection of luciferase reporter plasmid into cultured hamster cells. In 2000, Shohet et al.30 demonstrated that ultrasound can be used to direct transgene expression in the rat myocardium using albumin microbubbles. However, an adenoviral vector was used, and this had the disadvantage of low organ specificity, strong hepatic tropism, and immunogenicity which limited repeated applications. In 2003, Erikson et al.31 reported delivery of antisense oligonucleotides and perfluorocarbon-exposed sonicated dextrose albumin (PESDA) to rat hearts and demonstrated blunted ischaemia/reperfusion-induced tumour necrosis factor-α expression. In 2004, Hashiya et al.32 demonstrated effective transfection of rat carotid arteries with anti-oncogene (p53) plasmids in the presence of Optison®, to effect a decrease in intimal proliferation after balloon injury. In 2007, Suzuki et al.33 reported development of a novel polyethyleneglycol-modified liposome encapsulating nanobubbles of perfluoropropane which can be used as a vector for gene delivery into mouse femoral arteries. Interestingly, gene uptake using their ‘bubble liposome’ was limited to the area exposed to ultrasound, with implications for ultrasound-directed site-specific gene delivery. These studies, and many others, laid the foundation for in vivo work in larger animal models. In 2001, Porter et al.34 demonstrated a reduction in both per cent area stenosis measured by intravascular ultrasound and intimal thickness measured at histology, in the carotid arteries of a chronic balloon-injured pig model after treatment with intravenous antisense oligonucleotide bound to PESDA and targeted ultrasound, compared with antisense oligonucleotide without microbubbles, despite similar ultrasound application. In 2002, Vannan et al. used intravenous cationic lipid microbubbles containing perfluorobutane gas to deliver plasmid chloramphenicol acetyltransferase (pCAT), a marker gene, to the myocardium in closed-chest mongrel dogs.35 In this study, the authors demonstrated CAT activity in the myocardium of only those animals that received microbubble-linked pCAT plus ultrasound exposure and not in the groups which received pCAT alone, or pCAT with either microbubble or ultrasound application, but not both. Despite substantial efforts in this field, however, concerns over safety and low transfection efficiencies remain. Most in vivo experiments employed artificial delivery methods, such as isolation of arterial segments31 or cessation of blood flow,32 which are not adaptable to clinical studies or chronic disease conditions, and to date, only few studies have been carried out in large animal models.34,35 Despite its great potential, much work remains before this technique can be taken into the clinical arena for gene therapy.
3.1.2. Drug therapy
In contrast, ultrasound-mediated drug therapy with thrombolytics has already reached the clinical arena, not only for the treatment of deep venous thrombosis54 and lower limb arterial ischaemia,55 but also in the treatment of acute coronary syndromes56,57 and acute ischaemic strokes.58,59 The addition of UCAs to augment ultrasound-mediated thrombolysis is a natural progression of this technique. The synergistic effect of ultrasound and microbubbles on sonothrombolysis has been demonstrated in canine models of arteriovenous dialysis graft thrombosis.36,37 Recently, Molina et al.38 demonstrated acceleration of ultrasound-enhanced thrombolysis in patients with acute ischaemic stroke by the addition of Levovist, resulting in more complete arterial recanalization and a trend towards better in-hospital and 3-month outcomes. In a separate study, Perren et al.39 also demonstrated improved neurological scores and residual flow in patients who received microsphere-potentiated ultrasound-enhanced thrombolysis compared with those treated with lytic and ultrasound only. Further clinical studies are underway looking into other UCAs, such as more stable perflutren-lipid microspheres, which may give us more information regarding safety and efficacy.
Another application of ultrasound and UCAs is in the delivery of proteins that induce growth of endothelial cells, such as vascular endothelial growth factor (VEGF). In 2000, Mukherjee et al.60 demonstrated a 13-fold increase in VEGF uptake in the rat myocardium using ultrasound and PESDA. In 2003, the same investigators demonstrated reduced infarct area/risk area ratio in a canine model of chronic myocardial ischaemia treated with intravenous infusion of VEGF, albumin microbubbles, and directed ultrasound.61 They also demonstrated increased myocardial blood flow to the ischaemic territory, suggesting a new approach for therapeutic angiogenesis. On the other hand, ultrasound and UCAs are also being investigated to induce vascular endothelial damage in an innovative strategy for non-invasive, highly targeted therapeutic thrombosis of small vessels.62 In a rabbit model, Hwang et al.62 demonstrated thrombus formation in auricular veins treated with ultrasound and Optison® with (subthrombogenic doses of) thrombin. No clot formation was observed in the vessels treated with ultrasound and Optison® only or in control vessels treated with thrombin only. Although sample size was small, the authors hypothesized that this technique causes targeted endothelial injury, exposing thrombogenic surfaces along the vessel wall, thus activating vascular thrombosis.62 Clinical applications include restriction of blood flow to malignant tumours and occlusion of gastric varices.
3.1.3. Stem cell therapy
An exciting novel application of UCAs is for the enhancement of stem cell delivery. Therapeutic approaches to stem cell therapy include stimulating stem cells by restoring the capacity of the bone marrow to produce precursor cells or the administration of exogenous precursor cells to treat the specific disease state. The intramuscular transplantation of bone-marrow-derived endothelial progenitor cells has already been investigated in clinic trials to induce angiogenesis in the ischaemic limb.63 Other clinical applications include intracoronary delivery for acute myocardial infarction64,65 and intramyocardial injection for ischaemic cardiomyopathy.66,67 There is evidence, however, that vascular permeability and vasodilation are critical for enhancement of the cardiovascular effects of stem cell therapy.68 In 2006, Zen et al.69 reported targeted delivery of bone-marrow-derived mononuclear cells, co-administered with Optison®, to the myocardium of a hamster model of cardiomyopathy with the use of pulsed ultrasound applied to the anterior chest of the animal. The authors demonstrated induction of regional angiogenic response, which they hypothesized may be related to the enhanced attachment of the progenitor cells to the targeted vascular endothelium, in the group treated with ultrasound and microbubble. This increased neovessel formation and blood flow in turn resulted in an improvement in cardiac function with inhibition of myocyte apoptosis and interstitial fibrosis.69
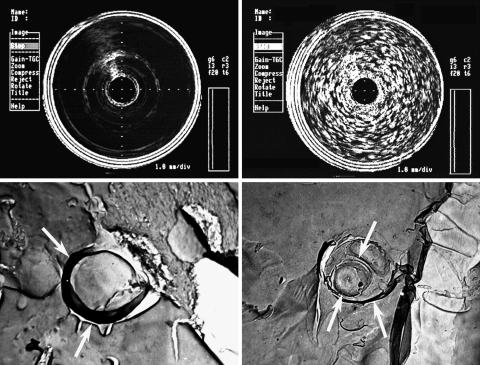
Our group has developed a targeted UCA, which we term ‘echogenic liposomes’ (ELIP) (Figure 3).70 By a specific process that includes lyophilization, we are able to stably entrap air in between the lipid bilayers, rendering the agent echogenic.71 During manufacture of these agents, we manipulate their targeting capabilities by the specific attachment of ligands.72 Recently, we have developed ‘bifunctional-ELIP’ for bridging stem cell markers and cellular adhesion molecules.73 Using dual ligand attachment, we have demonstrated facilitated binding of CD34+ haematopoietic stem cells and CD146+ mesenchymal stem cells to atherosclerotic porcine arterial segments.74 Ultrasound treatment not only enhanced stem cell attachment to the vascular endothelium, but also appeared to promote migration/penetration of the stem cells into the aortic wall in apoprotein-E knock-out mice.75 Interestingly, histopathological studies also demonstrated increased vascular outgrowth in the vessels treated with stem cells, bifunctional-ELIP and ultrasound. It is evident, however, that the use of ultrasound and UCAs for stem cell therapy is still in its infancy, and many questions remain unanswered, including mechanism of effect, duration of activity, and optimal dosing strategies. Nevertheless, we and others have demonstrated that this technique appears to be feasible with potential as an efficient non-invasive approach for stem cell therapy.
Figure 3.
(Top) Intravascular ultrasound images of 0.1 M phosphate buffer (left; control) and highly echogenic liposomes (right; 10 mg lipid/mL buffer) in a 10 mL glass vial. (Bottom) Freeze fracture electron microscopy of a liposome formulation that is poorly acoustically active (left) and highly acoustically active (right). Note the spheres within the acoustically active formulation vs. the single outer layer of the non-acoustically active formulation. Arrows point to the membrane.
3.2. De novo manufacture of microbubbles and encapsulated therapeutics
The third methodology to use UCAs as therapeutic delivery agents is to manufacture the microbubbles de novo, incorporating the gene or drug into the shell or lumen (Figure 2C). Theoretically, this allows design flexibility to prefabricate a diagnostic/therapeutic agent for a specific purpose. If we envision the cavitation event to be centred about the bubble nucleus, then the destruction of the bubble itself may impart a ‘ballistic’ effect to drive the encapsulated drug or gene locally nearer to the target tissue.76 Kodama et al. demonstrated that transient membrane permeability induced by ultrasonic cavitation occurs between 5 µm from the centre of the bubble up to a certain distance in which exogenous molecules may be delivered into the cytoplasm.77 Hence, a cavitation agent that itself contains the therapeutic may be advantageous in this respect. Entrapping the drug or gene within the contrast agent may also protect it from degradation or metabolism in the circulation. Making the agent de novo also allows manipulation of the microbubble itself for site-specific targeting. Finally, drug/gene delivery can be monitored in real-time with ultrasound in the diagnostic mode as the drug/gene carrier themselves are UCAs. An important concept, however, is that the bioactive substance is amenable to being released from the microbubble by UTMD in order to increase local concentration of the gene or drug at the target site. This concept exacts specific requirements on the microbubble including bubble shell rigidity and acoustic activity.
Several agents are currently in development or already in pre-clinical studies that are both targeted diagnostic UCAs and therapeutic vehicles to encapsulate drugs or genes. In 1998, Unger et al.78 described the development of acoustically active lipospheres (MRX-552) carrying paclitaxel for chemotherapeutic drug delivery. This agent consists of a thick fluid shell in which a drug may be suspended. This shell permitted a relatively large payload of drugs to be carried; destruction by an external acoustic field then released the drug, with stability decreasing as ultrasound energy was increased. Furthermore, acute toxicity studies in mice showed a 10-fold reduction in toxicity of this agent compared with free paclitaxel.78
Unger et al.79 have also developed a perfluorocarbon nanoemulsion (a mean diameter of 200 nm) with cationic lipids and DNA (FluoroGene™) which can also act as a cavitation nucleus. Electron microscopy studies have shown that the DNA is trapped in the centre of the fluorocarbon material and condensed into small electron-dense structures within the fluorocarbon core. These investigators have incorporated targeting ligands such as fibroblast growth factor (FGF) to these agents and have shown in cell culture studies enhanced transfection of cells bearing the receptor for FGF.79 Lanza et al.80 have also developed liquid perfluorocarbon nanoparticle emulsions which can be used as a targeted UCA. These investigators are exploring non-cavitational approaches to augment drug/gene delivery through lipid membrane fusion and lipid exchange using this agent. They have demonstrated marked enhancement of lipid delivery to C32 melanoma cells in vitro using this agent targeted to αvβ3-integrins and ultrasound.81 They concluded that cellular interaction of these nanoparticles is more likely under a combination of targeting and ultrasound, and that this technique has potential for delivery of lipophilic drugs without untoward ultrasound bioeffects.
As described earlier, our group has also developed a targeted UCA, which we term ELIP (Figure 3).70,71 These ELIP can be manipulated to entrap genes,82,83 fluorophores,84 antibiotics,85 thrombolytics,86 and peroxisomal proliferator-activated receptor agonists.87 During manufacture of these agents, we maintain their targeting capabilities to allow for high local concentrations of the drug or gene at the desired site, as well as their echogenicity properties, despite accepting the drug/gene payloads.88 In contrast to other gas-filled microbubbles that require that much of the microbubble volume be made up of gas in order to be acoustically active, our ELIP have the capacity to carry drugs or genes within the aqueous core. Release of the contents can then be controlled to produce (i) a bolus release using a single high-amplitude ultrasonic pulse, (ii) sustained release by a series of low-amplitude pulses delivered over a specific time period, or (iii) a combination of the two.88,89 Being echogenic, we can also use ultrasound in its diagnostic mode to image the microbubble in real-time during drug and gene delivery to the target site.
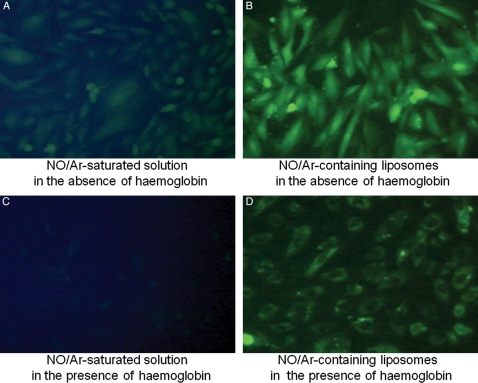
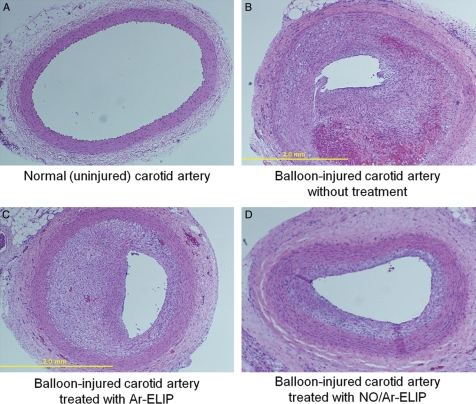
Recently, we have also demonstrated inclusion of a vasoactive gas, nitric oxide (NO), into our ELIP. We have demonstrated controlled release of NO to vascular smooth muscle cells with ultrasound, with effective uptake even in the presence of an NO-scavenging agent, haemoglobin (Figure 4).90,91 Furthermore, we have demonstrated inhibition of intimal hyperplasia in balloon-injured carotid arteries in rabbits by administration of our NO-ELIP agent and transcutaneous ultrasound (Figure 5).91 Future work will involve novel applications of our UCA as well as elucidation of the ultrasound parameters necessary to produce the desired therapeutic effects.
Figure 4.
Nitric oxide delivery into cultured vascular smooth muscle cells by ELIP in the presence and absence of the NO-scavenging agent, haemoglobin. Vascular smooth muscle cells were pre-loaded with a fluorescent probe, diaminofluorescein-2 diacetate (DFA-2DA), which reacts with NO to produce a fluorescent signal. Cultured cells were then treated with free NO (A and C) or NO encapsulated in ELIP (B and D), in the absence (A and B) or presence (C and D) of haemoglobin. NO-loaded ELIP were able to efficiently deliver NO into cultured cells even in the presence of a potent NO-scavenging agent such as haemoglobin. Published in J Am Coll Cardiol 2009, 54, Huang SL et al, Nitric oxide loaded echogenic liposomes for nitric oxide delivery and inhibition of intimal hyperplasia. Copyright Elsevier (2009).
Figure 5.
Inhibition of intimal hyperplasia in balloon-injured carotid arteries in rabbits by administration of NO-loaded ELIP and transcutaneous ultrasound. Representative histological sections of H&E-stained common carotid arteries in rabbits: (A) negative control (i.e. no balloon injury), (B) positive control (14 days after balloon injury of the artery), (C) sham (14 days after balloon injury of the artery plus pre-treatment with argon-loaded ELIP), and (D) treatment group (14 days after balloon injury of the artery plus pre-treatment with NO-loaded ELIP). NO ELIP significantly inhibited the development of intimal hyperplasia as a consequence of balloon injury to carotid arteries in rabbits. Published in J Am Coll Cardiol 2009, 54, Huang SL et al, Nitric oxide loaded echogenic liposomes for nitric oxide delivery and inhibition of intimal hyperplasia. Copyright Elsevier (2009).
4. Clinical concerns
As with any new technique, before this exciting modality can be translated into clinical practice, its safety needs to be first demonstrated, even in the early stages of development. Some investigators have raised concern about the same bioeffects that we want to harness for the therapeutic use of ultrasound.92,93 Capillary rupture and local extravasation of red blood cells after UTMD have been demonstrated;93 however, these were seen at much higher doses of microbubbles and higher local ultrasound pressures than are used clinically. Certain sensory organs such as the eyes, ears, and nervous tissue may be particularly sensitive to ultrasound as they are situated close to bone and, therefore, may be particularly vulnerable to conductive heat transfer. The recent reports on increased symptomatic intracranial haemorrhage with the use of ultrasound and thrombolytics for acute ischaemic stroke59,94 are worrisome and highlight the need for careful dose escalation studies as well as bench research on optimal frequencies and ultrasound parameters prior to translation to the bedside. Another area of concern is the induction of arrhythmias; cardiac premature contractions have been noted in frogs with ultrasound exposure as early as 199395 and were hypothesized to be related to cavitation effects. Some reports have demonstrated increased premature ventricular contractions in patients receiving UCAs and application of high ultrasound energies,96,97 raising concern about the need for cardiac monitoring during administration of these agents. On 10 October 2007, the United States FDA issued new warnings and contraindications for the use of the UCAs Optison® and Definity®.98 These warnings were largely based upon the reports of four fatal cardiac arrests during or within 30 min of Definity® injection in post-marketing surveillance, which equated to a 1:500 000 risk of death based on total patient doses since product approval.99 Following discussions with imaging specialists, the FDA relaxed these warnings on 17 July 2008;100 however, concern remains regarding the safety of these agents and the revised warning continues to recommend monitoring of high-risk patients with pulmonary hypertension or unstable cardiopulmonary conditions after administration of these agents.100 Hence, although the safety of UCAs for diagnostic echocardiography has been established,101–104 these issues will need to be revisited as we move this technique into the therapeutic realm.
Most of the work evaluating the use of UCAs for therapeutic delivery done to date has been performed either in vitro or in small animal models. Studies will need to be repeated in larger animal models with demonstration of efficacy before clinical application. From a technical perspective, there is still a need to identify optimal ultrasound settings for the highest delivery efficiencies for each therapeutic application. Some investigators have placed a plea for more transparency of reporting of specific ultrasound methodologies in order to be able to compare studies more readily. It may very well be that these parameters are different for different desired delivery sites and that the ultrasound system has to be optimized for each agent and target site. Much work is still needed with regards to optimization of microbubble composition to allow for the maximal payload of drug or gene to be delivered, with retention of acoustic properties for ultrasound-mediated enhanced therapeutic delivery. Finally, it is unclear if two-dimensional ultrasound is the ideal modality for this technique. The recent introduction of real-time three-dimensional ultrasound may, perhaps, result in more efficient ultrasound therapeutic delivery as it allows insonification of the entire organ, for instance, rather than a tissue slice.
5. Advantages
Therapeutic-targeted UCAs for drug and gene delivery afford several advantages, including (i) low toxicity—as lower concentrations of the drug are given systemically, concentrating the drug only to where it is needed; (this improved therapeutic index may allow expansion of the use of drugs with severe systemic effects such as cytotoxic agents); (ii) lower immunogenicity compared with viral gene delivery; (iii) low invasiveness with potential for repeated applications; (iv) organ specificity; (v) broad availability; (vi) broad applicability to organs or tissue amenable to sonication, and (vii) portability and being relatively cheap. Ultrasonography also avoids hazardous ionizing radiation, making repeated ‘treatments’ clinically acceptable. Unlike other imaging modalities, ultrasound can be optimized to allow triggered, controlled, and targeted interventions. With the possibility of decreasing the amount of therapeutic agent given, there is the potential not only to decrease systemic side effects, but also to decrease the cost of therapy. Hence, treatments that may have been thought to be too toxic or too expensive to be practical clinically may be revisited by packaging them into microbubbles and delivering them to the diseased site with the aid of therapeutic ultrasound.
6. Summary
The therapeutic use of UCAs is an emerging technique with high potential for enhanced directed gene and drug delivery. Molecular imaging using ultrasound and UCAs is in rapid dynamic evolution, and harnessing this technique for therapeutics is a logical corollary. This review focused on the cardiovascular uses of targeted UCAs; however, these agents are also actively being evaluated for cancer and respiratory pathologies. UTMD has already demonstrated feasibility for plasmid DNA delivery. The use of ultrasound for therapeutic thrombolysis has been taken into the clinical setting, and the addition of UCAs for therapeutic delivery or enhanced effect through cavitation is a natural progression to this investigation. UCAs can be designed as safe vehicles for encapsulating or co-transporting drugs or genes. Furthermore, the contrast agent can be targeted to cell-specific receptors for site-specific delivery. Being echogenic, delivery of the microbubble can be confirmed by diagnostic ultrasound, which is useful for the timing and spatial orientation of therapeutic ultrasound delivery. Ultrasound energy can then be used to rupture the microbubble and deliver the therapeutic agent locally to a tissue. Cavitation can be exploited to increase transvascular passage of macromolecules, and the cellular uptake or passage of therapeutic agents.
Much work needs to be done, however, including optimization of microbubble development to efficiently carry drug/gene payloads while maintaining acoustic activity, prolonging circulation time to prevent removal by the reticuloendothelial system, refining targeting techniques to enhance tissue attachment in areas of high shear stress, and elucidation of optimal ultrasound parameters for each UCA and its intended application. More importantly, due to the multiple interacting modalities involved, in order to move this field forward, there needs to be increased collaboration between membrane chemists, ultrasound engineers, and cardiovascular biologists.
Funding
This work was supported in part by the National Institutes of Health RO1 HL-059586 and HL-074002.
Acknowledgement
We would like to thank Mr Chris Akers for his invaluable help with the illustrations in this manuscript.
Conflict of interest: none declared.
References
- 1.Ng K, Liu Y. Therapeutic ultrasound: its application in drug delivery. Med Res Rev. 2002;22:204–223. doi: 10.1002/med.10004. [DOI] [PubMed] [Google Scholar]
- 2.Mitragotri S, Edwards DA, Blankschtein D, Langer R. A mechanistic study of ultrasonically-enhanced transdermal drug delivery. J Pharm Sci. 1995;84:697–706. doi: 10.1002/jps.2600840607. [DOI] [PubMed] [Google Scholar]
- 3.Tyle P, Agrawala P. Drug delivery by phonophoresis. Pharm Res. 1989;6:355–361. doi: 10.1023/a:1015967012253. [DOI] [PubMed] [Google Scholar]
- 4.Tachibana K. Transdermal delivery of insulin to alloxan-diabetic rabbits by ultrasound exposure. Pharm Res. 1992;9:952–954. doi: 10.1023/a:1015869420159. [DOI] [PubMed] [Google Scholar]
- 5.Mitragotri S, Blankschtein D, Langer R. Ultrasound-mediated transdermal protein delivery. Science. 1995;269:850–853. doi: 10.1126/science.7638603. [DOI] [PubMed] [Google Scholar]
- 6.Miller MW, Miller DL, Brayman AA. A review of the in vitro bioeffects of inertial ultrasonic cavitation from a mechanistic perspective. Ultrasound Med Biol. 1996;22:1131–1154. doi: 10.1016/s0301-5629(96)00089-0. [DOI] [PubMed] [Google Scholar]
- 7.Gramiak R, Shah PM. Echocardiography of the aortic root. Invest Radiol. 1968;3:356–366. doi: 10.1097/00004424-196809000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Feinstein SB, Cheirif J, Ten Cate FJ, Silverman PR, Heidenreich PA, Dick C, et al. Safety and efficacy of a new transpulmonary ultrasound contrast agent: initial multicenter clinical results. J Am Coll Cardiol. 1990;16:316–324. doi: 10.1016/0735-1097(90)90580-i. [DOI] [PubMed] [Google Scholar]
- 9.Leclercq F, Messner-Pellenc P, Descours Q, Daures JP, Pasquie JL, Hager FX, et al. Combined assessments of reflow and collateral blood flow by myocardial contrast echocardiography after acute reperfused myocardial infarction. Heart. 1999;82:62–67. doi: 10.1136/hrt.82.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leong-Poi H, Le E, Rim SJ, Sakuma T, Kaul S, Wei K. Quantification of myocardial perfusion and determination of coronary stenosis severity during hyperemia using real-time myocardial contrast echocardiography. J Am Soc Echocardiogr. 2001;14:1173–1182. doi: 10.1067/mje.2001.115982. [DOI] [PubMed] [Google Scholar]
- 11.Djikmans PA, Senior R, Becher H, Porter TR, Wei K, Visser CA, et al. Myocardial contrast echocardiography evolving as a clinically feasible technique for accurate, rapid, and safe assessment of myocardial perfusion: the evidence so far. J Am Coll Cardiol. 2006;48:2168–2177. doi: 10.1016/j.jacc.2006.05.079. [DOI] [PubMed] [Google Scholar]
- 12.Bhatia VK, Senior R. Contrast echocardiography: evidence for clinical use. J Am Soc Echocardiogr. 2008;21:409–416. doi: 10.1016/j.echo.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 13.Vogel R, Indermühle A, Reinhardt J, Meier P, Siegrist PT, Namdar M, et al. The quantification of absolute myocardial perfusion in humans by contrast echocardiography: algorithm and validation. J Am Coll Cardiol. 2005;45:754–762. doi: 10.1016/j.jacc.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 14.Kamp O, Lepper W, Vanoverschelde JL, Aeschbacher BC, Rovai D, Assayag P, et al. Serial evaluation of perfusion defects in patients with a first acute myocardial infarction referred for primary PTCA using intravenous myocardial contrast echocardiography. Eur Heart J. 2001;22:1485–1495. doi: 10.1053/euhj.2001.2604. [DOI] [PubMed] [Google Scholar]
- 15.Coggins MP, Le DE, Wei K, Goodman NC, Lindner JR, Kaul S. Noninvasive prediction of ultimate infarct size at the time of acute coronary occlusion based on the extent and magnitude of collateral-derived myocardial blood flow. Circulation. 2001;104:2471–2477. doi: 10.1161/hc4501.098954. [DOI] [PubMed] [Google Scholar]
- 16.Kaul S. Evaluating the ‘no reflow’ phenomenon with myocardial contrast echocardiography. Basic Res Cardiol. 2006;101:391–399. doi: 10.1007/s00395-006-0618-z. [DOI] [PubMed] [Google Scholar]
- 17.Sakuma T, Hayashi Y, Sumii K, Imazu M, Yamakido M. Prediction of short- and intermediate-term prognoses of patients with acute myocardial infarction using myocardial contrast echocardiography one day after recanalization. J Am Coll Cardiol. 1998;32:890–897. doi: 10.1016/s0735-1097(98)00342-8. [DOI] [PubMed] [Google Scholar]
- 18.Rinkevich D, Kaul S, Wang X-Q, Tong KL, Belcik T, Kalvaitis S, et al. Incremental value of regional perfusion over regional function in patients presenting to the emergency department with suspected cardiac chest pain and non-diagnostic electrocardiographic changes. Eur Heart J. 2005;26:1606–1611. doi: 10.1093/eurheartj/ehi335. [DOI] [PubMed] [Google Scholar]
- 19.Tong KL, Kaul S, Wang XQ, Rinkevich D, Kalvaitis S, Belcik T, et al. Myocardial contrast echocardiography versus thrombolysis in myocardial infraction score in patients presenting to the emergency department with chest pain and a nondiagnostic electrocardiogram. J Am Coll Cardiol. 2005;46:920–927. doi: 10.1016/j.jacc.2005.03.076. [DOI] [PubMed] [Google Scholar]
- 20.Wei K. Assessment of myocardial viability using myocardial contrast echocardiography. Echocardiography. 2005;22:85–94. doi: 10.1111/j.0742-2822.2005.04034.x. [DOI] [PubMed] [Google Scholar]
- 21.Hayat SA, Senior R. Contrast echocardiography for the assessment of myocardial viability. Curr Opin Cardiol. 2006;21:473–478. doi: 10.1097/01.hco.0000240585.68720.e7. [DOI] [PubMed] [Google Scholar]
- 22.Cosgrove D. Ultrasound contrast agents: an overview. Eur J Radiol. 2006;60:324–330. doi: 10.1016/j.ejrad.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 23.Hauff P, Reinhardt M, Foster S. Ultrasound contrast agents for molecular imaging. Handb Exp Pharmacol. 2008:223–245. doi: 10.1007/978-3-540-72718-7_11. [DOI] [PubMed] [Google Scholar]
- 24.Dayton PA, Rychak JJ. Molecular ultrasound imaging using microbubble contrast agents. Front Biosci. 2007;12:5124–5142. doi: 10.2741/2553. [DOI] [PubMed] [Google Scholar]
- 25.Villanueva FS. Molecular imaging of cardiovascular disease using ultrasound. J Nucl Cardiol. 2008;15:576–586. doi: 10.1016/j.nuclcard.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klibanov AL. Ultrasound molecular imaging with targeted microbubble contrast agents. J Nucl Cardiol. 2007;14:876–884. doi: 10.1016/j.nuclcard.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Holland CK, Apfel RE. Thresholds for transient cavitation produced by pulsed ultrasound in a controlled nuclei environment. J Acoust Soc Am. 1990;88:2059–2069. doi: 10.1121/1.400102. [DOI] [PubMed] [Google Scholar]
- 28.Tachibana K, Tachibana S. Albumin microbubble echo-contrast material as an enhancer for ultrasound accelerated thrombolysis. Circulation. 1995;92:1148–1150. doi: 10.1161/01.cir.92.5.1148. [DOI] [PubMed] [Google Scholar]
- 29.Bao S, Thrall BD, Miller DL. Transfection of a reporter plasmid into cultured cells by sonoporation in vitro. Ultrasound Med Biol. 1997;23:953–957. doi: 10.1016/s0301-5629(97)00025-2. [DOI] [PubMed] [Google Scholar]
- 30.Shohet RV, Chen S, Zhou YT, Wang Z, Meidell RS, Unger RH, et al. Echocardiographic destruction of albumin microbubbles directs gene delivery to the myocardium. Circulation. 2000;101:2554–2556. doi: 10.1161/01.cir.101.22.2554. [DOI] [PubMed] [Google Scholar]
- 31.Erikson JM, Freeman GL, Chandrasekar B. Ultrasound-triggered antisense oligonucleotide attenuates ischemia/reperfusion-induced myocardial tumor necrosis factor-alpha. J Moll Cell Cardiol. 2003;35:119–130. doi: 10.1016/s0022-2828(02)00289-4. [DOI] [PubMed] [Google Scholar]
- 32.Hashiya N, Aoki M, Tachibana K, Taniyama Y, Yamasaki K, Hiraoka K, et al. Local delivery of E2F decoy oligodeoxynucleotides using ultrasound with microbubble agent (Optison) inhibits intimal hyperplasia after balloon injury in rat carotid artery model. Biochem Biophys Res Commun. 2004;317:508–514. doi: 10.1016/j.bbrc.2004.03.070. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki R, Takizawa T, Negishi Y, Hagisawa K, Tanaka K, Sawamura K, et al. Gene delivery by combination of novel liposomal bubbles with perfluoropropane and ultrasound. J Control Rel. 2007;15:531–537. doi: 10.1016/j.jconrel.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Porter TR, Hiser WL, Kricskefd D, Deligonul U, Xie F, Inversen P, et al. Inhibition of carotid artery neointimal formation with intravenous microbubbles. Ultrasound Med Biol. 2001;27:259–265. doi: 10.1016/s0301-5629(00)00338-0. [DOI] [PubMed] [Google Scholar]
- 35.Vannan M, McCreery T, Li P, Han Z, Unger E, Kuersten B, et al. Ultrasound-mediated transfection of canine myocardium by intravenous administration of cationic microbubble-linked plasmid DNA. J Am Soc Echocardiogr. 2002;15:214–218. doi: 10.1067/mje.2002.119913. [DOI] [PubMed] [Google Scholar]
- 36.Xie F, Tsutsui JM, Lof J, Unger EC, Johanning J, Culp WC, et al. Effectiveness of lipid microbubbles and ultrasound in declotting thrombosis. Ultrasound Med Biol. 2005;21:979–985. doi: 10.1016/j.ultrasmedbio.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 37.Culp WC, Porter TR, McCowan TC, Roberson PK, James CA, Matchett WJ, et al. Microbubble-augmented ultrasound declotting of thrombosed arteriovenous dialysis grafts in dogs. J Vasc Interv Radiol. 2003;14:343–347. doi: 10.1097/01.rvi.0000058409.01661.b4. [DOI] [PubMed] [Google Scholar]
- 38.Molina CA, Ribo M, Rubiera M, Montaner J, Santamarina E, Delgado-Mederos R, et al. Microbubble administration accelerates clot lysis during continuous 2-MHz ultrasound monitoring in stroke patients treated with intravenous tissue plasminogen activator. Stroke. 2006;37:425–429. doi: 10.1161/01.STR.0000199064.94588.39. [DOI] [PubMed] [Google Scholar]
- 39.Perren F, Loulidi J, Poglia D, Landis T, Sztajzel R. Microbubble potentiated transcranial duplex ultrasound enhances IV thrombolysis in acute stroke. J Thromb Thrombolysis. 2008;25:219–223. doi: 10.1007/s11239-007-0044-6. [DOI] [PubMed] [Google Scholar]
- 40.Lindner JR. Evolving applications for contrast ultrasound. Am J Cardiol. 2002;90:72J–80J. doi: 10.1016/s0002-9149(02)02951-x. [DOI] [PubMed] [Google Scholar]
- 41.Tiukinhoy-Laing SD, Buchanan K, Parikh D, Huang S, MacDonald RC, McPherson DD, et al. Fibrin targeting of tissue-plasminogen activator-loaded echogenic liposomes. J Drug Target. 2007;15:109–114. doi: 10.1080/10611860601140673. [DOI] [PubMed] [Google Scholar]
- 42.Klegerman ME, Zou Y, McPherson DD. Fibrin-targeting of echogenic liposomes with inactivated tissue plasminogen activator. J Liposome Res. 2008;18:95–112. doi: 10.1080/08982100802118482. [DOI] [PubMed] [Google Scholar]
- 43.Kaufmann BA, Sanders JM, Davis C, Xie A, Aldred P, Sarembock IJ, et al. Molecular imaging of inflammation in atherosclerosis with targeted ultrasound detection of vascular cell adhesion molecule-1. Circulation. 2007;116:276–284. doi: 10.1161/CIRCULATIONAHA.106.684738. [DOI] [PubMed] [Google Scholar]
- 44.Takalkar AM, Klibanov AL, Rychak JJ, Lindner JR, Ley K. Binding and detachment dynamics of microbubbles targeted to P-selectin under controlled shear flow. J Control Release. 2004;96:473–482. doi: 10.1016/j.jconrel.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 45.Hamilton AJ, Huang SL, Warnick D, Rabbat M, Kane B, Nagaraj A, et al. Intravascular ultrasound molecular imaging of atheroma components in vivo. J Am Coll Cardiol. 2004;43:453–460. doi: 10.1016/j.jacc.2003.07.048. [DOI] [PubMed] [Google Scholar]
- 46.Demos SM, Alkan-Onyuksel H, Kane BJ, Ramani K, Nagaraj A, Greene R, et al. In vivo targeting of acoustically reflective liposomes for intravascular and transvascular ultrasonic enhancement. J Am Coll Cardiol. 1999;33:867–875. doi: 10.1016/s0735-1097(98)00607-x. [DOI] [PubMed] [Google Scholar]
- 47.Rychak JJ, Klibanov AL, Ley KF, Hossak JA. Enhanced targeting of ultrasound contrast agents using acoustic radiation force. Ultrasound Med Biol. 2007;33:1132–1139. doi: 10.1016/j.ultrasmedbio.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 48.Dayton P, Klibanov A, Brandenburger G, Ferrara K. Acoustic radiation force in vivo: a mechanism to assist targeting of microbubbles. Ultrasound Med Biol. 1999;25:1195–1201. doi: 10.1016/s0301-5629(99)00062-9. [DOI] [PubMed] [Google Scholar]
- 49.Stride E, Saffari N. On the destruction of microbubble ultrasound contrast agents. Ultrasound Med Biol. 2003;29:563–673. doi: 10.1016/s0301-5629(02)00787-1. [DOI] [PubMed] [Google Scholar]
- 50.Marin A, Sun H, Husseini GA, Pitt WG, Christensen DA, Rapoport NY. Drug delivery in pluronic micelles: Effect of high-frequency ultrasound on drug release from micelles and intracellular uptake. J Control Release. 2002;84:39–47. doi: 10.1016/s0168-3659(02)00262-6. [DOI] [PubMed] [Google Scholar]
- 51.Liu Y, Yang H, Sakanishi A. Ultrasound: mechanical gene transfer into plant cells by sonoporation. Biotech Adv. 2006;24:1–16. doi: 10.1016/j.biotechadv.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 52.Tachibana K, Uchida T, Ogawa K, Yamashita N, Tamura K. Induction of cell-membrane porosity by ultrasound. Lancet. 1999;353:1409. doi: 10.1016/S0140-6736(99)01244-1. [DOI] [PubMed] [Google Scholar]
- 53.Djikmans PA, Juffermans LJ, Musters RJ, van Wamel A, ten Cate FJ, van Gilst W, et al. Microbubbles and ultrasound: from diagnosis to therapy. Eur J Echocardiogr. 2004;5:245–256. doi: 10.1016/j.euje.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 54.Parikh S, Motarjeme A, McNamara T, Raabe R, Hagspiel K, Benenati JF, et al. Ultrasound-accelerated thrombolysis for the treatment of deep vein thrombosis: initial clinical experience. J Vasc Interv Radiol. 2008;19:521–528. doi: 10.1016/j.jvir.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 55.Wissgott C, Richter A, Kamusella P, Steinkamp HJ. Treatment of critical limb ischemia using ultrasound-enhanced thrombolysis (PARES trial): final results. J Endovasc Ther. 2007;14:438–443. doi: 10.1177/152660280701400402. [DOI] [PubMed] [Google Scholar]
- 56.Rosenschein U, Roth A, Rassin T, Basan S, Laniado S, Miller HI. Analysis of coronary ultrasound thrombolysis endpoints in acute myocardial infarction (ACUTE Trial): results of the feasibility phase. Circulation. 1997;95:1411–1416. doi: 10.1161/01.cir.95.6.1411. [DOI] [PubMed] [Google Scholar]
- 57.Rosenschein U, Gaul G, Erbel R, Amann F, Velasguez D, Stoerger H, et al. Percutaneous transluminal therapy of occluded saphenous vein grafts: can the challenges be met with ultrasound thrombolysis? Circulation. 1999;99:26–29. doi: 10.1161/01.cir.99.1.26. [DOI] [PubMed] [Google Scholar]
- 58.Alexandrov AV, Molina CA, Grotta JC, Garami Z, Ford SR, Alvarez-Sabin J, et al. CLOTBUST Investigators. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351:2170–2178. doi: 10.1056/NEJMoa041175. [DOI] [PubMed] [Google Scholar]
- 59.Eggers J, Konig IR, Koch B, Handler G, Seidel G. Sonothrombolysis with transcranial color-coded sonography and recombinant tissue-type plasminogen activator in acute middle cerebral artery main stem occlusion: results from a randomized study. Stroke. 2008;39:1470–1475. doi: 10.1161/STROKEAHA.107.503870. [DOI] [PubMed] [Google Scholar]
- 60.Mukherjee D, Wong J, Griffin B, Ellis SG, Porter T, Sen S, et al. Ten-fold augmentation of endothelial uptake of vascular endothelial growth factor with ultrasound after systemic administration. J Am Coll Cardiol. 2000;53:1678–1686. doi: 10.1016/s0735-1097(00)00575-1. [DOI] [PubMed] [Google Scholar]
- 61.Zhou Z, Mukherjee D, Wang K, Zhou X, Tarakji K, Ellis K, et al. Induction of angiogenesis in a canine model of chronic myocardial ischemia with intravenous infusion of vascular endothelial growth factor (VEGF) combines with ultrasound energy and echo contrast agent. (Abstract) J Am Coll Cardiol. 2003;39:396A. [Google Scholar]
- 62.Hwang JH, Brayman AA, Reidy MA, Matula TJ, Kimmey MB, Crum LA. Vascular effects induced by combines 1-MHz ultrasound and microbubble contrast agent treatments in vivo. Ultrasound Med Biol. 2005;31:553–564. doi: 10.1016/j.ultrasmedbio.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 63.Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, et al. Therapeutic angiogenesis for patients with limb ischemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360:427–435. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- 64.Schachinger V, Assmus B, Britten MB, Honold J, Lehmann R, Teupe C, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI trial. J Am Coll Cardiol. 2004;44:1690–1699. doi: 10.1016/j.jacc.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 65.Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 66.Fuchs S, Satler LF, Kornowski R, Okubagzi P, Weisz G, Baffour R, et al. Catheter-based autologous bone marrow myocardial injection in no-option patients with advanced coronary artery disease: a feasibility study. J Am Coll Cardiol. 2003;41:1721–1724. doi: 10.1016/s0735-1097(03)00328-0. [DOI] [PubMed] [Google Scholar]
- 67.Perin EC, Dohmann HF, Borojevic R, Silva SA, Sousa AL, Mesquita CT, et al. Transendocardial, autologous bone marrow cell transplantation for severe, chronic, ischemic heart failure. Circulation. 2003;107:2294–2302. doi: 10.1161/01.CIR.0000070596.30552.8B. [DOI] [PubMed] [Google Scholar]
- 68.Geng YJ, Madonna R. Stem cells in atherosclerosis-related vascular diseases. In: De Caterina R, Libby P, editors. Endothelial Dysfunction in Vascular Disease. Malden, MA: Blackwell Publishing; 2007. pp. 350–364. [Google Scholar]
- 69.Zen K, Okigaki M, Hosokawa Y, Adachi Y, Nozawa Y, Takamiya M, et al. Myocardium-targeted delivery of endothelial progenitor cells by ultrasound-mediated microbubble destruction improves cardiac function via an angiogenic response. J Mol Cell Cardiol. 2006;40:799–809. doi: 10.1016/j.yjmcc.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 70.Lanza GM, Alkan-Onyuksel H, Klegerman ME, Vonesh MJ, McPherson DD, Kane B, et al. Acoustically reflective liposomes and methods to make and use the same. Continuation issued 12 January 1999. Patent #5,858,399. [Google Scholar]
- 71.Huang SL, Hamilton AJ, Nagaraj A, Tiukinhoy SD, Klegerman ME, McPherson DD, et al. Improving ultrasound reflectivity and stability of echogenic liposomal dispersions for use as targeted ultrasound contrast agents. J Pharm Sci. 2001;90:1917–1926. doi: 10.1002/jps.1142. [DOI] [PubMed] [Google Scholar]
- 72.Klegerman ME, Hamilton AJ, Huang SL, Tiukinhoy SD, Khan AA, MacDonald RC, et al. Quantitative immunoblot assay for assessment of liposomal antibody conjugation efficiency. Anal Biochem. 2002;300:46–52. doi: 10.1006/abio.2001.5443. [DOI] [PubMed] [Google Scholar]
- 73.Klegerman ME, Zou Y, Huang S, Shelat HS, Geng YJ, McPherson DD. Bifunctional targeting of echogenic immunoliposomes for directed stem cell delivery. (Abstract) J Am Coll Cardiol. 2008;51:A288. [Google Scholar]
- 74.Kim H, Huang S, Shelat HS, Kee PH, Smulevitz B, Geng YJ, et al. Tracking and enhancement of mesenchymal stem cell homing to vascular wall with echogenic liposomes. (Abstract) Arterioscler Thromb Vasc Biol. 2008;28:P238. [Google Scholar]
- 75.Herbst SM, Klegerman ME, Kim H, Shelat HS, Moody MR, Yang C, et al. Targeted stem cell delivery to atheroma using bifunctional echogenic immunoliposomes. (Abstract) Arterioscler Thromb Vasc Biol. 2008;28:P611. [Google Scholar]
- 76.Unger EC, Matsunaga TO, McCreery T, Schumann P, Sweitzer R, Quigley R. Therapeutic applications of microbubbles. Eur J Radiol. 2002;42:160–168. doi: 10.1016/s0720-048x(01)00455-7. [DOI] [PubMed] [Google Scholar]
- 77.Kodama T, Yomita Y, Koshiyama K, Blomley MJ. Transfection effect of microbubbles on cells in superposed ultrasound waves and behavior of cavitation bubble. Ultrasound Med Biol. 2006;32:905–914. doi: 10.1016/j.ultrasmedbio.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 78.Unger EC, McCreery TP, Sweitzer RH, Caldwell VE, Wu Y. Acoustically active lipospheres containing paclitaxel: a new therapeutic ultrasound contrast agent. Invest Radiol. 1998;12:886–892. doi: 10.1097/00004424-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 79.Unger EC, Porter T, Culp W, Labell R, Matsunaga T, Zutshi R. Therapeutic applications of lipid-coated microbubbles. Adv Drug Deliv Rev. 2004;56:1291–1314. doi: 10.1016/j.addr.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 80.Lanza GM, Wallace KD, Scott MJ, Caheris WP, Abendschein DR, Christy DH, et al. A novel site-targeted ultrasonic contrast agent with broad biomedical application. Circulation. 1997;95:3334–3340. doi: 10.1161/01.cir.94.12.3334. [DOI] [PubMed] [Google Scholar]
- 81.Crowder KC, Hughes MS, Marsh JN, Barbieri AM, Fuhrhop RW, Lanza GM, et al. Sonic activation of molecularly-targeted nanoparticles accelerates transmembrane lipid delivery to cancer cells through contact-mediated mechanisms: implications for enhanced local drug delivery. Ultrasound Med Biol. 2005;31:1693–1700. doi: 10.1016/j.ultrasmedbio.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 82.Tiukinhoy SD, Mahowald ME, Shively VP, Nagaraj A, Kane BJ, Klegerman ME, et al. Development of echogenic, plasmid-incorporated, tissue-targeted cationic liposomes that can be used for directed gene delivery. Invest Radiol. 2000;35:732–738. doi: 10.1097/00004424-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 83.Huang S, Tiukinhoy S, McPherson DD, MacDonald RC. Combined use of ultrasound and acoustic cationic liposomes results in improved gene delivery into smooth muscle cells. (Abstract) Mol Ther. 2002;5:S9. [Google Scholar]
- 84.Huang SL, McPherson DD, MacDonald EC. A method to co-encapsulate gas and drugs in liposomes for ultrasound-controlled drug delivery. Ultrasound Med Biol. 2008;34:1272–1280. doi: 10.1016/j.ultrasmedbio.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tiukinhoy SD, Khan AA, Huang S, Klegerman ME, MacDonald RC, McPherson DD. Novel echogenic drug-immunoliposomes for drug delivery. Invest Radiol. 2004;39:104–110. doi: 10.1097/01.rli.0000111207.92580.44. [DOI] [PubMed] [Google Scholar]
- 86.Tiukinhoy-Laing SD, Huang S, Klegerman M, Holland CK, McPherson DD. Ultrasound-facilitated thrombolysis using tissue-plasminogen activator-loaded echogenic liposomes. Thromb Res. 2007;119:777–784. doi: 10.1016/j.thromres.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang S, McPherson DD, MacDonald RC. Multi-functional echogenic liposomes for image-guided and ultrasound-controlled PPAR agonist delivery. (Abstract) J Am Coll Cardiol. 2007;49:365A. [Google Scholar]
- 88.Huang S, MacDonald RC. Acoustically active liposomes for drug encapsulation and ultrasound-triggered release. Biochim Biophys Acta. 2004;1665:134–141. doi: 10.1016/j.bbamem.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 89.Smith D, Porter TM, Martinez J, Huang S, MacDonald RC, McPherson DD, et al. Destruction thresholds of echogenic liposomes with clinical diagnostic ultrasound. Ultrasound Med Biol. 2007;33:797–809. doi: 10.1016/j.ultrasmedbio.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 90.Huang S, Moody MR, Kee PH, Kim H, Geng YJ, McPherson DD. A novel gateway for vascular drug delivery: controlled release of nitric oxide from echogenic liposomes. (Abstract) J Am Coll Cardiol. 2008;51:A344. [Google Scholar]
- 91.Huang SL, Kee P, Moody M, Kim H, Chrzanowski S, MacDonald RC, et al. Nitric oxide loaded echogenic liposomes for nitric oxide delivery and inhibition of intimal hyperplasia. J Am Coll Cardiol. 2009;54 doi: 10.1016/j.jacc.2009.04.039. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Skyba DM, Price RJ, Linka AZ, Skalak TC. Direct in vivo visualization of intravascular destruction of microbubbles by ultrasound and its local effects on tissue. Circulation. 1998;98:290–293. doi: 10.1161/01.cir.98.4.290. [DOI] [PubMed] [Google Scholar]
- 93.Price RJ, Skyba DM, Kaul S, Skalak TC. Delivery of colloidal particles and red blood cells to tissue through microvessel ruptures created by targeted microbubble destruction with ultrasound. Circulation. 1998;98:1264–1267. doi: 10.1161/01.cir.98.13.1264. [DOI] [PubMed] [Google Scholar]
- 94.Daffertshofer M, Gass A, Ringleb P, Sitzer M, Sliwka U, Els T, et al. Transcranial low frequency ultrasound-mediated thrombolysis in brain ischemia (TRUMBI Trial). Increased risk of hemorrhage with combined ultrasound and tissue plasminogen activator: results of a phase II clinical trial. Stroke. 2005;36:1441–1446. doi: 10.1161/01.STR.0000170707.86793.1a. [DOI] [PubMed] [Google Scholar]
- 95.Dalecki D, Keller BB, Raeman CH, Carstensen EL. Effects of pulsed ultrasound on the frog heart: I. Thresholds for changes in cardiac rhythm and aortic pressure. Ultrasound Med Biol. 1993;19:385–390. doi: 10.1016/0301-5629(93)90057-u. [DOI] [PubMed] [Google Scholar]
- 96.Chapman S, Windle J, Xie F, McGrain A, Porter TR. Incidence of cardiac arrhythmias with therapeutic versus diagnostic ultrasound and intravenous microbubbles. J Ultrasound Med. 2005;24:1099–1107. doi: 10.7863/jum.2005.24.8.1099. [DOI] [PubMed] [Google Scholar]
- 97.Okasaki J, Ishikura F, Asanuma T, Otani K, Beppu S. Premature ventricular contraction during myocardial contrast echocardiography: relationship with imaging method, acoustic power and dose of contrast agent. J Cardiol. 2004;43:69–74. [PubMed] [Google Scholar]
- 98.New US Food and Drug administration prescribing information for Definity. approved 10 October 2007 http://www.fda.gov/cder/foi/label/2007/021064s007lbl.pdf. (2 May 2008) [Google Scholar]
- 99.Main ML, Goldman JH, Grayburn PA. Thinking outside the ‘box’—the ultrasound contrast controversy. J Am Coll Cardiol. 2007;50:2434–2437. doi: 10.1016/j.jacc.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 100.Information for Healthcare Professionals. Micro-bubble Contrast Agents marketed as Definity (Perflutren Lipid Microsphere) Injectable Suspension and Optison (Perflutren Protein-Type A Microspheres for Injection) Date created: 12 October 2007, updated 17 July 2008 http://www.fda.gov/cder/drug/InfoSheets/HCP/microbubbleHCP.htm. (2 May 2008) [Google Scholar]
- 101.Main ML, Ryan AC, Davis TE, Albano MP, Kusnetzky LL, Hibberd M. Acute mortality in hospitalized patients undergoing echocardiography with and without an ultrasound contrast agent (multicenter registry results in 4,300,966 consecutive patients) Am J Cardiol. 2008;102:1742–1746. doi: 10.1016/j.amjcard.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 102.Kusnetzky LL, Khalid A, Khumri TM, Moe TG, Jones PG, Main ML. Acute mortality in hospitalized patients undergoing echocardiography with and without an ultrasound contrast agent: results in 18,671 consecutive studies. J Am Coll Cardiol. 2008;51:1704–1706. doi: 10.1016/j.jacc.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 103.Gabriel RS, Smyth YM, Menon V, Klein AL, Grimm RA, Thomas JD, et al. Safety of ultrasound contrast agents in stress echocardiography. Am J Cardiol. 2008;102:1269–1272. doi: 10.1016/j.amjcard.2008.06.066. [DOI] [PubMed] [Google Scholar]
- 104.Piscaglia F, Bolondi L. The safety of Sonovue in abdominal applications: retrospective analysis of 23188 investigations. Ultrasound Med Biol. 2006;32:1369–1375. doi: 10.1016/j.ultrasmedbio.2006.05.031. [DOI] [PubMed] [Google Scholar]