Abstract
Aims
Imaging methods to track the fate of progenitor cells after their delivery would be useful in assessing the efficacy of cell-based therapies. We hypothesized that contrast-enhanced ultrasound (CEU) using microbubbles targeted to a genetically engineered cell-surface marker on endothelial progenitor cells (EPCs) would allow the targeted imaging of vascular engraftment.
Methods and results
Rodent bone marrow-derived EPCs were isolated, cultured, and transfected to express the marker protein, H-2Kk, on the cell surface. Non-transfected EPCs and EPCs transfected with either null plasmid or Firefly luciferase served as controls. Control microbubbles (MBC) and microbubbles targeted to H-2Kk expressed on EPCs (MBH-2Kk) were constructed. Binding of targeted microbubbles to EPCs was assessed in vitro using a parallel plate flow chamber system. CEU imaging of EPC-targeted microbubbles was assessed in vivo using subcutaneously implanted EPC-supplemented Matrigel plugs in rats. In flow chamber experiments, there was minimal attachment of microbubbles to plated control EPCs. Although numbers of adhered MBC were also low, there was greater and more diffuse attachment of MBH-2Kk to plated H-2Kk-transfected EPCs. Targeted CEU demonstrated marked contrast enhancement at the periphery of the H-2Kk-transfected EPC-supplemented Matrigel plug for MBH-2Kk, whereas contrast enhancement was low for MBC. Contrast enhancement was also low for both microbubbles within control mock-transfected EPC plugs. The signal intensity within the H-2Kk-transfected EPC plug was significantly greater for MBH-2Kk when compared with MBC.
Conclusion
Microbubbles targeted to a genetically engineered cell-surface marker on EPCs exhibit specific binding to EPCs in vitro. These targeted microbubbles bind to engrafted EPCs in vivo within Matrigel plugs and can be detected by their enhancement on CEU imaging.
Keywords: Molecular imaging, Contrast ultrasound, Endothelial progenitor cells
1. Introduction
Recent advances in cellular therapies, such as endothelial progenitor cell (EPC)- and mesenchymal stem cell-based strategies, have demonstrated great potential for the regeneration of ischaemic tissue and the promotion of neovascularization.1 Despite a wealth of basic research and ongoing clinical trials proceeding at a rapid pace, much debate still exists over the precise mechanisms for the beneficial effects of progenitor cell therapy.2 Although many studies have shown that bone marrow (BM)-derived cells incorporate into newly formed microvessels, the engraftment rate within tissue of genetically labelled BM-derived cells which co-express endothelial marker proteins varies widely from 0 to 90% incorporation.3 Meanwhile, several studies have shown that BM-derived progenitor cells will engraft mainly in the perivascular regions surrounding vessels, where they release a wide array of pro-angiogenic cytokines.4 Thus, the efficiency of neovascularization may not solely be attributable to the physical incorporation of cells into neovessels, but may also be influenced by the concomitant release of pro-angiogenic factors that can induce arteriogenesis, through paracrine mechanisms. A non-invasive imaging technique capable of spatially and temporally assessing EPC engraftment into vessels in vivo would be important in determining the role of vascular incorporation in the neovascularization response to cell-based therapy.5,6
Non-invasive imaging of pathophysiological molecular and cellular processes using contrast-enhanced ultrasound (CEU) has become possible with the development of novel ‘site-targeted’ microbubbles.7,8 We have previously developed microbubble contrast agents targeted against neovessel formation by conjugating ligands for αv-integrins, which are expressed by endothelial cells within these vessels, to the microbubble surface.9,10 Upon iv injection, these targeted microbubbles bind to the luminal surface of neovessels expressing the integrin αvβ3, where they can be detected using CEU.9 In our present study, we hypothesized that microbubbles targeted specifically to a unique cell-surface marker expressed on delivered exogenous EPCs would allow the tracking of their incorporation into new or existing vessels using CEU imaging.
2. Methods
2.1. Cell preparation and transfection
EPCs used for all experiments were isolated from the tibias and femurs of 3- to 5-week-old ‘syngeneic’ Fisher 344 rats (Charles River). The aspirated marrow was centrifuged and plated on fibronectin (Sigma)-coated flasks at a density of >1 × 106 cells/mL and grown in growth factor-supplemented endothelial cell basal medium 2 (Clonetics). Cells were allowed to adhere for 72 h, with subsequent growth media changes every 48 h.
For in vitro flow chamber studies, cultured EPCs were transfected with a plasmid encoding for the MACS Kk.HA(N)/H-2Kk gene (Miltenyibiotec).11 Non-transfected EPCs, cells transfected with a null-plasmid carrying an empty cassette (mock-transfected), and EPCs transfected with a plasmid encoding for the Firefly luciferase gene served as negative and positive controls, respectively. For in vivo Matrigel studies, EPCs were transfected with a bicistronic plasmid encoding for both the H-2Kk gene and a gene encoding for the marker enhanced green fluorescent protein (eGFP). Mock-transfected EPCs were used in control plugs.
Transfection was carried out via electroporation using the Amaxa Nucleaofector I (Amaxa), with 1.5 µg of plasmid per 3.0 × 106 cells in 100 µL aliquots, to achieve high transfection efficiency. The cells were allowed to recover overnight before being further used in subsequent experiments. Transfection efficiency was assayed 24, 48 h, and 7 days post-transfection via fluorescent-activated cell sorting (FACS) and immunohistochemistry, utilizing both anti-H-2Kk-FITC and/or anti-H-2Kk-PE antibodies (Cell Lab/Beckman–Coulter).
Phenotypic and functional characterization of EPCs was performed at 48 h post-transfection. Cells were stained for the presence of endothelial markers, VEGFR-2 (1:50) (R&D Systems), CD31 (1:100) (abcam), and the endothelial-specific lectin (1:200) (Sigma). Surface marker expression profiles were generated using FACS. For functional analysis, we performed standard cell migration assays using Boyden chambers. (See Supplementary material online, Methods, for further details.)
2.2. Microbubble preparation and assembly
Targeted microbubbles were prepared by conjugating a biotinylated-anti-H-2Kk antibody (Ab) (abcam) to their surface using a biotin–streptavidin link (MBH-2Kk).10 Biotinylated microbubbles were incubated with streptavidin (Sigma) (30 µg/1 × 108 bubbles) and washed to remove free streptavidin. Aliquots were further incubated with biotinylated H-2Kk Ab (75 µg/1 × 108 bubbles) and washed to remove free Ab. A control microbubble, bearing an isotype control Ab (abcam) (MBC), was also constructed. For perfusion imaging, lipid-shelled decafluorobutane microbubbles were used. Microbubbles had a median diameter of 2.03 µm (range 1.5–6 µm). Microbubble concentrations were determined using a Coulter Multisizer IIe (Beckman–Coulter), prior to iv administration.
2.3. Flow chamber studies
Flow chamber experiments (n = 6 for each) on adhered EPCs, H-2Kk-transfected, mock-transfected (null-plasmid), Firefly luciferase-transfected, and control non-transfected EPCs, were performed. Cells were plated to confluence on µ-Slide I (ibidi) flow chamber slides coated with fibronectin and allowed to grow and adhere overnight. The slides were mounted on a parallel plate flow chamber with controlled gasket thickness (400 µm) and a channel width of 5 mm and channel length of 50 mm. The flow chamber was then placed in an inverted position on a live cell microscope (Nikon Eclipse TE300) with a ×40 objective and a high-resolution CCD camera for video recording. A mixture of fluorescent-targeted (MBH-2Kk) and non-targeted control (MBC) bubbles (3 × 106 for each microbubble) was drawn through the flow chamber at a constant rate (800 µL/min), using a withdrawal pump (PHD 2000, Harvard Apparatus). Red (DiI) fluorescent-biotinylated microbubbles were used to assemble MBH-2Kk bubbles, whereas green (DiO) fluorescent-biotinylated microbubbles were used for MBC. Constant flow (1 dyne/cm2) of the microbubble suspension was maintained for 5 min and was followed by a 5 min flush with PBS at the same rate. The number of microbubbles (MBH-2Kk or MBC) attached to plated cells was determined for 20 optical fields, after flushing with PBS.
2.4. Animal preparation and Matrigel plug formation
The study protocol was approved by the Animal Care and Use Committee at St Michael's Hospital Research Centre, in accordance with the NIH Guide for the Care and Use of Laboratory Animals. F-344 rats (n = 15) were anaesthetized with intraperitoneal injection of ketamine hydrochloride (10 mg/kg) and xylazine (8 mg/kg) for Matrigel plug implantation. Under sterile conditions, dual Matrigel plugs (each 1 mL in volume) supplemented with 50 ng/mL of VEGF, mixed with either 1 × 106 H-2Kk/eGFP transfected EPCs or 1 × 106 mock-transfected EPCs were implanted subcutaneously adjacent to each other, within the ventral surface of the abdomen utilizing a 23 G needle. A further subset (n = 15) of animals underwent single Matrigel plug implantation with 50 ng/mL of VEGF mixed with 1 × 106 H-2Kk/eGFP transfected EPCs, for evaluation of H-2Kk gene expression in vivo at multiple time points after implantation. Matrigel plugs were removed at intermittent time points, days 1, 3, 7, 14, and 28 (n = 3 for each) for PCR analysis. (See Supplementary material online, Methods, for further details.)
2.5. CEU perfusion imaging
CEU imaging of the Matrigel plugs was performed with pulse inversion imaging (HDI 5000, Philips Ultrasound) at a mechanical index of 1.0 and a transmit frequency of 3.3 MHz (L7-4 transducer), during iv infusion of lipid microbubbles (1 × 107 min−1), to obtain quantitative perfusion data.12 (See Supplementary material online, Methods, for further details.)
2.6. Targeted CEU imaging
Targeted CEU imaging of Matrigel plugs was performed with a high-frequency linear-array probe (L7-4 transducer, transmit frequency of 3.3 MHz), using pulse inversion imaging (HDI 5000, Philips Ultrasound) at a mechanical index of 1.0 (n = 12). A bolus (5 × 107) of MBH-2Kk or MBC was injected intravenously in random order via a catheter in the left jugular vein and CEU imaging was suspended for 10 min to allow for circulation and attachment of site-specific microbubbles to their targets. On resumption of imaging, the first frame detects signal from both retained targeted microbubbles and the few remaining circulating microbubbles. After destruction of all microbubbles within the ultrasound beam with continuous ultrasound (20 Hz), the pulsing interval is increased to 10 s to detect the signal from remaining freely circulating microbubbles alone.9,10 Data was recorded digitally, saved to magnetic-optical disk, and transferred to a computer workstation for off-line analysis. The signal intensity from retained microbubbles is obtained by subtracting the signal from freely circulating microbubbles obtained at a pulsing interval of 10 s, from the initial frame obtained upon resumption of ultrasound imaging.
2.7. Immunohistochemistry and histology
In three animals, at 7-day post-implantation of the Matrigel plugs, an iv bolus of 5 × 107 H-2Kk-targeted bubbles (red fluorescent—DiI) and 5 × 107 isotype control bubbles (green fluorescent—DiO) was injected. Bubbles were allowed to circulate for 10 min post-injection at which time the plugs were explanted, cryo-embedded in OCT (Sakura, Japan), flash frozen in liquid nitrogen, and stored at −80°C until analysis.
Tissue was processed for H&E according to standard techniques. In vivo EPC engraftment and spatial localization were determined using immunohistochemistry. VEGFR-2 staining was performed with anti-VEGFR-2 antibodies (abcam) and anti-mouse secondary antibodies (1:200) (Invitrogen) to visualize endothelial cells. TOPRO-3 (Sigma) was used as a nuclear marker, whereas GFP staining was indicative of transfected EPCs. Stained sections were visualized under fluorescent confocal microscopy. (See Supplementary material online, Methods, for further details.)
2.8. In vivo gene expression
Semi-quantitative real-time RT–PCR for exogenous H-2Kk transcript was performed. H-2Kk and cyclophilin-specific primers and SYBR green (Applied BioSystems) were used to detect amplicon production using an ABI system. (See Supplementary material online, Methods, for further details.)
2.9. Statistical analysis
Unless specified, data are expressed as mean ± SD. Comparisons for per cent transfection between days post-transfection were made by one-way ANOVA, and data for EPC-targeted and isotype control bubbles in flow chamber studies with various EPCs were compared using two-way ANOVA; if significant, inter-stage comparisons were performed using non-paired Student's t-test with the Bonferroni correction. Comparisons between signal intensity from EPC-targeted and isotype control microbubbles in Matrigel plug imaging studies were made by the Mann–Whitney rank sum test. Differences were considered significant at P < 0.05 (two-sided).
3. Results
3.1. EPC transfection
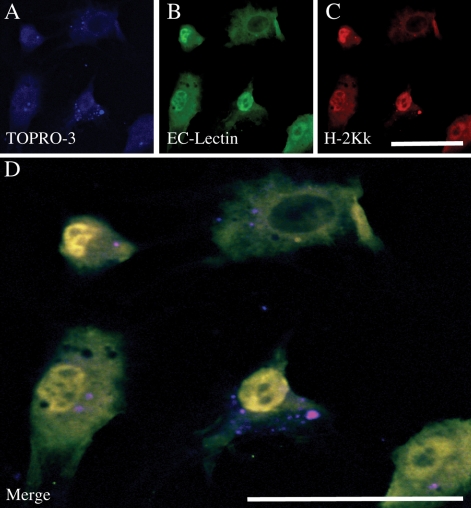
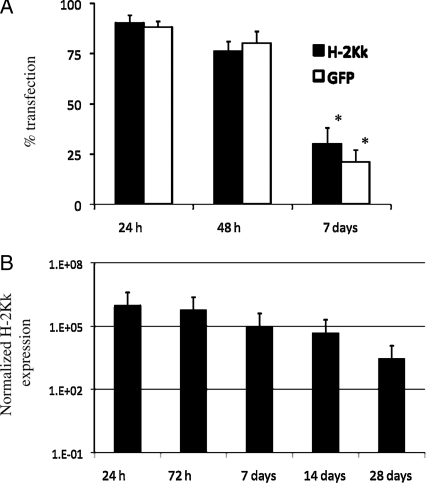
The in vitro efficacy of H-2Kk/eGFP transfection of BM EPCs via electroporation was assessed by fluorescent confocal microscopy and FACS analysis. After 7 days in culture, bone marrow EPCs expressed endothelial-specific lectin, and upon transfection, showed a high expression of H-2Kk (Figure 1). FACS data showed that >90% of cells transfected after 24 h, decreasing to ∼75% at 48 h, and <50% at day 7 post-transfection (Figure 2A). Data on in vivo transfection at various time points after Matrigel plug implantation are shown in Figure 2B. H-2Kk mRNA expression within cells implanted in the Matrigel plug is seen up to 28 days post-implantation, with good cellular expression seen up to 14 days.
Figure 1.
Immunohistochemical assessment of EPC transfection. Cultured EPCs were transfected with the H-2Kk gene and 48 h later exposed to an endothelial-specific lectin (B) and an anti-H-2Kk antibody conjugated with PE (C). Blue staining (A) is the nuclear dye TOPRO-3. There is robust expression of both H-2Kk and lectin. (D) The co-localization of H-2Kk and lectin staining, indicating that the cultured cells are predominantly of an endothelial lineage and strongly expressed H-2Kk on their surface. (Scale bar: 50 µm.)
Figure 2.
Quantification of in vitro and in vivo H-2Kk transfection. FACS analysis confirmed both H-2Kk and GFP expression after 24 h (>90%), falling to ∼75% after 48 h, and ∼25% after 7 days in culture, *P < 0.005 vs. 24 h (A). (B) PCR analysis of in vivo implanted EPC-supplemented Matrigel plugs grown for up to 28 days. Matrigel samples were run alongside a known standard to yield expression data on an individual cell basis. H-2Kk mRNA expression is seen up 28 days post-transfection with a strong signal seen up to 14 days.
3.2. EPC phenotypic and functional analysis
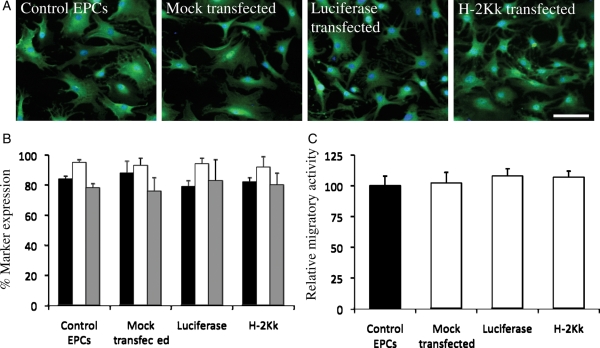
Immunohistochemical staining demonstrated strong endothelial cell-specific lectin staining in all EPC types at 24 h post-transfection (Figure 3A). FACS analysis revealed high (>80% of cells) expression of CD31, VEGFR-2, and UEA-1 after 7 days in culture for all EPCs (Figure 3B). Functional analysis showed no differences in migratory potential in the transfected groups when compared with control non-transfected EPCs. Mock-transfected EPCs (102 ± 9%) compared with control EPCs, luciferase-transfected EPCs (108 ± 6%) compared with control EPCs, and H-2Kk-transfected EPCs (107 ± 5%) compared with control EPCs (Figure 3C).
Figure 3.
Phenotypic and functional analysis of control and transfected EPCs. (A) No difference in endothelial cell-specific lectin staining in all EPCs at 24 h post-transfection. (B) FACS analysis of all EPCs at 24 h post-transfection, showing high (>80%) expression of specific endothelial markers. (C) Boyden chamber migration assay data performed 24 h post-transfection. There were no differences in the migratory responses to chemotactic agents between control and transfected EPCs. (Scale bar: 50 µm.)
3.3. Microbubble attachment to transfected EPCs in vitro
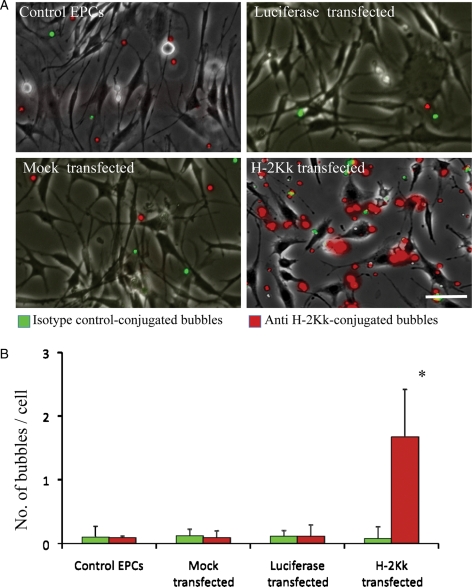
During flow chamber studies at a continuous shear rate of 1 dyne/cm2, there was minimal attachment of MBC and MBH-2Kk to either control non-transfected cells, mock-transfected cells, or luciferase-transfected cells (Figure 4A). Similarly, there was minimal attachment of MBC- to H-2Kk-transfected EPCs (Figure 4A). In comparison, there was a greater than 100-fold increase adherence of MBH-2Kk- to H-2Kk-transfected EPCs, both qualitatively (Figure 4A) and quantitatively (Figure 4B). As EPC-targeted microbubbles traversed around plated cells within the flow chamber, they were seen to adhere to EPCs in clusters, likely around focal sites of H-2Kk expression on the cell surface (Figure 4A).
Figure 4.
Data from flow chamber experiments on plated EPCs, after infusion of a mixture of targeted (red fluorescent) and non-targeted control (green fluorescent) bubbles. (A) Minimal attachment of MBC and MBH-2Kk to control, mock-transfected, and luciferase-transfected EPCs. Although there was minimal attachment of MBC to plated H-2Kk-transfected EPCs, there was a >100-fold increase adherence of MBH-2Kk- to H-2Kk-transfected EPCs. (B) Quantitative data of targeted microbubble binding to control, mock-transfected, luciferase-transfected, and H-2Kk-transfected EPCs, *P < 0.001 vs. MBC. (Scale bar: 50 µm.)
3.4. CEU perfusion and targeted imaging of EPC-supplemented Matrigel plugs
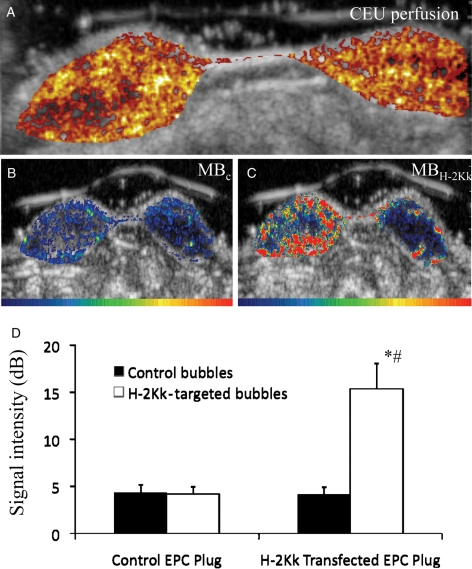
After 7 days post-implantation, the CEU imaging demonstrated perfusion within the plugs, with blood flow from the outer periphery of the plug characterized by larger feeder vessels, and extending into the central portion of the plugs as the pulsing interval was increased (Figure 5A). Many plugs showed an avascular central core, where CEU perfusion was absent. There were no significant differences in CEU perfusion between control mock-transfected EPC-enriched plugs when compared with H-2Kk/eGFP-transfected EPC-enriched plugs (2.7 ± 1.6 vs. 2.6 ± 1.3, respectively).
Figure 5.
(A) Background-subtracted colour-coded images of CEU perfusion in paired EPC-supplemented Matrigel plugs (left plug, H-2Kk-transfected EPC supplemented; right plug, control mock-transfected EPC supplemented). Perfusion to both plugs was similar, with perfusion highest at the periphery of the plug and lower in the central core. (B) Background-subtracted colour-coded images of CEU molecular imaging of EPC engraftment in supplemented Matrigel plugs, (left plug, H-2Kk-transfected EPC supplemented; right plug, control mock-transfected EPC supplemented) after iv bolus injection of control bubbles, MBC, and H-2Kk-targeted bubbles, MBH-2Kk. Although there was minimal signal in both plugs after MBC, there was a strong contrast ultrasound signal from H-2Kk-targeted bubbles in the left plug (H-2Kk-transfected EPC supplemented) localized predominantly to the periphery of the plug, with only minimal signal in the mock-transfected EPC-supplemented right plug. (C) Summary data of CEU EPC-targeted imaging data. There was minimal signal for control bubbles in both control EPC- and H-2Kk-EPC-supplemented plugs (black bars). In comparison, there was a significantly greater signal from H-2Kk-targeted bubbles (white bars) in the H-2Kk-transfected EPC supplemented, when compared with the control mock-transfected EPC-supplemented right plug. *P < 0.001 vs. MBC, #P < 0.005 vs. control mock-transfected EPC plug.
With targeted CEU imaging, there was minimal signal for MBC in both plugs as shown in Figure 5B. In comparison, there was strong signal enhancement from MBH-2Kk in the H-2Kk-transfected EPC-supplemented plug, with only minimal signal in the adjacent control EPC-supplemented plug (Figure 5C). Occasionally, small focal areas of signal enhancement from MBH-2Kk were noted in the adjacent control mock-transfected EPC-supplemented plug, which was attributed to the migration of H-2Kk/eGFP-transfected EPCs into the adjacent control plug (Figure 5C). Quantitative targeted CEU data are summarized in Figure 5D. CEU signal from retained MBC was low in both Matrigel plugs, whereas signal from retained MBH-2Kk was significantly greater in the H-2Kk/eGFP-transfected EPC Matrigel plug, when compared with the control mock-transfected EPC plug.
3.5. Histology and immunohistochemistry
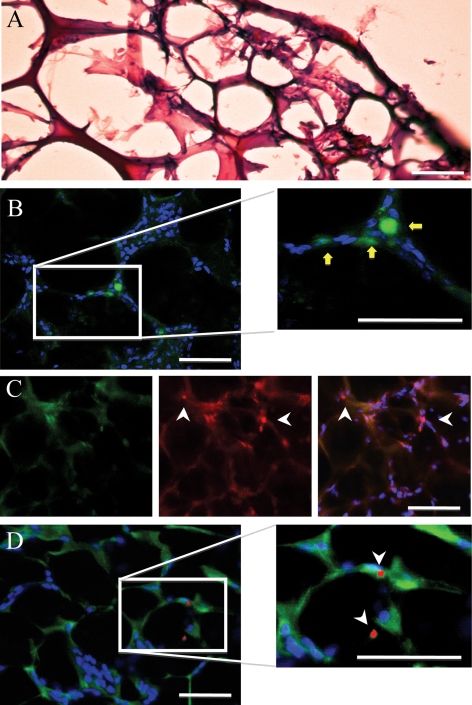
H&E staining showed a rich vascular network and tissue infiltration into the Matrigel plugs (Figure 6A). Figure 6B–D shows immunostaining of an H-2Kk/eGFP-transfected EPC-supplemented Matrigel plug that was removed after an iv bolus injection of red fluorescent MBH-2Kk microbubbles, without ultrasonic imaging. As expected, EPCs expressing eGFP were found incorporated into the vascular networks within H-2Kk/eGFP-transfected EPC-supplemented Matrigel plugs (Figure 6B), where they also stained positive for endothelial-specific lectin (Figure 6C). Fluorescent MBH-2Kk (red—DiI) microbubbles are seen adhering to H-2Kk/eGFP-positive cells, within the dense vascular network (Figure 6C or D).
Figure 6.
Post-mortem tissue analysis of eGFP/H-2Kk EPC-supplemented Matrigel plugs 7-day post-implantation, after an iv bolus of 5 × 107 red fluorescent H-2Kk-targeted microbubbles and green fluorescent isotype control microbubbles. H&E staining showed a rich vascular network within the plug (A), whereas immunohistochemistry illustrated eGFP/H-2Kk transfected EPC incorporation into vascular networks (yellow arrows) in (B). Sections were further stained with the endothelial marker VEGFR-2 to show co-localization with eGFP/H-2Kk-positive EPCs (C—red). The white arrows illustrate red fluorescent MBH-2Kk attachment to GFP-positive (green) and VEGFR-2 positive (red) endothelial cells (C). (D) Red fluorescent MBH-2Kk microbubble attachment to eGFP/H-2Kk-positive cells (white arrows), 'without' VEGFR-2 staining, to allow better distinction of adhered red fluorescent MBH-2Kk microbubbles. Blue staining is TOPRO-3 (nuclear stain). (Scale bar: 50 µm.)
4. Discussion
With the rapid increase in clinical studies of adult progenitor cells to treat cardiovascular diseases, it has become apparent that a non-invasive method to track the fate of delivered stem cells in vivo is urgently needed.5 The ability to image engrafted stem cells would allow the non-invasive monitoring of cell-based therapies in patients and be an important endpoint in ongoing clinical trials. In our present study, we have constructed site-specific contrast microbubbles targeted to a genetically engineered cell-surface marker expressed on delivered exogenous EPCs. These EPC-targeted microbubbles adhere to the surface of plated EPCs in an in vitro flow chamber system, and their binding to engrafted EPCs within angiogenic vessels of implanted Matrigel plugs can be detected in vivo using CEU imaging. Our study is the first to date investigating CEU techniques for the molecular imaging of progenitor cell engraftment.
4.1. Imaging techniques to track stem cells
The development of imaging techniques to track cell engraftment in the clinical setting has focused predominantly on single photon emission computerized tomography (SPECT),13 positron emission tomography (PET),14 and magnetic resonance imaging (MRI) techniques.15 SPECT and PET techniques are highly sensitive and can be used to assess early kinetics and tissue distribution after cell injection.16 The use of computed tomography (CT) for attenuation correction improves sensitivity even further, whereas overlaying the data on the CT image allows better spatial localization.17 However, the effects of ionizing radiation on cellular viability and function remain a concern, and despite high sensitivity, spatial resolution remains limited. MRI has the advantage of superior spatial resolution when compared with SPECT and ultrasound techniques; however, sensitivity appears to be lower.17 Although previous MRI techniques used traditional proton (1H) imaging of iron oxide-labelled cells, more recently, fluorine (19F) MRI of stem cells labelled with liquid perfluorocarbon nanoparticles has been shown to provide a more sensitive cell marker with minimal tissue background signal.18 In addition, newer high-field magnets may substantially reduce the threshold number of cells that can be detected.19 There are some theoretical disadvantages to magnetic labelling. Detection of MRI signal is not linked to cell viability, and release of the magnetic label, iron oxide, after cell death and its uptake by adjacent endogenous cells may yield misleading information about the kinetics of delivered cells. In addition, prior to clinical application, the effects of iron loading on cell proliferation and differentiation have to be further defined.20 Importantly, current MRI techniques cannot be used in patients with implanted pacemakers and cardiac defibrillators. These devices are now routinely implanted in patients with ischaemic heart disease and heart failure, the population in whom cell-based therapies are likely to be targeted, and ultimately most likely to benefit. Finally, although these techniques can track tissue engraftment, they lack the ability to differentiate the specific site of cell engraftment, specifically whether they are incorporated within vessels, in the surrounding perivascular region, or within the interstitial space. This information would be critical in determining the importance of vascular engraftment in the angiogenic response to cell-based therapies.
Cardiac ultrasound, or echocardiography, remains the most commonly available and utilized non-invasive imaging modality to evaluate cardiac structure and function. The introduction of commercially available microbubble contrast agents has improved endocardial border delineation and has allowed the assessment of myocardial perfusion using echocardiography. Non-invasive imaging of pathophysiological molecular and cellular processes using CEU has recently become possible with the development of novel ‘site-targeted’ microbubbles.7 Since microbubbles remain entirely within the intravascular space throughout their circulation, the processes that can be targeted are characterized by events that occur within the vascular compartment, such as endothelial dysfunction,21 angiogenesis,9,10 and thrombosis.22 Targeting results in retention of microbubbles within the vasculature at sites where these events occur and, hence, enhancement on ultrasound imaging. Thus, one novel aspect of targeted imaging of cell engraftment using CEU when compared with other molecular imaging techniques may be its unique ability to specifically target the vascular component of EPC engraftment.
4.2. Targeted CEU imaging of vascular EPC engraftment
In order to target exogenous delivered EPCs in a specific fashion, we required a unique cell-surface marker or ligand that is expressed on the luminal surface once engrafted into the vasculature. Although stem cells may express select markers once harvested, upon culture or engraftment, they tend to lose these early stem cell markers, undergoing differentiation to a more mature cellular phenotype. For example, BM-derived EPCs will develop an endothelial cell phenotype upon culture, expressing mature endothelial cell markers such as VEGFR2 and CD31. Thus, to allow distinction between engrafted exogenous EPCs and adjacent endogenous mature endothelial cells, we transfected BM-derived EPCs with a marker gene, H-2Kk. This gene encodes for the mouse MHC class 1 H-2Kk protein with a truncated cytoplasmatic domain, which is restricted to rarely used mouse strains, not used in our study. Transduction of this gene results in the expression of the H-2Kk marker on the cell surface where it can be easily recognized by our targeted microbubbles, bearing the anti-H-2Kk Ab on the outer surface of the microbubble shell. Similar monoclonal Ab strategies have been used to target microbubbles to cell-surface ligands for site-specific CEU imaging of inflammation23 and angiogenesis.10 We also used a bicistronic vector for our in vivo Matrigel plug studies, which allowed intracellular GFP expression for the identification of delivered EPCs ex vivo by fluorescent confocal microscopy. Similar strategies of reporter gene transduction have been used in other molecular imaging techniques to label stem cells for in vivo tracking, including optical bioluminescence imaging and PET imaging techniques.14 The genetic modulation of cells, in particular using viral transfer techniques that result in genomic incorporation, as a means to track engraftment may have potential disadvantages. Thus, prior to clinical application, the effects of transfection on the functionality and malignant potential of delivered pluripotent progenitor cells need to be thoroughly studied and potential safety issues carefully addressed.
We tested our targeting strategy in an in vitro flow chamber model and in an in vivo Matrigel model of therapeutic angiogenesis. At physiological shear rates, we demonstrated selective and robust binding with our H-2Kk-targeted microbubbles, with minimal non-specific microbubble binding. We next developed a Matrigel plug model of EPC engraftment and angiogenesis, by supplementing the Matrigel with growth factors and BM-derived EPCs prior to implantation. In this model, neovascularization occurred along the outer border of the plug with extension into the centre of the plug. Using fluorescent confocal microscopy, we found our eGFP/H-2Kk-transfected EPCs associated with the neovasculature, compatible with vascular engraftment of our exogenous EPCs. Similar to the flow chamber model, H-2Kk-targeted microbubble adherence was found in the in vivo Matrigel plug model. The number of adhered targeted microbubbles found on post-mortem examination of Matrigel plugs was relatively low compared with the numbers seen in flow chamber studies, likely reflecting the substantial microbubble loss that occurs during tissue harvesting, processing, and staining. Using targeted CEU imaging of H-2Kk-targeted microbubbles, we found a strong signal enhancement within the vascularized peripheral portions of plugs supplemented with H-2Kk-transfected EPCs, with relatively little signal in plugs supplemented with mock-transfected EPCs, demonstrating selective binding to our exogenous EPCs. We also noted the occasional focal signal enhancement within control EPCs plugs and found these corresponded to areas where H-2Kk-transfected EPCs had migrated across to the adjacent Matrigel plug, where binding of our targeted microbubbles occurred.
4.3. Study limitations
There are several important limitations of our present study. We used electroporation for EPC transduction, which does not result in permanent expression of our marker gene H-2Kk. Although this resulted in 90% of EPCs transduced within the first 24 h, this fell to <50% after 7 days in culture. This reduction in EPC transduction was in part due to active cellular division in culture. This was confirmed from our Matrigel gene expression studies, which showed good in vivo transfection efficiency out to day 14 post-implantation, with a significant loss of H-2Kk gene expression only occurring by day 28. Alternatively, reduced expression of H-2Kk in vivo may be due to immune responses against the mouse protein or CMV promoter silencing. Thus, our present technique for cell labelling would be useful to track early (up to 14 days) cell engraftment, as opposed to longer-term tracking.
As targeted microbubbles remain purely intravascular during their circulatory transit, CEU molecular imaging techniques to track EPC engraftment will only allow the detection of EPCs that are engrafted within the vasculature, and cannot assess the components that are engrafted within the perivascular or interstitial compartments. Although this may be less of a limitation for tracking EPC engraftment given their endothelial-like lineage, CEU molecular imaging may be a less robust technique for tracking engraftment of other progenitor cells, such as mesenchymal stem cells or embryonic stem cells.
Our in vivo model of Matrigel angiogenesis may not be fully representative of the clinical setting of cell therapy for ischaemia or infarction. As the entire dose of administered EPCs was within the implanted plug, even taking into account cell death and migration, a large proportion of delivered EPCs were capable of engraftment. Due in part to relatively inefficient cell delivery methods, the number of engrafted EPCs within ischaemic tissue is generally low and decreases over time. Thus, future studies to assess the sensitivity of CEU molecular imaging of EPC engraftment using current delivery techniques in models of ischaemia and infarction, where EPC engraftment may be lower, are required. Finally, microbubble ultrasound signal has many variable determinants, and we do not address whether our technique can quantify EPC engraftment at multiple points over time. Our present study does serve to confirm proof-of-principle for this technique and will be the basis of ongoing studies to optimize labelling and quantitative cell tracking using targeted CEU imaging.
4.4. Conclusions
In summary, contrast microbubbles targeted to a unique engineered cell-surface marker expressed on delivered exogenous EPCs allows the detection of their engraftment within angiogenic vessels using targeted CEU imaging. The ability to image the specific vascular component of EPC engraftment may provide further insights into the beneficial effects of cell therapies, and the relative importance of directed vascular engraftment vs. paracrine effects in the overall angiogenic response. Finally, as strategies are developed to augment EPC engraftment and determine the most effective strategy for progenitor cell therapy, CEU molecular imaging of cell engraftment may play an important role in their evaluation. Further studies evaluating the utility of EPC-targeted microbubbles to spatially and temporally track vascular engraftment in physiological models of ischaemia and angiogenesis will be necessary to explore these potential applications.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work was supported by Operating Grants from the Heart and Stroke Foundation of Ontario and the Canadian Institutes of Health Research (MOP 89836), Ottawa, Ontario, Canada. H.F. is supported by a Career Development Award from The American Society of Echocardiography. J.R.L. is supported by grants R01-HL-074443, R01-HL-078610 and R01-DK-063508 from the National Institutes of Health, Bethesda, Maryland. H.L.-P. is supported by a Clinician Scientist Award from the Heart and Stroke Foundation and an Early Researcher Award from the Ministry of Research and Innovation, Ontario, Canada.
References
- 1.Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 2.Melo LG, Pachori AS, Kong D, Gnecchi M, Wang K, Pratt RE, et al. Molecular and cell-based therapies for protection, rescue, and repair of ischemic myocardium: reasons for cautious optimism. Circulation. 2004;109:2386–2393. doi: 10.1161/01.CIR.0000128597.37025.00. [DOI] [PubMed] [Google Scholar]
- 3.Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 5.Frangioni JV, Hajjar RJ. In vivo tracking of stem cells for clinical trials in cardiovascular disease. Circulation. 2004;110:3378–3383. doi: 10.1161/01.CIR.0000149840.46523.FC. [DOI] [PubMed] [Google Scholar]
- 6.Ly HQ, Frangioni JV, Hajjar RJ. Imaging in cardiac cell-based therapy: in vivo tracking of the biological fate of therapeutic cells. Nat Clin Pract Cardiovasc Med. 2008;5(Suppl. 2):S96–S102. doi: 10.1038/ncpcardio1159. [DOI] [PubMed] [Google Scholar]
- 7.Lindner JR. Molecular imaging with contrast ultrasound and targeted microbubbles. J Nucl Cardiol. 2004;11:215–221. doi: 10.1016/j.nuclcard.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Villanueva FS, Wagner WR. Ultrasound molecular imaging of cardiovascular disease. Nat Clin Pract Cardiovasc Med. 2008;5(Suppl. 2):S26–S32. doi: 10.1038/ncpcardio1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leong-Poi H, Christiansen J, Heppner P, Lewis CW, Klibanov AL, Kaul S, et al. Assessment of endogenous and therapeutic arteriogenesis by contrast ultrasound molecular imaging of integrin expression. Circulation. 2005;111:3248–3254. doi: 10.1161/CIRCULATIONAHA.104.481515. [DOI] [PubMed] [Google Scholar]
- 10.Leong-Poi H, Christiansen J, Klibanov AL, Kaul S, Lindner JR. Noninvasive assessment of angiogenesis by ultrasound and microbubbles targeted to alpha(v)-integrins. Circulation. 2003;107:455–460. doi: 10.1161/01.cir.0000044916.05919.8b. [DOI] [PubMed] [Google Scholar]
- 11.Wang CY, Huang L. pH-sensitive immunoliposomes mediate target-cell-specific delivery and controlled expression of a foreign gene in mouse. Proc Natl Acad Sci USA. 1987;84:7851–7855. doi: 10.1073/pnas.84.22.7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation. 1998;97:473–483. doi: 10.1161/01.cir.97.5.473. [DOI] [PubMed] [Google Scholar]
- 13.Chin BB, Nakamoto Y, Bulte JW, Pittenger MF, Wahl R, Kraitchman DL. 111In oxine labelled mesenchymal stem cell SPECT after intravenous administration in myocardial infarction. Nucl Med Commun. 2003;24:1149–1154. doi: 10.1097/00006231-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Wu JC, Chen IY, Sundaresan G, Min JJ, De A, Qiao JH, et al. Molecular imaging of cardiac cell transplantation in living animals using optical bioluminescence and positron emission tomography. Circulation. 2003;108:1302–1305. doi: 10.1161/01.CIR.0000091252.20010.6E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill JM, Dick AJ, Raman VK, Thompson RB, Yu ZX, Hinds KA, et al. Serial cardiac magnetic resonance imaging of injected mesenchymal stem cells. Circulation. 2003;108:1009–1014. doi: 10.1161/01.CIR.0000084537.66419.7A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aicher A, Brenner W, Zuhayra M, Badorff C, Massoudi S, Assmus B, et al. Assessment of the tissue distribution of transplanted human endothelial progenitor cells by radioactive labeling. Circulation. 2003;107:2134–2139. doi: 10.1161/01.CIR.0000062649.63838.C9. [DOI] [PubMed] [Google Scholar]
- 17.Kraitchman DL, Tatsumi M, Gilson WD, Ishimori T, Kedziorek D, Walczak P, et al. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation. 2005;112:1451–1461. doi: 10.1161/CIRCULATIONAHA.105.537480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Partlow KC, Chen J, Brant JA, Neubauer AM, Meyerrose TE, Creer MH, et al. 19F magnetic resonance imaging for stem/progenitor cell tracking with multiple unique perfluorocarbon nanobeacons. FASEB J. 2007;21:1647–1654. doi: 10.1096/fj.06-6505com. [DOI] [PubMed] [Google Scholar]
- 19.Shapiro EM, Sharer K, Skrtic S, Koretsky AP. In vivo detection of single cells by MRI. Magn Reson Med. 2006;55:242–249. doi: 10.1002/mrm.20718. [DOI] [PubMed] [Google Scholar]
- 20.Bulte JW, Kostura L, Mackay A, Karmarkar PV, Izbudak I, Atalar E, et al. Feridex-labeled mesenchymal stem cells: cellular differentiation and MR assessment in a canine myocardial infarction model. Acad Radiol. 2005;12(Suppl. 1):S2–S6. doi: 10.1016/j.acra.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 21.Weller GE, Villanueva FS, Klibanov AL, Wagner WR. Modulating targeted adhesion of an ultrasound contrast agent to dysfunctional endothelium. Ann Biomed Eng. 2002;30:1012–1019. doi: 10.1114/1.1513565. [DOI] [PubMed] [Google Scholar]
- 22.Schumann PA, Christiansen JP, Quigley RM, McCreery TP, Sweitzer RH, Unger EC, et al. Targeted-microbubble binding selectively to GPIIb IIIa receptors of platelet thrombi. Invest Radiol. 2002;37:587–593. doi: 10.1097/00004424-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Lindner JR, Song J, Christiansen J, Klibanov AL, Xu F, Ley K. Ultrasound assessment of inflammation and renal tissue injury with microbubbles targeted to P-selectin. Circulation. 2001;104:2107–2112. doi: 10.1161/hc4201.097061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.