Abstract
Hilar basal dendrites form on dentate granule cells following seizures. To determine whether other brain insults cause the formation of hilar basal dendrites, a model of global cerebral hypoxia/ischemia was used. Rats underwent a transient induction of ischemia by occlusion of both common carotid arteries followed by reperfusion. Hippocampal slices were prepared from these animals 1 month after the ischemic insult, and granule cells were labeled with a retrograde tracing technique after biocytin injections into stratum lucidum of CA3b. Ischemic rats had numerous biocytin-labeled granule cells with hilar basal dendrites located at the hilar border of the granule cell layer. Quantitative analysis of ischemic rats compared to controls showed a significant increase in the percentage of biocytin-labeled granule cells with hilar basal dendrites. These data demonstrate that other brain insults in addition to epilepsy may result in the formation of hilar basal dendrites on granule cells.
Keywords: stroke, ischemia, Rat, hippocampus, dentate gyrus, granule cells
Introduction
Granule cells in the rodent dentate gyrus are organized in a densely packed cell layer with their apical dendrites extending into the molecular layer and their axons arising from the opposite pole of the soma and projecting into the hilus (Lorente de No, 1934). Although most granule cells have this regular appearance, other granule cells may have an apical dendrite arising from the base of their cell body or an axon originating from either the apical pole of the soma or an apical dendrite (Ribak et al., 2000; Yan et al., 2001; Dashtipour et al., 2002). These morphological findings suggest that granule cells are more heterogeneous than previously indicated. In addition, granule cells respond to seizures with two different neuroplastic changes, mossy fiber sprouting (Tauck and Nadler, 1985; Sutula et al., 1989) and the formation of hilar basal dendrites (Spigelman et al., 1998; Buckmaster and Dudek, 1999). These studies show that the granule cells of the dentate gyrus can have both their dendritic and axonal morphology modified to form increased recurrent excitatory circuitry (Okazaki et al., 1995; Sutula et al. 1998; Zhang and Houser; 1999; Ribak et al., 2000; Shapiro et al., 2008; Thind et al., 2008).
Rats subjected to global ischemia are similar to those undergoing recurrent seizures in that both groups show a similar pattern of hilar cell death, comparable increases in neurotrophic factors and in high affinity receptors in granule cells (Lindvall et al., 1994). However, current evidence suggests that global ischemia does not lead to behaviorally observed status epilepticus or recurrent spontaneous limbic seizures (Epsztein et al., 2008), but EEG observed seizure activity was reported in vivo after global ischemia and during the reperfusion phase (Caruana et al., 2008). In contrast, unilateral hypoxia-ischemia does lead to mossy fiber sprouting and the development of spontaneous recurrent seizures in a small (15%) proportion of animals 6-12 months after the ischemic insult (Williams and Dudek, 2007).
The present study was undertaken to determine whether hilar cell death without status epilepticus is a necessary and sufficient stimulus to induce the formation of hilar basal dendrites on dentate granule cells. We examined biocytin-labeled granule cells using a retrograde labeling approach at the light microscopic level to determine whether bilateral hypoxia-ischemia induces the formation of hilar basal dendrites on granule cells.
Results
The appearance of granule cell bodies and the arborization pattern of their apical dendrites within the molecular layer of the dentate gyrus in ischemic rats were similar to those of the control group (Spigelman et al., 1998; Yan et al., 2001). However, biocytin-labeled granule cells were identified with thick and long basal dendrites in ischemic rats, as shown in Fig. 1. Furthermore, the axons of these granule cells arose from their hilar pole and entered the hilus (Fig. 2). These basal dendrites most frequently arose from the hilar pole of the granule cell bodies, and were easily distinguished from the axons of these cells (Figs. 1 and 2). Basal dendrites were less commonly seen to originate from the base of the apical dendrites. Some granule cells only gave rise to a single basal dendrite but it would extend into the hilus for various lengths, usually 200–500 μm, whereas other granule cells had basal dendrites that branched less than 20 μm from the soma (Fig. 2). However, in all cases, the basal dendrites were restricted to the subgranular region of the hilus that was previously defined as the first 50 μm subjacent to the granule cell layer. The diameter of basal dendrites could be as thick as that of the apical dendrites (e.g., Figs. 1C, D and 2C, D).
Fig. 1.
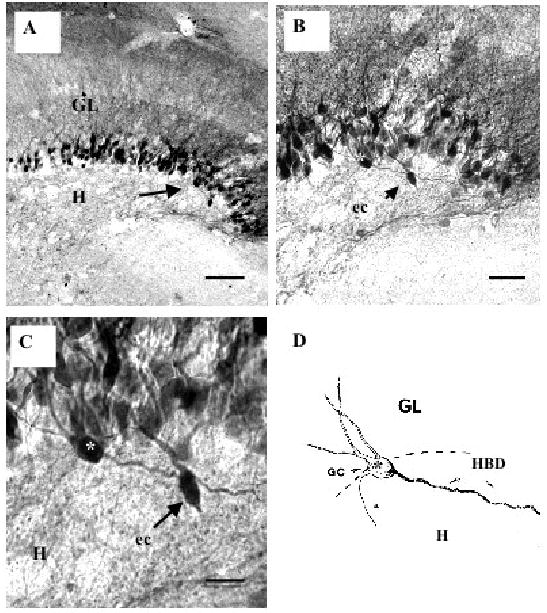
Biocytin-labeled granule cells (GC) from ischemic rats. A low magnification of the dentate gyrus shows the granule cell layer (GL) and hilus (H) with many labeled granule cells and their dendrites. The area indicated by the arrow in A is enlarged in B and in C to show a granule cell with a hilar basal dendrite (white asterisk) and an ectopic (ec) granule cell (arrow) in the subgranular zone. In D, a camera lucida drawing of the granule cell GC (*) with the long and thick hilar basal dendrite (HBD) extending into the hilus (H). The thinner process arising from this granule cell is its axon (a). Scale bars: in A= 40 μm; B = 25 μm and C = 10 μm.
Fig. 2.
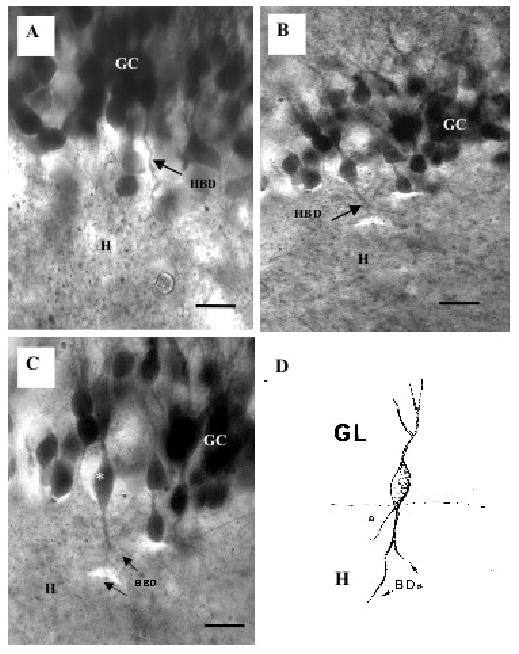
Two examples of biocytin-labeled granule cells with hilar basal dendrites from ischemic rats. A shows many labeled granule cells (GC) and one of these shows a large hilar basal dendrite (arrow, HBD) that enters the hilus (H). B and C show another example of a granule cell with a hilar basal dendrite (arrows, HBD). Note that this HBD is thick and it branches in the hilus (H). D is a camera lucida drawing of the same granule cell (*) with a hilar basal dendrite in C (asterisk). The basal dendrites (BDs) extend into the hilus while a thinner process arising from the base of this cell is the axon (a). Scale bars in A,C = 10 μm and B = 25 μm.
A quantitative analysis of the frequency of hilar basal dendrites was made because their presence on many labelled granule cells was a consistent morphological finding in ischemic rats. Quantitative data from ischemic and control rats showed a significant increase (p < 0.05; unpaired Student's t-test) in the percentage of granule cells with hilar basal dendrites for the ischemic rats (Table 1). We found a mean of 4.0% of granule cells with hilar basal dendrites in ischemic rats as compared to 0% in control rats.
Table 1.
Frequency of hilar basal dendrites (HBDs) in ischemic and control rats
| Rat | Total* | Cells with HBDs | % of cells with HBDs |
|---|---|---|---|
| Ischemic 1 | 2890 | 26 | 0.9 |
| Ischemic 2 | 51 | 4 | 7.8 |
| Ischemic 3 | 122 | 5 | 4.1 |
| Ischemic 4 | 187 | 6 | 3.2 |
| Control 1 | 694 | 0 | 0 |
| Control 2 | 510 | 0 | 0 |
| Control 3 | 200 | 0 | 0 |
| Control 4 | 156 | 0 | 0 |
Number of biocytin-labeled granule cells that were counted
Discussion
The major finding of the present study is that transient global hypoxia-ischemia results in the formation of hilar basal dendrites on granule cells within the hippocampal dentate gyrus. The frequency of hilar basal dendrites in ischemic rats indicates significant increases compared to control rats (Table 1). The frequency of granule cells with hilar basal dendrites in the dentate gyrus of ischemic rats is similar to that found for epileptic rats (Spigelman et al., 1998; Buckmaster and Dudek, 1999; Ribak et al., 2000). It is highly likely that these ischemia-induced dendritic changes are accompanied by changes in the synaptic connections of the granule cells with hilar basal dendrites. Further, electron microscopic studies of hilar basal dendrites from ischemic animals will be needed to determine whether these dendrites contribute to recurrent excitatory circuits similar to that found in epileptic animals (Ribak et al., 2000; Thind et al., 2008).
Ischemia results in permanent learning and memory impairments similar to those found in human stroke (Volpe et al., 1984,1992; Block 1999). While the underlying mechanisms of these impairments are not known, neuronal pathology in the hippocampus after ischemia would be consistent with this change in learning and memory. An increase in extracellular glutamate and aspartate within the hippocampus following ischemia may be a contributing factor that leads to the damage of hippocampal neurons (Pulsinelli, 1985; Benveniste, 2009). While the most severe pathology is observed primarily in the CA1 region of the hippocampus (Dugan and Choi, 1994), there is also prominent loss of hilar neurons in the dentate gyrus in ischemic rats. Because both ischemic and epileptic animals show similar patterns of hilar cell death and hilar basal dendrite formation, we suggest that hilar neuronal loss could play a causal role in the formation of hilar basal dendrites. However, several other possible common mechanisms may exist for the formation of hilar basal dendrites. Recently, Nakahara et al. (2009) provided evidence that neuronal hyperactivity contributes to the formation of hilar basal dendrites by stabilizing the basal dendrites on immature dentate granule cells.
Following global ischemia, many CA1 pyramidal cells die, whereas the number of BrdU-labeled, newly generated cells in the subgranular zone of the dentate gyrus increases in response to ischemia (Sharp et al., 2002). It should be noted that increased neurogenesis also occurs after seizures (Parent et al., 1997), and many of the newly generated granule cells display hilar basal dendrites (Shapiro et al., 2005). In addition, the increase in adult neurogenesis of granule cells that occurs after ischemia may contribute to partial memory recovery after a global insult (Bernabeu and Sharp, 2000). Thus, one possibility is that the granule cell remodelling found following global ischemia is another hippocampal adaptive mechanism to maintain its function after injury. It has also been shown that hypoxia-ischemia can result in mossy fiber sprouting and spontaneous motor seizures many months after the initial ischemic-hypoxic event (Williams & Dudek, 2007). Although we only studied rats which did not exhibit signs of motor deficits or seizures, we cannot exclude the possibility that seizures occurred outside our observation periods. Thus, we suggest that hilar basal dendrite formation after an ischemic insult could contribute to the recurrent excitatory circuitry and may play a role in spontaneous seizures long after the initial injury. These results and others provide evidence that common cellular and molecular pathways may contribute to the formation of hilar basal dendrites in the dentate gyrus.
Methods
Ischemia
Prior to the experiments, rats (male Sprague Dawley, 79-85 days old) were fasted for 7 ± 2 hours. Rats were anesthetized with halothane (2.5% induction and 1.2% maintenance in 30% O2 and 70% N2O). The left femoral artery was cannulated for measurements of arterial blood pressure and blood gases. The common carotid arteries were temporarily occluded with two vascular clamps per artery. The halothane level was lowered to 0.8% prior to induction of ischemia and maintained at 0.8% throughout. Animals were allowed to breathe 10% O2 and 90% N2 during the ischemic episode (10-17 min). At 10 min post ischemia, the femoral artery was tied off, and clamps were removed. Respiratory rate, blood pressure, core temperature, and PO2 were recorded before, during, and after ischemia. Rats were monitored daily for seizures and signs of motor deficits for 1 week, and then, every second day for 1 month. At 1 month after ischemia, rats were anesthetized with halothane, decapitated and hippocampal slices prepared for biocytin injections. Only those ischemic rats which showed no signs of seizures or motor deficits were used for retrograde labeling studies.
Biocytin Injections
Brain slices (400 μm thick) obtained from the caudal third of the hippocampal formation, transverse to the long axis, were cut with a vibrating blade slicer (Campden Instruments). They were then transferred to a custom-made recording chamber and perfused with artificial cerebrospinal fluid (ACSF) composed of (in mM): 124 NaCl, 3.5 KCl, 1.25 NaH2PO4, 2.0 CaCl2, 2.0 MgCl2, 26 NaHCO3, and 10 dextrose. The ACSF was continuously bubbled with a 95/5% mixture of O2/CO2 to ensure adequate oxygenation of slices and pH of 7.4. A 10-15% solution of biocytin was iontophoretically ejected into stratum lucidum of CA3b of the hippocampus to label the mossy fibers of dentate granule cells. A glass micropipette with a filament (WPI, 1.5 mm external diameter, 10-50 μm tip diameter) was filled with a freshly prepared solution of 10-15% biocytin in 0.9% NaCl. The tip of the glass micropipette was inserted into the hippocampus 200 μm below the surface and biocytin was ejected into the extracellular space with intermittent positive current pulses (15 μA, 3 s on, 3 s off) for 15-25 min. Three-four hours following biocytin injection, slices were immersed in a fixative solution containing 4% paraformaldehyde and 0.5% glutaraldehyde in 0.1M phosphate buffer (PB), pH 7.4 and stored overnight at 4°C.
To visualize the biocytin-labeled granule cells, the fixed slices were sectioned on a Vibratome at a thickness of 40 μm. The sections were collected in 0.1 M PB and treated with 1% H2O2 to suppress endogenous peroxidase activity. Then, the sections were washed in 0.1M PB for 30 min and incubated in avidin-biotin horseradish peroxidase solution at room temperature overnight. Finally, they were washed several times in 0.1 M PB and incubated in 0.025% diaminobenzidine and 0.01% nickel ammonium sulfate for 15-20 min. Sections for light microscopy were incubated in 0.1% osmium tetroxide to stabilize the reaction product, then mounted onto slides and dehydrated. Coverslips were applied over the sections with Polymount.
Data Collection and Statistics
Biocytin-labeled granule cells in hippocampal sections were viewed with a Zeiss Axioplan light microscope. Images were captured with an Axiocam digital camera and prepared with Jasc Paint Shop Pro 8. Hilar basal dendrites were identified using criteria as previously described (Ribak et al., 2000). Drawings of labeled granule cells with hilar basal dendrites were made with the use of a drawing tube attachment to the light microscope. The number of labeled granule cells with hilar basal dendrites was expressed as a percentage of the total number of labeled granule cells that were counted for each animal. Statistical analysis of the percentage of granule cells with hilar basal dendrites was performed using an unpaired Student's t-test.
Acknowledgments
We are grateful to Dr. D. Pless for proofreading the manuscript, Z. Shan and S. Castro for technical assistance, NIH grant NS038331 and grants from DGAPA-UNAM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benveniste H. Glutamate, microdialysis, and cerebral ischemia: lost in translation? Anesthesiology. 2009;110:422–425. doi: 10.1097/ALN.0b013e318194b620. [DOI] [PubMed] [Google Scholar]
- Bernabeu R, Sharp FR. NMDA and AMPA7Kainate glutamate receptors modulate dentate neurogenesis and CA3 synapsin-I in normal and ischemic hippocampus. J Cerb Blood Flow Metab. 2000;20:1669–1680. doi: 10.1097/00004647-200012000-00006. [DOI] [PubMed] [Google Scholar]
- Block F. Global ischemia and behavioral deficits. Prog Neurobiology. 1999;58:279–295. doi: 10.1016/s0301-0082(98)00085-9. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Dudek FE. In vivo intracellular analysis of granule cell axon reorganization in epileptic rats. J Neurophysiol. 1999;81:712–721. doi: 10.1152/jn.1999.81.2.712. [DOI] [PubMed] [Google Scholar]
- Caruana DA, Nesbitt C, Mumby DG, Chapman CA. Seizure activity in the rat hippocampus, perirhinal and prefrontal cortex associated with transient global cerebral ischemia. J Neural Transm. 2008;115:401–411. doi: 10.1007/s00702-007-0847-9. [DOI] [PubMed] [Google Scholar]
- Dashtipour K, Yan XX, Dinh TT, Okazaki MM, Nadler JV, Ribak CE. Quantitative and morphological analysis of dentate granule cells with recurrent basal dendrites from normal and epileptic rats. Hippocampus. 2002;12:235–244. doi: 10.1002/hipo.1114. [DOI] [PubMed] [Google Scholar]
- Dugan LL, Choi DW. Excitotoxicity, free radicals, and cell membrane changes. Ann Neurol. 1994;35(Suppl):S17–21. doi: 10.1002/ana.410350707. [DOI] [PubMed] [Google Scholar]
- Epsztein J, Milh M, Bihi RI, Jorquera I, Ben-Ari Y, Represa A, Crépel V. Ongoing epileptiform activity in the post-ischemic hippocampus is associated with a permanent shift of the excitatory-inhibitory synaptic balance in CA3 pyramidal neurons. J Neurosci. 2006;26:7082–7092. doi: 10.1523/JNEUROSCI.1666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z, Bengzon J, Elmér E, Kokaia M. Neurotrophins and brain insults. Trends Neurosci. 1994;17:490–496. doi: 10.1016/0166-2236(94)90139-2. [DOI] [PubMed] [Google Scholar]
- Lorente de Nó R. Studies on the structure of the cerebral cortex. II. Continuations of the study of the Ammonic system. J Psychol Neurol Lpz. 1934;46:113–177. [Google Scholar]
- Nakahara S, Tamura M, Matsuki N, Koyama R. Neuronal hyperactivity sustains the basal dendrites of immature dentate granule cells: time-lapse confocal analysis using hippocampal slice cultures. Hippocampus. 2009;19:379–391. doi: 10.1002/hipo.20529. [DOI] [PubMed] [Google Scholar]
- Okazaki MM, Evenson DA, Nadler JV. Hippocampal mossy fiber sprouting and synapse formation after status epilepticus in rats: visualization after retrograde transport of biocytin. J Comp Neurol. 1995;352:515–534. doi: 10.1002/cne.903520404. [DOI] [PubMed] [Google Scholar]
- Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulsinelli WA. Selective neuronal vulnerability: morphological and molecular characteristics. Prog Brain Res. 1985;63:29–37. doi: 10.1016/S0079-6123(08)61973-1. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Tran PH, Spigelman I, Okazaki MM, Nadler JV. Status epilepticus-induced hilar basal dendrites on rodent granule cells contribute to recurrent excitatory circuitry. J Comp Neurol. 2000;428:240–53. doi: 10.1002/1096-9861(20001211)428:2<240::aid-cne4>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Shapiro LA, Korn MJ, Ribak CE. Newly generated dentate granule cells from epileptic rats exhibit elongated hilar basal dendrites that align along GFAP-immunolabeled processes. Neuroscience. 2005;136:823–831. doi: 10.1016/j.neuroscience.2005.03.059. [DOI] [PubMed] [Google Scholar]
- Shapiro LA, Ribak CE, Jessberger S. Structural changes for adult-born dentate granule cells after status epilepticus. Epilepsia. 2008;49 5:13–18. doi: 10.1111/j.1528-1167.2008.01633.x. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Liu J, Bernabeu R. Neurogenesis following brain ischemia. Brain Res Dev Brain Res. 2002;134:23–30. doi: 10.1016/s0165-3806(01)00286-3. [DOI] [PubMed] [Google Scholar]
- Spigelman I, Yan XX, Obenaus A, Lee EY, Wasterlain CG, Ribak CE. Dentate granule cells form novel basal dendrites in a rat model of temporal lobe epilepsy. Neuroscience. 1998;86:109–120. doi: 10.1016/s0306-4522(98)00028-1. [DOI] [PubMed] [Google Scholar]
- Sutula T, Zhang P, Lynch M, Sayin U, Golarai G, Rod R. Synaptic and axonal remodeling of mossy fibers in the hilus and supragranular region of the dentate gyrus in kainate-treated rats. J Comp Neurol. 1998;390:578–594. doi: 10.1002/(sici)1096-9861(19980126)390:4<578::aid-cne9>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Sutula T, Cascino G, Cavazos J, Parada I, Ramirez L. Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Ann Neurol. 1989;26:321–330. doi: 10.1002/ana.410260303. [DOI] [PubMed] [Google Scholar]
- Tauck DL, Nadler JV. Evidence of functional mossy fiber sprouting in hippocampal formation of kainic acid-treated rats. J Neurosci. 1985;5:1016–1022. doi: 10.1523/JNEUROSCI.05-04-01016.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thind KK, Ribak CE, Buckmaster PS. Synaptic input to dentate granule cell basal dendrites in a rat model of temporal lobe epilepsy. J Comp Neurol. 2008;509:190–202. doi: 10.1002/cne.21745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe BT, Pulsinelli WA, Tribuna J, Davis HP. Behavioral performance of rats following transient forebrain ischemia. Stroke. 1984;15:558–562. doi: 10.1161/01.str.15.3.558. [DOI] [PubMed] [Google Scholar]
- Volpe BT, Towle A, Davis HP, Dunlap WP. Loss of hippocampal CA1 pyramidal neurons correlates with memory impairment in rats with ischemic or neurotoxin lesions. Behav Neurosci. 1992;106:457–464. doi: 10.1037//0735-7044.106.3.457. [DOI] [PubMed] [Google Scholar]
- Williams PA, Dudek FE. A chronic histopathological and electrophysiological analysis of a rodent hypoxic-ischemic brain injury model and its use as a model of epilepsy. Neuroscience. 2007;149:943–961. doi: 10.1016/j.neuroscience.2007.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan XX, Spigelman I, Tran PH, Ribak CE. A typical features of rat dentate granule cells: recurrent basal dendrites and apical axons. Anat Embryol (Berl) 2001;2033:203–209. doi: 10.1007/s004290000150. [DOI] [PubMed] [Google Scholar]
- Zhang N, Houser CR. Ultrastructural localization of dynorphin in the dentate gyrus in human temporal lobe epilepsy: a study of reorganized mossy fiber synapses. J Comp Neurol. 1999;405:472–90. [PubMed] [Google Scholar]


