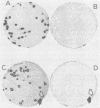
Abstract
Plaques formed by herpes simplex virus (HSV), pseudorabies virus, and varicella-zoster virus were studied by plaque autoradiography after [14C]thymidine labeling. Standard thymidine kinase-positive (TK+) viruses and TK- mutants of HSV types 1 and 2 and pseudorabies virus were studied, including cell cultured viruses and viruses isolated from animals. Autoradiography was performed with X-ray film with an exposure time of 5 days. After development of films, TK+ plaques showed dark rims due to isotope incorporation, whereas TK- plaques were minimally labeled. Plaque autoradiography of stock TK- viruses showed reversion frequencies to the TK+ phenotype of less than 10(-3). Autoradiography indicated that TK- virus retained the TK- phenotype after replication in vivo. In addition, it was shown that TK- HSV could be isolated from mouse trigeminal ganglion tissue after corneal inoculation of TK- HSV together with TK+ HSV. The plaque autoradiographic procedure was very useful to evaluate proportions of TK+ and TK- virus present in TK+-TK- virus mixtures.
Full text
PDF


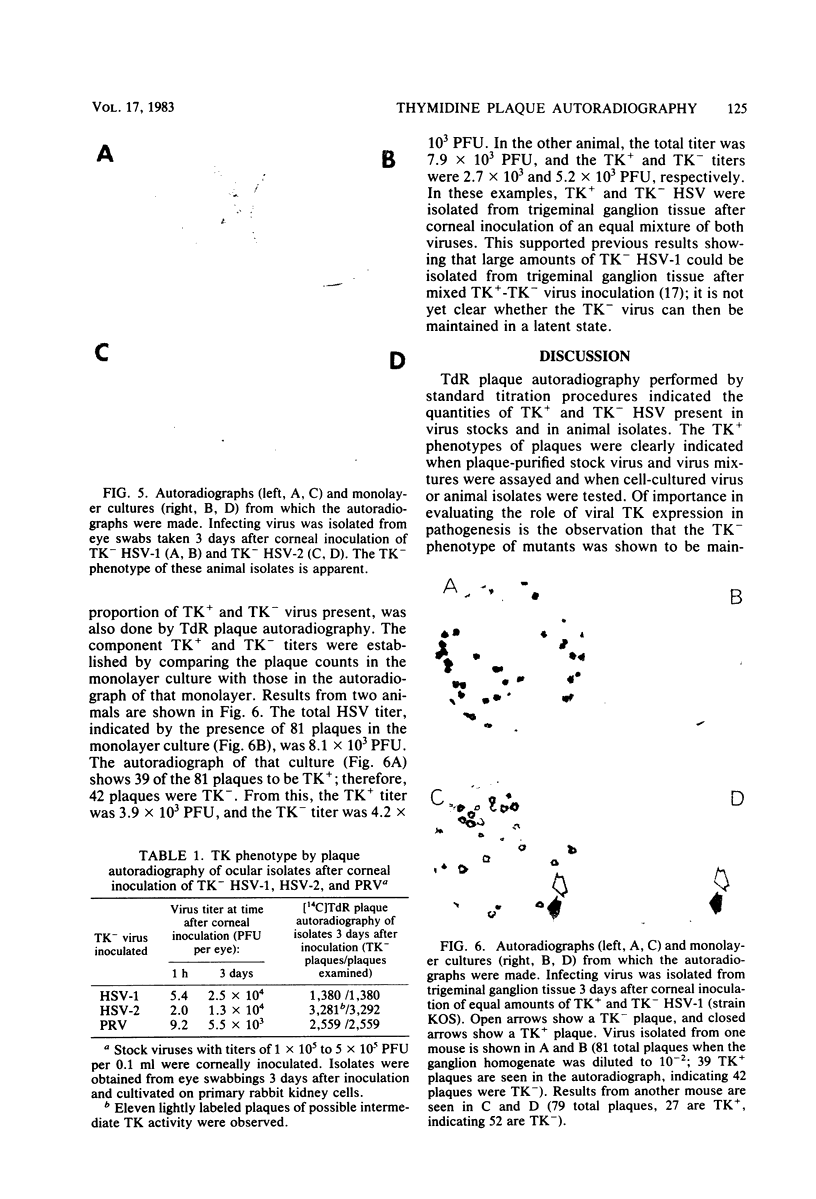


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burns W. H., Saral R., Santos G. W., Laskin O. L., Lietman P. S., McLaren C., Barry D. W. Isolation and characterisation of resistant Herpes simplex virus after acyclovir therapy. Lancet. 1982 Feb 20;1(8269):421–423. doi: 10.1016/s0140-6736(82)91620-8. [DOI] [PubMed] [Google Scholar]
- Cheng Y. C., Dutschman G., Fox J. J., Watanabe K. A., Machida H. Differential activity of potential antiviral nucleoside analogs on herpes simplex virus-induced and human cellular thymidine kinases. Antimicrob Agents Chemother. 1981 Sep;20(3):420–423. doi: 10.1128/aac.20.3.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Schaffer P. A. Two distinct loci confer resistance to acycloguanosine in herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2265–2269. doi: 10.1073/pnas.77.4.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumpacker C. S., Schnipper L. E., Marlowe S. I., Kowalsky P. N., Hershey B. J., Levin M. J. Resistance to antiviral drugs of herpes simplex virus isolated from a patient treated with acyclovir. N Engl J Med. 1982 Feb 11;306(6):343–346. doi: 10.1056/NEJM198202113060606. [DOI] [PubMed] [Google Scholar]
- Ecker J. R., Hyman R. W. Varicella zoster virus DNA exists as two isomers. Proc Natl Acad Sci U S A. 1982 Jan;79(1):156–160. doi: 10.1073/pnas.79.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman P. A., St Clair M. H., Fyfe J. A., Rideout J. L., Keller P. M., Elion G. B. Inhibition of herpes simplex virus-induced DNA polymerase activity and viral DNA replication by 9-(2-hydroxyethoxymethyl)guanine and its triphosphate. J Virol. 1979 Oct;32(1):72–77. doi: 10.1128/jvi.32.1.72-77.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson A. T., Subak-Sharpe J. H. Interallelic complementation of mutants of herpes simplex virus deficient in deoxypyrimidine kinase activity. Virology. 1978 Mar;85(1):109–117. doi: 10.1016/0042-6822(78)90415-4. [DOI] [PubMed] [Google Scholar]
- Larsson A., Oberg B. Selective inhibition of herpesvirus deoxyribonucleic acid synthesis by acycloguanosine, 2'-fluoro-5-iodo-aracytosine, and (E)-5-(2-bromovinyl)-2'-deoxyuridine. Antimicrob Agents Chemother. 1981 May;19(5):927–929. doi: 10.1128/aac.19.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. L., Iltis J. P., Rapp F. Differential effect of arabinofuranosylthymine of the replication of human herpesviruses. J Virol. 1977 Sep;23(3):679–684. doi: 10.1128/jvi.23.3.679-684.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnipper L. E., Crumpacker C. S. Resistance of herpes simplex virus to acycloguanosine: role of viral thymidine kinase and DNA polymerase loci. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2270–2273. doi: 10.1073/pnas.77.4.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley J. R., Wagner M. J., Summers W. P., Summers W. C. Genetic and physical evidence for the polarity of transcription of the thymidine kinase gene of herpes simplex virus. Virology. 1980 Apr 15;102(1):83–93. doi: 10.1016/0042-6822(80)90072-0. [DOI] [PubMed] [Google Scholar]
- St Clair M. H., Furman P. A., Lubbers C. M., Elion G. B. Inhibition of cellular alpha and virally induced deoxyribonucleic acid polymerases by the triphosphate of acyclovir. Antimicrob Agents Chemother. 1980 Nov;18(5):741–745. doi: 10.1128/aac.18.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers W. C., Summers W. P. [125I]deoxycytidine used in a rapid, sensitive, and specific assay for herpes simplex virus type 1 thymidine kinase. J Virol. 1977 Oct;24(1):314–318. doi: 10.1128/jvi.24.1.314-318.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenser R. B., Dunstan M. E. Herpes simplex virus thymidine kinase expression in infection of the trigeminal ganglion. Virology. 1979 Dec;99(2):417–422. doi: 10.1016/0042-6822(79)90021-7. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Miller R. L., Rapp F. Trigeminal ganglion infection by thymidine kinase-negative mutants of herpes simplex virus. Science. 1979 Aug 31;205(4409):915–917. doi: 10.1126/science.224454. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Ressel S., Dunstan M. E. Herpes simplex virus thymidine kinase expression in trigeminal ganglion infection: correlation of enzyme activity with ganglion virus titer and evidence of in vivo complementation. Virology. 1981 Jul 15;112(1):328–341. doi: 10.1016/0042-6822(81)90638-3. [DOI] [PubMed] [Google Scholar]