Abstract
To investigate the relationship of cutaneous leishmaniasis isolates from Sri Lanka to known species, we performed DNA sequencing and microsatellite analyses. We identified Leishmania donovani as the agent of Sri Lanka cutaneous leishmaniasis and showed that these parasites are closely related to those causing visceral leishmaniasis in the Indian subcontinent.
Keywords: Parasitic diseases, protozoan infections, leishmaniasis, cutaneous, leishmaniasis, visceral, zoonoses, Sri Lanka, dispatch
Infection with Leishmania protozoa can result in cutaneous, mucocutaneous, or visceral leishmaniasis (VL), depending on the parasite, host, and environmental factors (1). Globally, the disease results in ≈2 million new cases and 2.4 million disability-adjusted life years each year (2). The leishmaniases have received renewed interest because of an upsurge of cases in traditionally leishmaniasis-endemic areas and the emergence of new foci of disease (3,4). One of the most dramatic examples is a new focus of cutaneous leishmaniasis (CL) in Sri Lanka (5), from which >400 cases have been reported since 2001.
Previously, multilocus enzyme electrophoresis (MLEE) characterization of a small number of isolates led to the surprising conclusion that CL in Sri Lanka was caused by Leishmania donovani (5). However, L. donovani typically causes VL, a potentially fatal disease and ongoing public health problem in neighboring India, Bangladesh, and Nepal, as well as in East Africa (1,2). No cases of VL have been reported in Sri Lanka. Occasional cases of CL due to L. donovani have been described in other VL-endemic regions (6–9). Karunaweera et al. (5) examined a limited number of isolates and used a single technique, MLEE. Although this technique is usually reliable for characterizing isolates, important exceptions were found in a recent study on L. donovani in East Africa (10). Therefore, we further investigated Sri Lanka CL by examining more isolates and using 2 molecular techniques.
The Study
Suspected clinical diagnoses of CL were confirmed by demonstrating the presence of Leishmania amastigotes in skin lesions, promastigotes in cultures, or both (5). Ethical approval was obtained from the Ethics Review Committee, Faculty of Medicine, University of Colombo. PCR, performed as described (11), confirmed 15 primary isolates as members of the genus Leishmania. Eight of the Sri Lanka isolates originated from Welioya (northeast), 1 from Jaffna (north), and 2 from Galle (south).
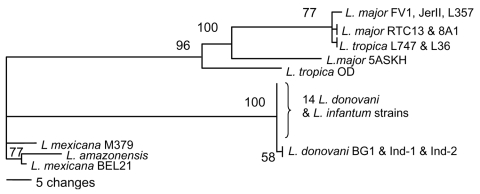
DNA sequencing of a single-copy gene was used to identify the Leishmania species (10). The 6-phosphogluconate dehydrogenase (6PGDH) gene was chosen because it shows a high degree of sequence polymorphism among Leishmania species (12), is well represented in sequence databases, and is known to differentiate the main zymodeme from L. donovani in India (MON-2) from that elsewhere (13). Primers for conserved regions of 6PGDH were designed by using full-length gene sequences of the L. major FV1 (MHOM/IL/1980/Friedlin) and L. mexicana BEL21 (MHOM/BZ/1982/BEL21) reference strains. Primers 6PGDH-F (AAT CGA GCA GCT CAA GGA AG) and 6PGDH-R (GAG CTT GGC GAG AAT CTG AC) were designed to generate a 997-bp amplicon incorporating the 822-nt partial 6PGDH sequence that is represented for multiple Leishmania species in GenBank. The partial sequences of 6PGDH genes were obtained from 11 Sri Lanka isolates from patients with CL, 2 India isolates from patients with VL, and 2 additional known L. donovani strains. These 15 new sequences and 10 publicly available sequences for species belonging to the genus Leishmania were used to construct a classification (Figure 1). Of 17 L. donovani and L. infantum sequences, 14 were >99% identical and could not be separated; the remaining 3 stocks were from India and Bangladesh (Ind-1, Ind-2, and BG1) and clustered together with 58% bootstrap support. Thus, L. donovani from Sri Lanka formed a strongly supported group with L. donovani and L. infantum from Europe and Africa. This group was quite distinct from the group that includes L. major and L. tropica, which are the parasite species most closely related to L. donovani and L. infantum and which both cause CL in Africa and Asia. This analysis provided convincing evidence that all 11 Sri Lanka isolates examined were L. donovani or L. infantum.
Figure 1.
Classification of Leishmania species according to the partial DNA sequence of the 6-phosphogluconate dehydrogenase gene constructed with PHYLIP (http://evolution.genetics.washington.edu/phylip.html) using parsimony. Numbers at branch points are bootstrap values compiled by using 100 replicates. Isolates examined and the accession numbers of their 6PGDH sequences in the GenBank/EMBL/DDBJ database are as follows: 11 Sri Lanka isolates, L59, L60, L75, L78, L80, L284, L304, L355, L330, L301, L348 (AJ888888-AJ888898); 2 India isolates, Ind-1, Ind-2 from splenic aspirates of visceral leishmaniasis patients in Muzafapur, Bihar (MHOM/IN/2004/Ind-1 and MHOM/IN/2004/Ind-2, AJ888900, AJ888901); 3 previously identified L. donovani isolates BG1 (MHOM/BD/1997/BG1, AJ888899), LEM719 (IMAR/KE/1962/LRC-L57; LEM719, AJ888902), LV9 (MHOM/ET/1967/HU3;LV9, AY168567); and L. infantum JPC (MCAN/ES/1998/LEM935;JPC;M5, GeneDB LinJ35.2940). Also analyzed were sequences from the following isolates: L. tropica (AY045763, AY168568), L. major (FV1, AF242436; 8A1, AF242436; RTC13, AY706106; JerII, AY706105; 5ASKH, AY706107), L. mexicana (M379, AY217723; BEL21, AY386372), and L. amazonensis (PH8, AY168562).
Strains of L. donovani from Sri Lanka were typed as zymodeme MON-37 by MLEE (5). This differs from the predominant India zymodeme (MON-2) in the mobility of 1 isoenzyme, 6PGDH. Therefore, the sequences were further analyzed to investigate the sequence variation underlying the isoenzyme identification. Translation of the 822-nt sequences showed 1 amino acid change that was consistent with the results of MLEE. A single nucleotide difference at position 976 was responsible for the occurrence of an uncharged asparagine (codon AAC) in MON-2 or a negatively charged aspartic acid (codon GAC) in MON-1, MON-18, and MON-37 sequences. This single change would explain the lower mobility of the MON-2 6PGDH isoenzyme, similar to the situation previously reported for glutamate oxaloacetate transaminase isoenzymes in East Africa L. donovani strains (10).
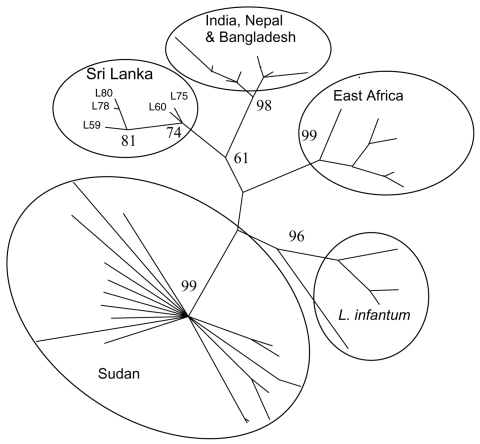
To more closely analyze the relationships of the L. donovani and L. infantum strains, we performed microsatellite analysis (10). These data were combined with a dataset comprising 40 previously examined L. donovani and L. infantum isolates (Figure 2). The Sri Lanka isolates clustered together and close to a group containing L. donovani isolates from India, Bangladesh, and Nepal. L. infantum isolates formed a distinct cluster, as did the L. donovani isolates from Sudan and Kenya. This analysis reconfirms recent observations (10,14) that L. donovani isolates tend to cluster on a geographic basis, which suggests that strains of this parasite are geographically distinct. Also, although the Sri Lanka isolates form 1 or possibly 2 distinct groups, they are most closely related to L. donovani, which causes VL in India, and distant from L. infantum parasites.
Figure 2.
Classification of Leishmania donovani and L. infantum isolates constructed by using microsatellite data with parsimony in PAUP (Sinauer Associates Inc., Sunderland, MA, USA). Numbers at branch points are bootstrap values compiled by using 100 replicates. Isolates formed geographically based groups (circled). Sri Lanka isolates L59, L60, L75, L78, and L80 are indicated. The tips of other branches are from a dataset of other previously analyzed isolates, including all those identified as L. donovani or L. infantum and isolates from the Indian subcontinent (10).
Conclusions
The results of this study led us to conclude that in Sri Lanka, CL is caused by L. donovani, which affects the epidemiology and clinical management of leishmaniasis. CL in Sri Lanka can no longer be regarded as a minor problem; an explosion of cases in the past 5 years, undoubtedly an underrepresentation of the true incidence of disease, has not included a single case of VL. However, the possibility that VL will emerge should be considered because subclinical infection is frequent in VL-endemic areas (15). The clinical management of CL is often self-cure, which may be preferable to active treatment because self-cure may promote natural immunity to reinfection. Alternatively, antileishmanial drugs may be administered topically or by intralesional injection (2). However, L. donovani is recognized as one of the great scourges of mankind (3,4), and if visceral disease does emerge as a problem, more aggressive treatment of CL in Sri Lanka should be considered, e.g., parenteral administration of antimonial compounds, amphotericin, or oral miltefosine. Unfortunately, no drugs are currently registered for the treatment of leishmaniasis in Sri Lanka, and cryotherapy is the only available option in most healthcare centers. Better availability of drugs to treat CL in Sri Lanka is needed, but their introduction must be carefully monitored and critically evaluated.
Our study also raises questions about how infection with apparently identical or very similar parasites can result in radically different types of disease. We speculate that the answers likely lie with the nature of the parasites, the genetics of the human population, or the contribution of sandfly vectors. The data presented here demonstrate the overall close genetic similarity among all L. donovani isolates examined. However, some critical genetic difference in Sri Lanka parasites may exist and render them less virulent than L. donovani from elsewhere. Clearly much work remains to be done, including PCR or serologic investigation of possible subclinical VL, to understand the factors behind the emergence of Sri Lanka CL due to L. donovani. Future studies must be a priority as the number of cases continues to increase.
Acknowledgments
We are grateful to J.P. Dedet, I. Mauricio¸ and S. Sundar for providing some of the isolates analyzed in this study.
H.V.Y.D.S. was supported by a Commonwealth Split-Site Doctoral Scholarship from the Association of Commonwealth Universities.
Dr Siriwardana qualified from the Faculty of Medicine, University of Colombo, and is currently completing postgraduate PhD training in medical parasitology. Her main research interest is in leishmaniasis.
Footnotes
Suggested citation for this article: Siriwardana HVYD, Noyes HA, Beeching NJ, Chance ML, Karunaweera ND, Bates PA. Leishmania donovani and cutaneous leishmaniasis, Sri Lanka. Emerg Infect Dis [serial on the Internet]. 2007 Mar [date cited]. Available from http://www.cdc.gov/eid/content/13/3/476.htm
References
- 1.Bates PA. Leishmania. In: Encyclopedia of life sciences. Hoboken (NJ): John Wiley & Sons, Inc; 2006. [cited 2007 Jan 25]. Available from http://els.wiley.com/els/ (doi:10.1038/npg.els.0001968)
- 2.Davies CR, Kaye P, Croft SL, Sundhar S. Leishmaniasis: new approaches to disease control. BMJ. 2003;326:377–82. 10.1136/bmj.326.7385.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashford RW. The leishmaniases as emerging and reemerging zoonoses. Int J Parasitol. 2000;30:1269–81. 10.1016/S0020-7519(00)00136-3 [DOI] [PubMed] [Google Scholar]
- 4.Desjeux P. The increase in risk factors for leishmaniasis worldwide. Trans R Soc Trop Med Hyg. 2001;95:239–43. 10.1016/S0035-9203(01)90223-8 [DOI] [PubMed] [Google Scholar]
- 5.Karunaweera ND, Pratlong F, Siriwardana HVYD, Ihalamulla RL, Dedet JP. Sri Lankan cutaneous leishmaniasis is caused by Leishmania donovani MON 37. Trans R Soc Trop Med Hyg. 2003;97:380–1. 10.1016/S0035-9203(03)90061-7 [DOI] [PubMed] [Google Scholar]
- 6.Mebrahtu YB, Van Eys G, Guizani I, Lawyer PG, Pamba H, Koech D, et al. Human cutaneous leishmaniasis caused by Leishmania donovani s.l. in Kenya. Trans R Soc Trop Med Hyg. 1993;87:598–601. 10.1016/0035-9203(93)90101-U [DOI] [PubMed] [Google Scholar]
- 7.Pratlong F, Bastein P, Perello R, Lami P, Dedet JP. Human cutaneous leishmaniasis caused by Leishmania donovani sensu stricto in Yemen. Trans R Soc Trop Med Hyg. 1995;89:398–9. 10.1016/0035-9203(95)90025-X [DOI] [PubMed] [Google Scholar]
- 8.Ben-Ami R, Schnur LF, Golan Y, Jaffe CL, Mardi T, Zeltser D. Cutaneous involvement in a rare case of adult visceral leishmaniasis acquired in Israel. J Infect. 2002;44:181–4. 10.1053/jinf.2002.0953 [DOI] [PubMed] [Google Scholar]
- 9.Sharma NL, Mahajan VK, Kanga A, Sood A, Katoch VM, Mauricio I, et al. Localized cutaneous leishmaniasis due to Leishmania donovani and Leishmania tropica: preliminary findings of the study of 161 new cases from a new endemic focus in Himachal Pradesh, India. Am J Trop Med Hyg. 2005;72:819–24. [PubMed] [Google Scholar]
- 10.Jamjoom MB, Ashword RW, Bates PA, Chance ML, Kemp SJ, Watts PC, et al. Leishmania donovani is the only cause of visceral leishmaniasis in East Africa; previous descriptions of L. infantum and L. archibaldi from this region are a consequence of convergent evolution in the iso-enzyme data. Parasitology. 2004;129:399–409. 10.1017/S0031182004005955 [DOI] [PubMed] [Google Scholar]
- 11.Lachaud L, Chabbert E, Dubessay P, Reynes J, Lamothe J, Bastien P. Comparison of various sample preparation methods for PCR diagnosis of visceral leishmaniasis using peripheral blood. J Clin Microbiol. 2001;39:613–7. 10.1128/JCM.39.2.613-617.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenblatt CL, Schnur LF, Bar-Gal GK, Ermolaev H, Peleg N, Barret MP. Polymorphism among alleles of the 6-phosphogluconate dehydrogenase gene from Leishmania major and Leishmania tropica. Mol Biochem Parasitol. 2002;125:185–8. 10.1016/S0166-6851(02)00213-X [DOI] [PubMed] [Google Scholar]
- 13.Pratlong F, Dereure J, Bucheton B, El-Saf S, Dessein A, Lanotte G, et al. Sudan: the possible original focus of visceral leishmaniasis. Parasitology. 2001;122:599–605. 10.1017/S0031182001007867 [DOI] [PubMed] [Google Scholar]
- 14.Zemanova E, Jirku M, Mauricio I, Miles MA, Lukes J. Genetic polymorphisms within the Leishmania donovani complex: correlation with geographic origin. Am J Trop Med Hyg. 2004;70:613–7. [PubMed] [Google Scholar]
- 15.Sundar S, Maurya R, Singh RK, Bharti K, Chakravarty K, Parekh A, et al. Rapid, noninvasive diagnosis of visceral leishmaniasis in India: comparison of two immunochromatographic strip tests for detection of anti-RK39 antibody. J Clin Microbiol. 2006;44:251–3. 10.1128/JCM.44.1.251-253.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]