Abstract
Recent emergence of a virulent strain of Clostridium difficile demonstrates the importance of tracking C. difficile incidence locally. Our survey of New Jersey hospitals documented increases in the rates of C. difficile disease (by 2-fold), C. difficile–associated complications (by 7-fold), and C. difficile outbreaks (by 12-fold) during 2000–2004.
Keywords: Clostridium difficile, hospital infections, surveillance, New Jersey, dispatch
Clostridium difficile, a gram-positive organism, is the most common cause of nosocomial infectious diarrhea in the United States (1). In 2005, the Centers for Disease Control and Prevention (CDC) reported on a new, epidemic, toxin gene–variant strain of C. difficile on the basis of a study of isolates collected from hospitals in multiple states, including New Jersey. CDC recommended that inpatient healthcare facilities track the incidence of C. difficile–associated disease (CDAD), including the clinical outcomes of patients (2).
The Study
To estimate the incidence of CDAD in hospitalized patients in New Jersey, we conducted a retrospective survey of acute-care hospitals. An Internet-based questionnaire was distributed to all 81 New Jersey hospitals in early 2005; hospitals that did not respond were contacted by telephone or electronic mail. We collected information on hospital characteristics, the number of CDAD cases, C. difficile–positive laboratory test results, C. difficile–associated complications, deaths due to any cause within 30 days of diagnosis with C. difficile infection, healthcare-associated C. difficile outbreaks, recurrent CDAD cases, diagnostic test methods, and surveillance activities. The proportion of community- versus healthcare-acquired cases was not assessed objectively; however, respondents provided their perceptions regarding trends. No individual patient information was obtained.
A CDAD case was defined as a patient with symptoms of diarrhea and at least 1 of the following: positive toxin assay result, diagnosis of pseudomembranous colitis on sigmoidoscopy or colonoscopy, or histopathologic diagnosis. An outbreak was defined as >3 cases of healthcare-associated CDAD in the same general area within 7 days. A complicated case was defined as a patient with CDAD in whom toxic megacolon, perforation of the colon, colectomy, or shock requiring vasopressor therapy subsequently developed within 30 days after diagnosis with CDAD. The definition of recurrent CDAD and the method of laboratory diagnoses were determined by each hospital.
Data were analyzed by using EpiInfo version 3.3.2 (CDC, Atlanta, GA, USA) and SAS version 8.02 (SAS Institute, Cary, NC, USA). The medians, means, ranges, frequencies, and totals reported are based on actual responses to the survey questions; hospitals that did not answer a question were excluded from the analysis of responses to that question. Tests for linear trend over the study period were performed by using linear regression. We also examined the association between CDAD rates and staffing levels of infection-control professionals (ICPs) by using a Poisson regression model.
Of the 81 hospitals contacted, 58 (72%), located in 20 of 21 New Jersey counties, responded to the survey. The median bed capacity of participating hospitals was 281 (range 77–683), and the median number of full-time equivalent ICPs per 250 beds was 1.2 (range 0–3).
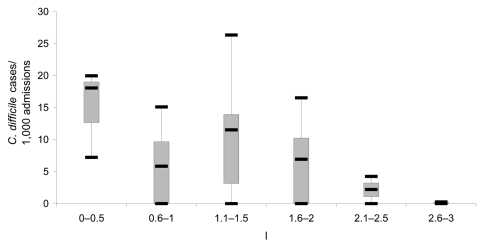
During 2000–2004, participating hospitals reported a total of 13,394 CDAD cases. The mean annual rate of CDAD increased from 3.7/1,000 admissions in 2000 to 7.7/1,000 admissions in 2004, which represented a >2-fold increase in CDAD rates during the 5-year period (p<0.05, Table 1). Of the hospitals that responded, the percentage that did not identify CDAD cases decreased from 55% in 2000 to 25% in 2004. A significant inverse association existed between the number of ICPs per 250 beds and CDAD rates in 2004 (p = 0.05, Figure). Table 1 indicates a similar increasing trend in the rates of positive C. difficile test results, CDAD outbreaks, complications, annual 30-day crude mortality (deaths due to any cause), and recurrent CDAD infection. Hospitals differed widely in their case definitions for recurrent cases. Most used an arbitrary period (from first episode to CDAD relapse), varying from 6 weeks to 1 year, to define a recurrent case.
Table 1. Clostridium difficile infection rates in acute-care hospitals, New Jersey, 2000–2004*.
| 2000 | 2001 | 2002 | 2003 | 2004 | Mean | Total | 2004 rate/ 2000 rate | |
|---|---|---|---|---|---|---|---|---|
| C. difficile cases | 1,585 | 1,540 | 2,201 | 2,974 | 5,094 | 2,679 | 13,394 | NA |
| C. difficile cases/1,000 admissions | 3.7 | 3.2 | 4.1 | 4.9 | 7.7 | 4.7 | NA | 2.1 |
| C. difficile–positive test results | 2,355 | 2,759 | 5,193 | 8,592 | 12,445 | 6,269 | 31,344 | NA |
| C. difficile–positive test results/1,000 admissions | 4.2 | 4.4 | 7.8 | 12.4 | 18.4 | 9.4 | NA | 4.4 |
| C. difficile–complicated cases | 2 | 3 | 5 | 12 | 43 | 13 | 65 | NA |
| C. difficile complication rate† (%) | 0.1 | 0.2 | 0.2 | 0.4 | 0.9 | 0.4 | NA | 6.8 |
| C. difficile healthcare-associated outbreaks (no. hospitals affected) | 2 (2) | 6 (1) | 10 (7) | 11 (7) | 25 (12) | 11 | 54 | NA |
| C. difficile healthcare-associated outbreak rate‡ | 3.7 | 10.9 | 18.2 | 19.6 | 44 | 19.3 | NA | 11.9 |
| Deaths reported within 30 days after diagnosis | 0 | 0 | 23 | 50 | 87 | 32 | 160 | NA |
| 30-day C. difficile crude mortality rate (%) | 0 | 0 | 1.2 | 1.9 | 1.8 | 1 | NA | 1.5§ |
| Recurrent C. difficile cases | 0 | 1 | 7 | 76 | 171 | 51 | 255 | NA |
| Recurrent C. difficile rate¶ (%) | 0 | 0.1 | 0.4 | 2.7 | 3.4 | 1.3 | NA | 34# |
*NA, not available. †Percentage of C. difficile patients in whom complications developed. ‡Outbreaks per 100 acute-care hospitals that responded. §2004 rate/2002 rate. ¶Percentage of C. difficile patients whose infections recurred. #2004 rate/2001 rate.
Figure.
Boxplot of Clostridium difficile rates by number of infection-control professionals (ICPs) per 250 beds, New Jersey, 2004. Each box shows the median, quartiles, and extreme values.
Most (60%) respondents thought that the number of cases of community-acquired CDAD increased in 2004 compared with previous years. Smaller proportions of respondents perceived increases in the numbers of recurrent cases (55%), healthcare-acquired cases (40%), complicated cases (28%), and deaths (19%) during the same period.
Hospital laboratories most commonly used enzyme immunoassay (EIA) tests for toxins A and B to identify C. difficile (88%), followed by stool culture (16%), cytotoxin testing using tissue culture (7%), and EIA for toxin A (7%). Forty (69%) hospital laboratories reported having written institutional policies for correct specimen collection, storage, and transportation of specimens for CDAD diagnosis. Surveillance methods used by hospitals to track CDAD in their institutions are detailed in Table 2.
Table 2. Clostridium difficile surveillance activities conducted by hospitals, New Jersey, 2000–2004.
| Surveillance activities | No. (%) hospitals |
|---|---|
| Monitors C. difficile–positive laboratory results | 55 (95) |
| Makes a distinction between community- and healthcare-acquired C. difficile cases | 44 (76) |
| Uses a standard C. difficile case definition for surveillance | 35 (60) |
| Monitors clinical outcome of patients with C. difficile infection | 28 (48) |
| Physicians notify infection-control professional of C. difficile diagnoses | 18 (31) |
The survey design had several limitations. First, the analysis was designed to measure the overall incidence of CDAD associated with acute-care hospitalization, regardless of acquisition site, and we did not distinguish between community- and healthcare-associated infections. Second, we did not collect information on nonresponding hospitals and therefore are unable to determine if substantial differences existed between responding and nonresponding hospitals. Third, the rates of CDAD infections were calculated from data provided by the hospitals; hospitals might not have consistently followed the case definitions that were provided for reporting. We also reviewed administrative (i.e., universal billing) data as a secondary data source and found similar trends to those observed in this study. However, this process has multiple weaknesses, including ambiguities in coding and misclassification, which limit its utility for surveillance (3). Finally, enhanced awareness of the disease among clinicians and ICPs might have contributed to increased reporting during the 5-year period.
Conclusions
Our results demonstrate that CDAD rates and associated complications rose rapidly among New Jersey hospitals during 2000–2004. How much of the increase reflects rising awareness and how much is a true increase in incidence is unclear. Nevertheless, the trend is dramatic and consistent with published reports in the United States, Canada, and Europe that evaluated CDAD rates during earlier periods (4–7).
Our observation that a higher ICP staffing level was associated with lower CDAD rates is consistent with previous studies demonstrating that a higher ICP-to-bed ratio is associated with reduction in rates of healthcare-acquired infections (8–10). We recommend that hospitals ensure that their infection-control programs employ sufficient personnel and other resources to implement adequate infection-control practices, with the goal of decreasing CDAD rates in their institutions.
In terms of surveillance activities, almost all participating hospitals tracked C. difficile laboratory results. However, a relatively low percentage of hospitals routinely monitored CDAD complications and deaths. Given recent reports of the emergence of hypertoxin-producing C. difficile strains that are more treatment-resistant and potentially more virulent than other strains (2), we recommend that hospitals implement or continue comprehensive surveillance programs to track the incidence of both healthcare-acquired and community-acquired CDAD, as well as patient outcomes. Surveillance of these entities will allow ICPs to identify quickly changes in CDAD incidence and severity that could be associated with the introduction of new, more virulent strains. In addition, rapid changes in incidence and detected outbreaks should be reported to public health officials.
Despite the survey’s limitations, the estimates provided from this substantial sample of acute-care hospitals are useful for hospitals to develop appropriate CDAD policies and can serve as comparison data for future infection prevention and control efforts in New Jersey and other states. Indeed, given the recent increase in the extent of C. difficile death and illness in North America and Europe, the findings in this study show that CDAD is an emerging problem, worthy of substantial investment in effective infection-control and monitoring systems.
Acknowledgments
We thank Beena Poonolly, Diana Bensyl, and Stella Tsai, as well as infection-control professionals and hospital epidemiology personnel at participating New Jersey institutions and members of the New Jersey Chapter of the Association of Professionals of Infection Control, whose time and effort made this project possible.
Biography
Dr Tan is a medical planning officer (pandemic influenza preparedness) at the Department of Peacekeeping Operations, United Nations. Her research interests include emerging infectious diseases, bioterrorism, emergency preparedness, tropical medicine, and the intersection between infectious disease and public health policy.
Footnotes
Suggested citation for this article: Tan ET, Robertson CA, Brynildsen S, Bresnitz E, Tan C, McDonald C. Clostridium difficile–associated disease in New Jersey hospitals, 2000–2004. Emerg Infect Dis [serial on the Internet]. 2007 Mar [date cited]. Available from http://www.cdc.gov/eid/content/13/3/498.htm
Presented in part at the 16th Annual Scientific Meeting of the Society for Healthcare Epidemiology of America, Chicago, Illinois, USA, March 20, 2006.
References
- 1.Barbut F, Corthier G, Charpak Y, Cerf M, Monteil H, Fosse T, et al. Prevalence and pathogenicity of Clostridium difficile in hospitalized patients. A French multicenter study. Arch Intern Med. 1996;156:1449–54. 10.1001/archinte.156.13.1449 [DOI] [PubMed] [Google Scholar]
- 2.McDonald LC, Killgore GE, Thompson A, Owens RC Jr, Kazakova SV, Sambol SP, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353:2433–41. 10.1056/NEJMoa051590 [DOI] [PubMed] [Google Scholar]
- 3.Virnig BA, McBean M. Administrative data for public health surveillance and planning. Annu Rev Public Health. 2001;22:213–30. 10.1146/annurev.publhealth.22.1.213 [DOI] [PubMed] [Google Scholar]
- 4.Archibald LK, Banerjee SN, Jarvis WR. Secular trends in hospital-acquired Clostridium difficile disease in the United States, 1987–2001. J Infect Dis. 2004;189:1585–9. 10.1086/383045 [DOI] [PubMed] [Google Scholar]
- 5.Pepin J, Valiquette L, Alary ME, Villemure P, Pelletier A, Forget K, et al. Clostridium difficile–associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. CMAJ. 2004;171:466–72. 10.1503/cmaj.1041104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Clostridium difficile Standards Group. Report to the Department of Health. J Hosp Infect. 2004;56(Suppl 1):1–38. [DOI] [PubMed] [Google Scholar]
- 7.McDonald LC, Owings M, Jernigan DB. Increasing rates of Clostridium difficile infection among patients discharged from US short-stay hospitals, 1996–2003. Emerg Infect Dis [serial on the internet]. 2006. Mar [cited 5 Jan 2007]. Available from http://www.cdc.gov/ncidod/EID/vol12no03/05-1064.htm [DOI] [PMC free article] [PubMed]
- 8.Centers for Disease Control and Prevention. Public health focus: surveillance, prevention and control of nosocomial infections. MMWR Morb Mortal Wkly Rep. 1992;41:783–7. [PubMed] [Google Scholar]
- 9.Culver DH, White JW, Morgan WM, Emori TG, Munn VP, Hooton TP. The efficacy of infection surveillance and control programs in preventing nosocomial infections in US hospitals. Am J Epidemiol. 1985;121:182–205. [DOI] [PubMed] [Google Scholar]
- 10.Scheckler WE, Brimhall D, Buck AS, Farr BM, Friedman C, Garibaldi RA, et al. Requirements for infrastructure and essential activities of infection control and epidemiology in hospitals: a consensus panel report. Am J Infect Control. 1998;26:47–60. 10.1016/S0196-6553(98)70061-6 [DOI] [PubMed] [Google Scholar]