Abstract
To determine the predominant staphylococcal cassette chromosome (SCC) mec element in methicillin-resistant Staphylococcus aureus, we typed 190 isolates from a hospital in Taiwan. We found a shift from type IV to type III SCCmec element during 1992–2003, perhaps caused by selective pressure from indiscriminate use of antimicrobial drugs.
Keywords: Methicillin-resistant Staphylococcus aureus, multilocus sequence typing, SCCmec, dispatch
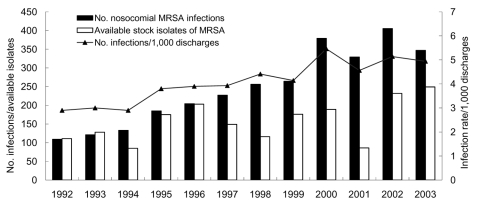
The high prevalence of methicillin-resistant Staphylococcus aureus (MRSA), which accounts for as much as 80% of all S. aureus isolates causing nosocomial infections in Taiwanese hospitals since 1998, has greatly affected infection control and medical treatment in Taiwan (1). At National Taiwan University Hospital (NTUH), a 2,200-bed major teaching hospital in northern Taiwan, MRSA has become a common nosocomial pathogen since the early 1990s. The annual number and incidence of nosocomial MRSA infections, as well as the number of available nonduplicate isolates for the past 12 years at NTUH, are shown in the Figure.
Figure.
Number and cumulative incidence of nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infections per 1,000 discharges and number of available nonduplicate MRSA isolates at National Taiwan University Hospital, 1992–2003.
In a previous study, we used pulsed-field gel electrophoresis typing of 140 randomly selected nosocomial MRSA isolates and samples from our collection of nosocomial isolates obtained from 1992 to 1996 to identify 3 major pulsotypes (A, B, and C) (2). Pulsotype A was predominant among all MRSA isolates in 1992 (accounting for 50%) and 1993 (52%), pulsotype B was predominant in 1994 (59%) and 1995 (49%), and pulsotype C was predominant in 1996 (83%) (2). Pulsotype C remained the predominant clone until 2003 (J.-T. Wang et al., unpub. data).
S. aureus acquires methicillin resistance through a mobile staphylococcal cassette chromosome (SCC) that contains the mecA gene complex (SCCmec) (3). Until now, 5 major types of SCCmec element have been characterized and studied (3–5). However, longitudinal studies of SCCmec elements in nosocomial MRSA isolates in a hospital have seldom been reported (6). We analyzed SCCmec elements in predominant nosocomial MRSA clones at NTUH from 1992 through 2003. Because the predominant MRSA clone at NTUH from 1996 through 2003 was pulsotype C, MRSA isolates obtained from 1997 through 2002 were not studied.
The Study
We analyzed all 140 MRSA isolates we obtained during a previous study (2) and 50 other isolates selected from our collection of nosocomial MRSA isolates obtained in 2003. Characteristics of these 190 isolates are shown in Table 1.
Table 1. Pulsotypes, characteristics, and sources of 190 methicillin-resistant Staphylococcus aureus (MRSA) isolates, Taiwan, 1992–2003.
| Year, clinical syndrome (no. isolates)* | Pulsotype (no. isolates) | Source specimen (no. isolates)† | Site of isolation (no. isolates)‡ |
|---|---|---|---|
| 1992 | |||
| NI (9) | A (4), B (3), F (1) | Bl (3), Ur (2), Sp (2), Pu (2) | ICU (6), ward (3) |
| NC (8) | A (3), B (4), F (1) | Sp (4), Wo (4) | ICU (3), ward (5) |
| IEOH (5) | A (4), B (1) | Sp (4), Pu (1) | ICU (3), ward (2) |
| 1993 | |||
| NI (10) | A (1), B (8), F(1) | Bl (4), Ur (1), Sp (2), Pu (3) | ICU (8), ward (2) |
| NC (14) | A (11), B (3) | Sp (5), Wo (5), Ns (3), Ct (1) | ICU (9), ward (5) |
| IEOH (1) | A (1) | Sp (1) | Ward (1) |
| 1994 | |||
| NI (9) | A (4), B (4), D (1) | Bl (7), Sp (1), Pu (1) | ICU (4), ward (5) |
| NC (8) | A (1), B (6), C (1) | Sp (4), Wo (3), Ns (1) | ICU (5), ward (3) |
| 1995 | |||
| NI (9) | B (3), C (5), E (1) | Bl (2), Sp (3), Pu (4) | ICU (6), ward (3) |
| NC (25) | A (1), B (13), C (9), D (2) | Sp (14), Wo (5), Ns (6) | ICU (19), ward (6) |
| IEOH (1) | B (1) | Sp (1) | Ward (1) |
| 1996 | |||
| NI (23) | A (1), B (1), C (20), D (1) | Bl (15), Sp (3), Pu (5) | ICU (16), ward (7) |
| NC (11) | A (1), C (10) | Sp (7), Wo (2), Ns (1), St (1) | ICU (11) |
| IEOH (7) | A (1), B (2), C (4) | Bl (2), Sp (5) | ICU (5), ward (2) |
| 2003 | |||
| NI (24) | C (10), D (2), K (5), L (2), M (3), other (2) | Bl (16), Sp (4), Pu (4) | ICU (10), ward (14) |
| NC (26) | A (1), C (10), K (6),L (3), M (5), other (1) | Sp (14), Wo (12) | ICU (12), ward (14) |
*NI, nosocomial infection acquired at National Taiwan University Hospital (NTUH); NC, nosocomial colonization at NTUH; IEOH, MRSA infection detected at NTUH within 48 h after transfer from another hospital. †Bl, blood; Ur, urine; Sp, sputum; Pu, pus; Wo, wound; Ns, nostril; Ct, catheter tip; St, stool. ‡ICU, intensive care unit.
Pulsed-field gel electrophoresis patterns were interpreted according to procedures previously reported (7,8). Thirty-four isolates belonged to pulsotype A, 49 to pulsotype B, 69 to pulsotype C, 6 to pulsotype D, 2 to pulsotype E, 3 to pulsotype F, 11 to pulsotype K, 5 to pulsotype L, 8 to pulsotype M, and 3 (all isolated in 2003) to 3 minor pulsotypes. All isolates were tested by SCCmec element typing and multilocus sequence typing (MLST) (9) and were analyzed for the Panton-Valentine leukocidin (PVL) gene (10) and drug susceptibility to erythromycin, clindamycin, gentamicin, amikacin, ciprofloxacin, levofloxacin, tetracycline, trimethoprim-sulfamethoxazole, rifampin, and vancomycin by using the disk diffusion method (11). SCCmec element typing was determined by previously described PCR methods (3–5).
Results of these analyses are shown in Table 2. MRSA isolates of the same pulsotype have the same MLST pattern and SCCmec types. Isolates with pulsotype A are sequence type 254 (ST254); those with pulsotype B are ST241; those with pulsotypes C, K, and L are ST239; and those with pulsotypes D, E, F, and M are ST59, ST 254, ST30, and ST5, respectively. All MRSA isolates with pulsotypes A, D, E, and F have the type IV SCCmec element. However, only isolates with pulsotypes D and F, as well as 2 isolates from 2003 with 2 minor pulsotypes, have the PVL gene. Isolates with pulsotypes B and C have the type III SCCmec element, and isolates with pulsotype M have the type II SCCmec element.
Table 2. Characteristics of 190 methicillin-resistant Staphylococcus aureus isolates, Taiwan, 1992–2003*.
| P (no. isolates) | SCC mec type | MLST | PVL | Year of isolation (no. isolates) | Drug susceptibility rate, %† |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OX | EM | CL | GM | AM | CP | LV | TC | TS | RF | VA | ||||||
| A (34) | IV | 254 | N | 1992 (11), 1993 (13), 1994 (5), 1995 (1), 1996 (3), 2003 (1) | 0 | 0 | 8.8 | 0 | 0 | 20.1 | 32.3 | 5.9 | 91.2 | 0 | 100 | |
| B (49) | III | 241 | N | 1992 (8), 1993 (11), 1994 (10), 1995 (17), 1996 (3) | 0 | 0 | 40.8 | 0 | 0 | 4.1 | 4.1 | 0 | 2.0 | 18.4 | 100 | |
| C (69) | III | 239 | N | 1994 (1), 1995 (14), 1996 (34), 2003 (20) | 0 | 0 | 5.8 | 2.9 | 0 | 1.4 | 1.4 | 2.9 | 1.4 | 95.7 | 100 | |
| D (6) | IV | 59 | Y | 1994 (1), 1995 (2), 1996 (1), 2003 (2) | 0 | 0 | 0 | 16.7 | 33.3 | 66.7 | 100 | 33.3 | 100 | 100 | 100 | |
| E (2) | IV | 254 | N | 1992 (1), 1995 (1) | 0 | 0 | 50 | 0 | 0 | 50 | 50 | 0 | 100 | 0 | 100 | |
| F (3) | IV | 30 | Y | 1992 (2), 1993 (1) | 0 | 0 | 0 | 33.3 | 66.7 | 100 | 100 | 33.3 | 100 | 100 | 100 | |
| K (11) | III | 239 | N | 2003 (11) | 0 | 0 | 9.1 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 100 | |
| L (5) | III | 239 | N | 2003 (5) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 100 | |
| M (8) | II | 5 | N | 2003 (8) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 100 | 25 | 100 | |
| Other‡ (3) | ||||||||||||||||
| I | 5 | N | 2003 (1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 100 | 0 | 100 | ||
| IV | 59 | Y | 2003 (1) | 0 | 0 | 0 | 0 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | ||
| IV | 59 | Y | 2003 (1) | 0 | 0 | 0 | 0 | 0 | 100 | 100 | 0 | 100 | 100 | 100 | ||
*P, pulsotype; SCC, staphylococcal cassette chromosome; MLST, multilocus sequence typing; PVL, Panton-Valentine leukocidin; N, no; Y, yes. †OX, oxacillin; EM, erythromycin; CL, clinidamycin; GM, gentamicin; AM, amikacin; CP, ciprofloxacin; LV, levofloxacin; TC, tetracycline; TS, trimethoprim-sulfamethoxazole; RF, rifampin; VA, vancomycin. ‡Including 3 pulsotypes each containing only 1 isolate.
Results of MLST and typing of SCCmec elements of the 3 isolates with 3 minor pulsotypes obtained in 2003 are shown in Table 2. The correlation between SCCmec element typing and MLST in this study corresponds to findings of previous reports (6,12–14). Enright et al. identified ST254-IV MRSA isolates in Germany and the United Kingdom (12), and Chongtrakool et al. identified ST239-III and ST241-III MRSA isolates in several Asian countries (14).
Conclusions
We demonstrate that the predominant MRSA clone at NTUH in early 1990s had the type IV SCCmec element. However, the predominant MRSA clones at NTUH from 1994 to 2003 had the type III SCCmec element. These findings differ from those of Wisplinghoff et al., who reported that that the SCCmec element in predominant MRSA clones at their institute changed from type III in 1984 to 1988 to type I in 1989 to 1998 (6). Differences between our findings and those of Wisplinghoff et al. might be caused by differences in location and epidemiologic characteristics.
MRSA isolates of pulsotypes B and C are more resistant than isolates of pulsotype A to certain antimicrobial drugs, especially fluoroquinolones and trimethoprim-sulfamethoxazole; MRSA isolates with pulsotype C are more resistant to clindamycin but less resistant to rifampin than those with pulsotype B (Table 2). From 1993 through 2000, annual use of fluoroquinolones increased ≈3× at NTUH; however, use of trimethoprim-sulfamethoxazole, clindamycin, and rifampin did not change (15). Therefore, the shift of predominant MRSA clones, which also led to the shift in types of SCCmec elements at NTUH, might be caused by selective pressure from antimicrobial drugs, especially fluoroquinolones.
The MRSA clone (pulsotype A) that predominated in 1992 and 1993 at NTUH has the type IV SCCmec element. Although the first study of MRSA with the type IV SCCmec element reported that this element was found in community-acquired MRSA (CA-MRSA) (5), some studies have reported MRSA isolates with this element in a hospital environment (12). However, to our knowledge, these reports did not demonstrate that MRSA isolates with the type IV SCCmec element became predominant among all MRSA isolates in an institution, especially before the mid-1990s.
Furthermore, 4 ST59 MRSA isolates obtained in 1994 and 1996 and 3 ST30 MRSA isolates obtained in 1992 and 1993 have the type IV SCCmec element and PVL gene. Recently, ST59 MRSA isolates were found to cause CA-MRSA infection in Taiwan (13). Among these ST59 CA-MRSA isolates, some had the type IV SCCmec element, and others had the type V SCCmec element (13). Although the type IV SCCmec element could be transferred to CA-MRSA clones with other genetic backgrounds, our finding supports the possibility that ST59 MRSA isolates with the SCCmec element type IV in Taiwan may originate from hospital strains but transfer into CA-MRSA strains.
Chongtrakool et al. recently reported the results of SCCmec typing of 615 MRSA isolates obtained in 1998 and 1999 from 11 Asian countries (14). The ST239-III, ST241-III, ST254-II, and ST5-II MRSA isolates were prevalent in many Asian countries. However, the ST254-IV, ST30-IV, and ST59-IV MRSA isolates from our study were not found in other Asian countries. In addition, ST254-IV MRSA isolates have been found in Germany and the United Kingdom (12). Whether ST254-IV MRSA isolates in our study were transmitted from those in Germany or the United Kingdom by international travel requires further study.
The first MRSA isolate with the type IV SCCmec element in our hospital appeared as early as 1992. The SCCmec element carried by predominant MRSA clones changed from type IV to type III SCCmec element during the period 1992–2003 at NTUH. Because the major MRSA clones isolated in 1994–2003 are more resistant to antimicrobial drugs, especially fluoroquinolones and trimethoprim-sulfamethoxazole, than those obtained in 1992 and 1993, this shift may be caused by selective pressure from indiscriminate use of antimicrobial drugs.
Biography
Dr Wang is an attending staff physician at National Taiwan University Hospital. His research interests include infectious diseases and infection control.
Footnotes
Suggested citation for this article: Wang J-T, Fang C-T, Chen Y-C, Wu C-L, Chen M-L, Chang S-C. Staphylococcal cassette chromosome mec in MRSA, Taiwan. Emerg Infect Dis [serial on the Internet]. 2007 Mar [date cited]. Available from http://www.cdc.gov/eid/content/13/3/494.htm
References
- 1.Ho M, McDonald LC, Lauderdale TL, Yeh LLL, Chen PC, Shiau YR. Surveillance of antibiotic resistance in Taiwan, 1998. J Microbiol Immunol Infect. 1999;32:239–49. [PubMed] [Google Scholar]
- 2.Chen ML, Chang SC, Pan HJ, Hsueh PR, Yang LS, Ho SW, et al. Longitudinal analysis of methicillin-resistant Staphylococcus aureus isolates at a teaching hospital in Taiwan. J Formos Med Assoc. 1999;98:426–32. [PubMed] [Google Scholar]
- 3.Ito T, Katayama Y, Asada K, Mori N, Tsutsumimoto K, Tiensasitorn C, et al. Structural comparison of the three types of staphylococcal cassette chromosome mec integrated in the chromosome in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2001;45:1323–36. 10.1128/AAC.45.5.1323-1336.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ito T, Ma XX, Takeuchi F, Okuma K, Yuzawa H, Hiramatsu K. Novel type V staphylococcal cassette chromosome mec driven by a novel cassette chromosome recombinase, ccrC. Antimicrob Agents Chemother. 2004;48:2637–51. 10.1128/AAC.48.7.2637-2651.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma XX, Ito T, Tiensasitorn C, Jamklang M, Chongtrakool P, Boyle-Vavra S, et al. Novel type of staphylococcal cassette chromosome mec identified in community-acquired methicillin-resistant Staphylococcus aureus strains. Antimicrob Agents Chemother. 2002;46:1147–52. 10.1128/AAC.46.4.1147-1152.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wisplinghoff H, Ewertz B, Wisplinghoff S, Stefanik D, Plum G, Perdreau-Remington F, et al. Molecular evolution of methicillin-resistant Staphylococcus aureus in the metropolitan area of Cologne, Germany, from 1984 to 1998. J Clin Microbiol. 2005;43:5445–51. 10.1128/JCM.43.11.5445-5451.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bannerman TL, Hancock GA, Tenover FC, Miller JM. Pulsed-field gel electrophoresis as a replacement for bacteriophage typing of Staphylococcus aureus. J Clin Microbiol. 1995;33:551–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jorgensen M, Givney R, Pegler M, Vickery A, Funnell G. Typing multidrug-resistant Staphylococcus aureus: conflicting epidemiological data produced by genotypic and phenotypic methods clarified by phylogenetic analysis. J Clin Microbiol. 1996;34:398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol. 2000;38:1008–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lina G, Piemont Y, Godail-Gamot F, Bes M, Peter MO, Gauduchon V, et al. Involvement of Panton-Valentine leukocidin–producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis. 1999;29:1128–32. 10.1086/313461 [DOI] [PubMed] [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards (NCCLS). Performance standards for antimicrobial disk susceptibility tests, 6th ed. Approved standard M2–A6. Wayne (PA): The Committee; 1998. [Google Scholar]
- 12.Enright MC, Robinson DA, Randle G, Feil EJ, Grundmann H, Spratt BG. The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA). Proc Natl Acad Sci U S A. 2002;99:7687–92. 10.1073/pnas.122108599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen FJ, Lauderdale TL, Huang IW, Lo HJ, Lai JF, Wang HY, et al. Methicillin-resistant Staphylococcus aureus in Taiwan. Emerg Infect Dis. 2005;11:1760–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chongtrakool P, Ito T, Ma XX, Kondo Y, Trakulsomboon S, Tiensasitorn C, et al. Staphylococcal cassette chromosome mec (SCCmec) typing of methicillin-resistant Staphylococcus aureus strains isolated in 11 Asian countries: a proposal for a new nomenclature for SCCmec elements. Antimicrob Agents Chemother. 2006;50:1001–12. 10.1128/AAC.50.3.1001-1012.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The antibiotics annual report, 2004. Taipei: National Taiwan University Hospital; 2005. [Google Scholar]