Abstract
Background:
Despite the extensive frontal atrophy and behavioral disinhibition that characterizes behavioral variant frontotemporal dementia (bvFTD), many studies of early bvFTD suggest normal executive functioning (EF). The current study examined cognitive control in patients with bvFTD who otherwise seemed cognitively normal.
Methods:
Subjects included 7 patients with bvFTD with normal neuropsychological test scores, 7 patients with bvFTD matched for Mini-Mental State Examination score but with impaired neuropsychological test scores, and 14 normal controls. A flanker paradigm and other measures of EF were administered to participants. A semiautomated parcellation program was used to analyze structural MRI scans.
Results:
On the flanker task, multivariate analysis of variance revealed a significant condition X diagnosis interaction. Both bvFTD groups showed a larger congruency effect than normal controls, i.e., they displayed disproportionately reduced speed and accuracy on incongruent trials relative to congruent trials. Imaging data illustrated significant orbitofrontal atrophy in patients with early bvFTD as compared with controls.
Conclusions:
Patients with behavioral variant frontotemporal dementia (bvFTD) who performed within normal limits on clinical tests of executive functioning demonstrated a select impairment on an experimental test of cognitive control, suggesting a subtle impairment in inhibiting attention or response to the irrelevant stimuli. Measures of neuropsychological functioning sensitive to the ventromedial prefrontal cortex may be useful in early diagnosis of patients with bvFTD. Our understanding of this syndrome may be increased by considering the efficiency of selective inhibition, a fundamental component of executive cognitive control.
GLOSSARY
- ANOVA
= analysis of variance;
- bvFTD
= behavioral variant frontotemporal dementia;
- CDR
= Clinical Dementia Rating;
- EF
= executive functioning;
- FTLD
= frontotemporal lobar dementia;
- MANCOVA
= multivariate analysis of covariance;
- MCI
= mild cognitive impairment;
- MMSE
= Mini-Mental State Examination;
- MP-RAGE
= magnetization-prepared rapid gradient echo;
- MR
= magnetic resonance.
Frontotemporal lobar dementia (FTLD) is a devastating neurodegenerative disease found to be as common as Alzheimer disease in patients younger than 65 years.1,2 Behavioral variant frontotemporal dementia (bvFTD) is the most common variant of FTLD and is characterized by profound disturbances in personality, social function, judgment, and insight. Imaging studies have shown early and disproportionate involvement of orbitofrontal cortex3 and associated networks involving the striatum, ventromedial prefrontal cortex, anterior cingulate cortex, and insula.4–6
Given the widespread frontal atrophy and behavioral disinhibition that characterizes bvFTD, impairments in executive functioning have been surprisingly difficult to measure.7 In fact, many patients with early bvFTD perform within the normal range on traditional “frontal-executive” tests measuring working memory, planning, mental flexibility, response inhibition, and concept formation.8 One potential explanation for this lack of sensitivity of traditional tasks of executive control is that most clinical measures of executive functioning either are not specific to the frontal lobes9 or are insensitive to the medial frontal involvement most affected in early bvFTD.10 Recent studies from cognitive neuroscience suggest that conflict monitoring tasks may have greater specificity for these regions.11–18
Conflict monitoring is engaged during decision making when it is necessary to override prepotent responses or choose from a set of equally permissible responses.11,12 The flanker test is a widely used conflict monitoring task that requires subjects to indicate the direction of a central arrow flanked by arrows pointing in the same or opposite direction.
The current study evaluated cognitive control in patients with bvFTD who otherwise seemed cognitively normal. We hypothesized that patients with bvFTD would show disproportionate impairment on the incongruent trials of the flanker task as compared with normal controls.
METHODS
Subjects.
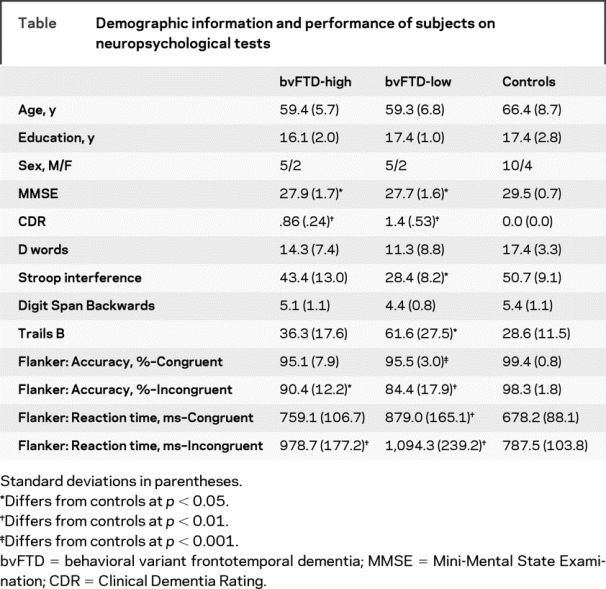
All subjects were evaluated at the University of California, San Francisco Memory and Aging Center by a team of experienced clinicians, including a behavioral neurologist, a neuropsychologist, and a nurse. Every patient was evaluated for capacity to consent, and then, once capacity was assured, each patient signed a consent form to participate in this research study. Institutional review board approval was received to conduct this study. A research diagnosis of bvFTD was made using Neary criteria.19 From a larger cohort of patients with bvFTD (n = 18), we established a smaller cohort of high-performing subjects with bvFTD by identifying all those who scored within normal limits on a set shifting task (modified Trails).20 This search yielded 7 patients with bvFTD, who we refer to as the high-scoring bvFTD group (bvFTD-high). Each patient in this group was matched with 1 patient with bvFTD who was impaired on the set shifting task (bvFTD-low) and 2 controls on the basis of age and sex (see table for demographic information). The mean Mini-Mental State Examination (MMSE) score of the bvFTD-high group was 27.9 (SD = 1.7); 2 subjects had Clinical Dementia Rating (CDR) scores of 0.5, and 5 subjects had CDR scores of 1.0. The mean MMSE of the bvFTD-low group was 27.7 (SD = 1.6); 4 subjects had CDR scores of 1.0, and 3 subjects had CDR scores of 2.0. Age, education, and sex did not significantly differ among the 3 diagnostic groups. In addition to set shifting (modified Trails B: completion time), subjects were also administered other measures of executive functioning, including verbal fluency (d-word generation: number correctly generated in 1 minute), response inhibition (Stroop Interference Test: total number correct), and working memory (Digit Span Backwards).
Table Demographic information and performance of subjects on neuropsychological tests
Participants were given a computer-administered flanker task and were asked to indicate the direction of a centrally presented arrow as quickly and accurately as possible. The central arrow was flanked on both sides by 2 arrows pointing in either the same direction (congruent condition) or opposite directions (incongruent condition). Each trial began with a central fixation cross presented from 400 to 1,600 ms. The fixation period was followed by a 100-ms warning cue and a 400-ms fixation period, and then the target stimulus (target arrow and flankers) appeared 1.06° above or below the fixation point (figure 1). Subjects were given up to 3 practice set trials of 16 (proceeding immediately to the test if they responded correctly to 12 of the 16 trials and proceeding to the next practice trial if accuracy was below 12 of 16). After passing the practice trials, participants were administered 128 experimental trials (64 with a 200-ms cue-to-stimulus interval and then 64 with a 600-ms cue-to-stimulus interval). The stimuli were presented until the subject pressed a key (with a maximum exposure of 4 seconds). The interstimulus interval was a random amount of time and varied between 1 and 3 seconds. Accuracy (percent correct) and reaction time were measured for each trial.
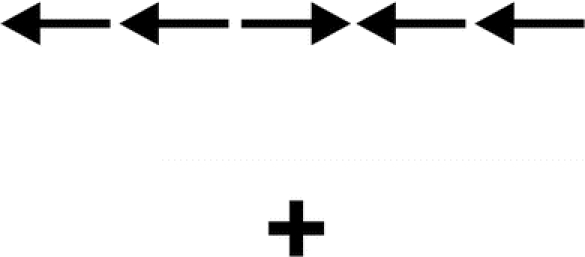
Figure 1 Incongruent trial on flanker task
Illustration of an incongruent trial above the fixation point on the flanker task.
MR image acquisition.
MRI scans were acquired on a 1.5-T Magnetom VISION system (Siemens Inc., Iselin, NJ) using a standard quadrature head coil. Volumetric magnetization-prepared rapid gradient echo (MP-RAGE) MRI (repetition time/echo time/inversion time = 10/4/300 ms) to obtain T1-weighted structural images of the entire brain. The T1 images were in a coronal orientation, with a 15° flip angle, 1.0 × 1.0-mm2 in-plane resolution, and 1.5-mm slab thickness.
Image analysis.
The T1 MP-RAGE structural magnetic resonance (MR) images were analyzed using Freesurfer, which is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu/). Previous publications have detailed and validated the software.21–24 Freesurfer is a surface-based structural MRI analysis tool that segments white matter and tessellates both grey and white matter surfaces. The procedure, in brief, involves the removal of nonbrain tissue using a hybrid watershed/surface deformation procedure21 and intensity normalization,25 followed by automated Talairach transformation and volumetric segmentation of cortical and subcortical gray and white matter, subcortical limbic structures, basal ganglia and ventricles, used to calculate total intracranial volume.26 The surfacing algorithm uses intensity and continuity data, and corrects topological defects to generate a continuous cortical ribbon used to calculate gray matter volume and thickness,21,24 a procedure validated against histologic analysis27 and manual measurements.28 This cortical surface is then inflated and registered to a spherical atlas and parcellated into regions of interest based on gyral and sulcal structure.23,29,30 The current study examined the orbitofrontal cortex and middle frontal gyrus cortical regions of interest delineated by Desikan et al.30
Our study used the LONI Pipeline environment31 (http://pipeline.loni.ucla.edu), which was developed by LONI and used to distribute Freesurfer processing tasks to an off-site central processing unit cluster located at the UCLA- LONI.
Statistical analysis.
Independent samples t tests were conducted to examine group differences between bvFTD-high patients and controls on measures of executive functioning (lexical fluency, response inhibition, working memory, set shifting). An analysis of variance (ANOVA) was used to examine differences between bvFTD-high patients, bvFTD-low patients, and controls on traditional measures of executive functioning. Flanker accuracy and median reaction time data were analyzed with a multivariate analysis of covariance (MANCOVA) with diagnosis as the between-subjects factor and condition (congruent vs incongruent) as the within-subject factor. Although there were no group differences in age, the subjects with bvFTD were slightly younger, so age and MMSE were included as covariates. These analyses were first completed in bvFTD-high patients and controls to explore whether group differences existed early in the disease. Analyses were then conducted between bvFTD-low patients and controls to examine group differences in more functionally impaired patients. bvFTD-high and bvFTD-low patients were compared as well. Orbitofrontal cortex and middle frontal gyrus volumes were analyzed with a MANCOVA with diagnosis as the between-subjects factor and brain region as the within-subject factor. Total intercranial volume was included as a covariate.
RESULTS
Neuropsychological functioning.
Performance on clinical neuropsychological tasks was first compared using independent sample t tests to determine whether there was a significant difference in performance between bvFTD-high patients and normal controls (table). There were no significant differences between the 2 groups’ performance on any of the measures (verbal fluency, response inhibition, working memory, and set shifting). ANOVA showed that bvFTD-low patients differed from bvFTD-high patients and controls on set shifting and response inhibition tasks and performed comparably to the other cohorts on verbal fluency and working memory tasks.
The MANCOVA for accuracy on the flanker in bvFTD-high patients and controls yielded an insignificant main effect for diagnosis and condition. The diagnosis by condition interaction was also not significant. The MANCOVA for accuracy on the flanker in bvFTD-low patients and controls yielded an insignificant main effect for condition and a significant main effect for diagnosis [F(1,15) = 9.5]. The diagnosis by condition interaction was significant [F(1,15) = 6.8], with bvFTD-low patients showing a larger congruency effect than normal controls (figure 2). Post hoc t tests revealed that bvFTD-low patients performed less accurately than normal controls on congruent (t = 4.6, p < 0.001) and incongruent (t = 3.0, p < 0.01) flanker trials.
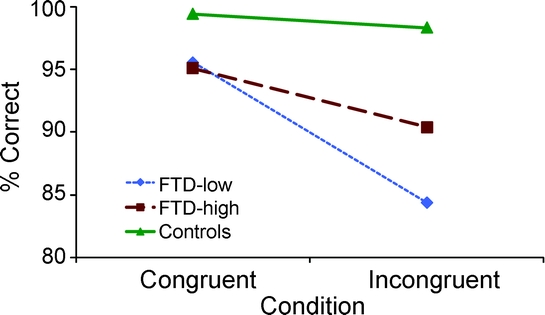
Figure 2 Condition by diagnosis interaction effect for accuracy
A condition by diagnosis interaction effect was found, demonstrating that patients with behavioral variant frontotemporal dementia (FTD) showed a larger congruency effect on accuracy than normal controls.
No subject had a total accuracy rate less than 74%, so all subjects were included in the reaction time analyses. Main effects for diagnosis (bvFTD-high and controls) and condition were not significant. However, there was a significant diagnosis-by-condition interaction on reaction time [F(1,17) = 5.6], with bvFTD-high patients showing a larger congruency effect than normal controls (figure 3). Post hoc t tests revealed no significant difference in reaction time between bvFTD-high patients and normal controls on congruent trials, but bvFTD-high patients had slower reaction times than controls during incongruent flanker trials (t = −3.1, p < 0.01). Normal controls showed a 16% increase in reaction time on incongruent trials, whereas bvFTD-high patients showed a 30% increase, almost twice that of controls. The MANCOVA for reaction time on the flanker in bvFTD-low patients and controls yielded an insignificant main effect for condition, and a significant main effect for diagnosis [F(1,15) = 12.0]. The diagnosis by condition interaction was also significant [F(1,15) = 8.1], with bvFTD-low patients showing a larger congruency effect than normal controls (figure 3). Post hoc t tests revealed that bvFTD-low patients had slower reaction times than normal controls on congruent (t = −3.7, p < 0.01) and incongruent (t = −4.2, p < 0.01) flanker trials. bvFTD-low patients showed a 25% increase in reaction time on incongruent trials as compared with congruent trials.
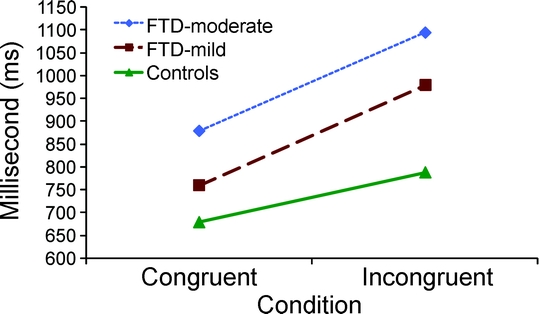
Figure 3 Condition by diagnosis interaction effect for reaction time
A condition by diagnosis interaction effect was found, demonstrating that patients with behavioral variant frontotemporal dementia (FTD) showed a larger congruency effect on reaction time than normal controls.
Of note, there were no significant differences between bvFTD-high and bvFTD-low patients on any flanker accuracy or reaction time measures. Even though bvFTD-high patients performed within normal limits on traditional neuropsychological measures, these patients displayed the same congruency effect as neuropsychologically impaired bvFTD-low patients when compared with normal controls.
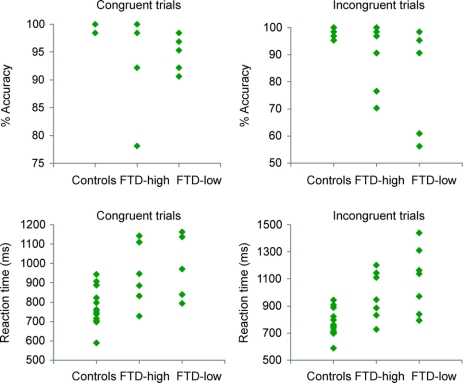
Scatter plots depicting the individual scores of bvFTD-high patients, bvFTD-low patients, and normal controls on the flanker tasks revealed a large degree of overlap between the data, but the bvFTD groups displayed more variability as compared with controls (figure 4). This suggests that the flanker test may have fairly high specificity but very low sensitivity for diagnosing bvFTD. Therefore, the flanker test would not be useful in diagnosing individual patients.
Figure 4 Scatter plots of individual scores on flanker tasks
Scatter plots displaying individual accuracy and reaction time scores on congruent and incongruent flanker trials. There is a large degree of overlap among the 3 groups, but the behavioral variant frontotemporal dementia (FTD) groups displayed greater variability in performance as compared with controls.
Imaging.
The MANCOVA for region of interest brain volumes in bvFTD-high patients and controls yielded an insignificant main effect for brain region and a significant main effect for diagnosis [F(1,13) = 5.5]. The diagnosis by brain region interaction was not significant. Post hoc t tests revealed a trend for differences in middle frontal gyrus volumes and a significant difference in orbitofrontal cortex volumes (t = 2.8). bvFTD-high patients had significantly smaller orbitofrontal cortex volumes (20.01 cm3) as compared with controls (23.75 cm3). bvFTD-high patients showed somewhat smaller middle frontal gyrus volumes (37.13 cm3) as compared with controls (42.23 cm3).
DISCUSSION
The main finding of this study is that patients with very mild bvFTD who performed within normal limits on clinical tests of executive functioning demonstrated selective impairment in conflict monitoring. On a flanker task, we found that while bvFTD-high and controls responded with comparable accuracy and speed on congruent trials, reaction time data showed an interaction between diagnosis and condition, whereby bvFTD-high patients showed a larger congruency effect (i.e., disproportionate slowing on incongruent trials) than normal controls. These results demonstrate that bvFTD is associated with deficits in executive cognitive control, even very early in the disease when standard clinical neuropsychological test scores are within normal limits. bvFTD-low patients also displayed a larger congruency effect than normal controls, suggesting that selective impairment in conflict monitoring continues as functional impairments progress. Although the flanker test yielded group-wise differences, it does not seem that this test can be used for diagnosis in individual patients.
Research has proposed that the medial frontal lobes detect and signal the occurrence of conflicts in information processing.32 A study examining the flanker interference effect as a function of the entire reaction time distribution in patients with mild cognitive impairment (MCI) as compared with normal controls found evidence that inefficient inhibition, rather than greater activation of the response induced by incongruent flankers, accounted for the enhanced interference effect in MCI patients.33 Accordingly, the greater interference effect found in patients with bvFTD as compared with normal controls is likely a function of inefficient response inhibition. Patients with early bvFTD may have subtle impairments in inhibiting their attention or response to irrelevant stimuli.
Functional imaging studies have demonstrated the role of the medial frontal circuits in response inhibition during the flanker task.11–13,17 Patients with diseases associated with impairment in these brain regions (e.g., Parkinson disease, MCI, attention-deficit hyperactivity disorder) show larger flanker interference effects as compared with healthy controls.33–37 The strong activation of medial frontal lobes, and specifically the rostral anterior cingulate, during response conflict trials of the flanker task in neurologic healthy controls suggests that the observed findings of impairment in conflict monitoring in patients with bvFTD as compared with normal controls in the current study are a result of dysfunction in this brain area and related anterior cingulate circuit. Research has illustrated the intrinsic corticocortical connections within the orbital and medial prefrontal cortex.38 Imaging results in the current study revealed significant orbitofrontal cortex atrophy in bvFTD-high patients as compared with controls. More lateral areas of the frontal lobes (middle frontal gyrus) were not as profoundly affected. The orbitofrontal cortex and associated network is likely the neuroanatomical substrate for the observed suppression difficulties evidenced in patients with bvFTD.39
Patients with bvFTD and normal controls differed on an experimental cognitive measure of executive control but did not deviate on widely used clinical measures of executive functioning. These results highlight the issue of task sensitivity in detecting executive cognitive control deficits in patients with bvFTD. Reaction time tasks may be more sensitive to these deficits than standard clinical measures. Current models of prefrontal function posit a dissociation between ventromedial or orbitofrontal areas that mediate behavioral control, and dorsolateral structures that are more heavily involved in cognition. Clinical neuropsychological tests of executive functioning sensitive to lateral prefrontal function may fail to test the distinct and separate functions of the areas of the prefrontal cortex that are predominantly affected in bvFTD.
One limitation to this study is the relatively small sample size; however, the sample size was comparable to other studies of this type, and statistically significant differences were found indicating fairly large effect sizes. In addition, although not a significant difference, controls were slightly older than subjects with bvFTD. Nevertheless, one might expect that the increased age in the controls would be associated with greater likelihood of neuropsychological deficits, if it had any effect at all.
Despite being a fairly common presenile neurodegenerative disease, bvFTD continues to be underdiagnosed and misdiagnosed.40 Early and accurate diagnosis remains essential to treat symptoms correctly and to help patients and families plan for the future. Identifying patients in the earliest stages of the disease not only allows for effective patient management, but also may be beneficial in the assessment of efficacious therapeutic interventions. Measures of neuropsychological functioning sensitive to the ventromedial prefrontal cortex may be useful in early diagnosis of patients with bvFTD. Future research examining the efficiency of selective inhibition, a fundamental component of executive cognitive control, may increase our understanding of this syndrome.
AUTHOR CONTRIBUTIONS
Dr. Krueger conducted the statistical analysis.
DISCLOSURE
Dr. Krueger reports no disclosures. Ms. Bird receives royalties from publishing Personality Disorders in Psychology: Contemporary Theory, Research, and Issues, Lawrence Erlbaum, 2004. Mr. Growdon reports no disclosures. Ms. Jang reports no disclosures. Dr. Miller serves on the speakers’ bureaus of Pfizer and Novartis Pharmaceuticals and is funded by NIH grants 01-154-20 and P50-AG05142. Dr. Kramer is funded by NIH grants AG22983 and HHSN-271200623661C. This study utilized the LONI Pipeline environment, which was partially funded by NIH grants P41 RR013642, R01 MH71940, and U54 RR021813.
Received January 13, 2009. Accepted in final form April 21, 2009.
Address correspondence and reprint requests to Dr. Casey E. Krueger, 350 Parnassus Ave., Suite 905, San Francisco, CA 94143-1207 ckrueger@memory.ucsf.edu
Disclosure: Author disclosures are provided at the end of the article.
REFERENCES
- 1.Knopman DS, Petersen RC, Edland SD, et al. The incidence of frontotemporal lobar degeneration in Rochester, Minnesota, 1990 through 1994. Neurology 2004;62:506–508. [DOI] [PubMed] [Google Scholar]
- 2.Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology 2002;58:1615–1621. [DOI] [PubMed] [Google Scholar]
- 3.Perry RJ, Graham A, Williams G, et al. Patterns of frontal lobe atrophy in frontotemporal dementia: a volumetric MRI study. Dement Geriatr Cogn Disord 2006;22:278–287. [DOI] [PubMed] [Google Scholar]
- 4.Liu W, Miller BL, Kramer JH, et al. Behavioral disorders in the frontal and temporal variants of frontotemporal dementia. Neurology 2004;62:742–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mummery C, Patterson K, Price C, et al. A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Ann Neurol 2000;47:36–45. [PubMed] [Google Scholar]
- 6.Rosen HJ, Gorno-Tempini ML, Goldman WP, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology 2002;58:198–208. [DOI] [PubMed] [Google Scholar]
- 7.Nedjam Z, Devouche E, Dalla Barba G. Confabulation, but not executive dysfunction discriminate AD from frontotemporal dementia. Eur J Neurol 2004;11:728–733. [DOI] [PubMed] [Google Scholar]
- 8.Gregory CA, Hodges JR. Clinical features of frontal lobe dementia in comparison to Alzheimer’s disease. J Neural Transm 1996;47:103–123. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez JA, Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev 2006;16:17–42. [DOI] [PubMed] [Google Scholar]
- 10.Rahman S, Sahakian BJ, Hodges JR, et al. Specific cognitive deficits in mild frontal variant frontotemporal dementia. Brain 1999;122(pt 8):1469–1493. [DOI] [PubMed] [Google Scholar]
- 11.Botvinick MM. Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cogn Affect Behav Neurosci 2007;7:356–366. [DOI] [PubMed] [Google Scholar]
- 12.Botvinick MM, Braver TS, Barch DM, et al. Conflict monitoring and cognitive control. Psychol Rev 2001;108:624–652. [DOI] [PubMed] [Google Scholar]
- 13.Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci 2004;8:539–546. [DOI] [PubMed] [Google Scholar]
- 14.Carter CS, MacDonald AW III, Ross LL, Stenger VA. Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. Am J Psychiatry 2001;158:1423–1428. [DOI] [PubMed] [Google Scholar]
- 15.Fan J, Hof PR, Guise KG, et al. The functional integration of the anterior cingulate cortex during conflict processing. Cereb Cortex 2008;18:796–805. [DOI] [PubMed] [Google Scholar]
- 16.Pochon JB, Riis J, Sanfey AG, et al. Functional imaging of decision conflict. J Neurosci 2008;28:3468–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn 2004;56:129–140. [DOI] [PubMed] [Google Scholar]
- 18.Luks TL, Simpson GV, Dale CL, Hough MG. Preparatory allocation of attention and adjustments in conflict processing. Neuroimage 2007;35:949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998;51:1546–1554. [DOI] [PubMed] [Google Scholar]
- 20.Kramer JH, Jurik J, Sha SJ, et al. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol 2003;16:211–218. [DOI] [PubMed] [Google Scholar]
- 21.Segonne F, Dale AM, Busa E, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage 2004;22:1060–1075. [DOI] [PubMed] [Google Scholar]
- 22.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis I: segmentation and surface reconstruction. Neuroimage 1999;9:179–194. [DOI] [PubMed] [Google Scholar]
- 23.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis II: Inflation, flattening, and a surface-based coordinate system. Neuroimage 1999;9:195–207. [DOI] [PubMed] [Google Scholar]
- 24.Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging 2001;20:70–80. [DOI] [PubMed] [Google Scholar]
- 25.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 1998;17:87–97. [DOI] [PubMed] [Google Scholar]
- 26.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002;33:341–355. [DOI] [PubMed] [Google Scholar]
- 27.Rosas HD, Liu AK, Hersch S, et al. Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology 2002;58:695–701. [DOI] [PubMed] [Google Scholar]
- 28.Salat DH, Buckner RL, Snyder AZ, et al. Thinning of the cerebral cortex in aging. Cereb Cortex 2004;14:721–730. [DOI] [PubMed] [Google Scholar]
- 29.Fischl B, van der Kouwe A, Destrieux C, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex 2004;14:11–22. [DOI] [PubMed] [Google Scholar]
- 30.Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006;31:968–980. [DOI] [PubMed] [Google Scholar]
- 31.Rex DE, Ma JQ, Toga AW. The LONI Pipeline Processing Environment. Neuroimage 2003;19:1033–1048. [DOI] [PubMed] [Google Scholar]
- 32.Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature 1999;402:179–181. [DOI] [PubMed] [Google Scholar]
- 33.Wylie SA, Ridderinkhof KR, Eckerle MK, Manning CA. Inefficient response inhibition in individuals with mild cognitive impairment. Neuropsychologia 2007;45:1408–1419. [DOI] [PubMed] [Google Scholar]
- 34.Praamstra P, Plat EM, Meyer AS, Horstink MW. Motor cortex activation in Parkinson’s disease: dissociation of electrocortical and peripheral measures of response generation. Mov Disord 1999;14:790–799. [DOI] [PubMed] [Google Scholar]
- 35.Praamstra P, Stegeman DF, Cools AR, Horstink MW. Reliance on external cues for movement initiation in Parkinson’s disease: evidence from movement-related potentials. Brain 1998;121(pt 1):167–177. [DOI] [PubMed] [Google Scholar]
- 36.Wylie SA, Stout JC, Bashore TR. Activation of conflicting responses in Parkinson’s disease: evidence for degrading and facilitating effects on response time. Neuropsychologia 2005;43:1033–1043. [DOI] [PubMed] [Google Scholar]
- 37.Ridderinkhof KR, Scheres A, Oosterlaan J, Sergeant JA. Delta plots in the study of individual differences: new tools reveal response inhibition deficits in AD/Hd that are eliminated by methylphenidate treatment. J Abnorm Psychol 2005;114:197–215. [DOI] [PubMed] [Google Scholar]
- 38.Carmichael ST, Price JL. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol 1996;371:179–207. [DOI] [PubMed] [Google Scholar]
- 39.Viskontas IV, Possin KL, Miller BL. Symptoms of frontotemporal dementia provide insights into orbitofrontal cortex function and social behavior. Ann NY Acad Sci 2007;1121:528–545. [DOI] [PubMed] [Google Scholar]
- 40.Wittenberg D, Possin KL, Rascovsky K, Rankin KP, Miller BL, Kramer JH. The early neuropsychological and behavioral characteristics of frontotemporal dementia. Neuropsychol Rev 2008;18:91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]