Abstract
Emerging data suggest that a wide array of measurable biomarkers in blood may provide a novel window into the pathophysiology of stroke. In this review, we survey the state of progress in the field. Three specific questions are assessed. Can biomarkers augment the clinical examination and powerful brain imaging tools to enhance the accuracy of the diagnostic process? Can biomarkers be used to help triage patients for thrombolytic therapy? Can biomarkers help predict patients who are most susceptible to malignant infarction? Many encouraging molecular candidates have been found that appear to match the known cascades of neurovascular injury after stroke. However, whether these putative biomarkers may indeed have direct clinical utility remains to be quantitatively validated. Larger clinical trials are warranted to establish the sensitivity and specificity of biomarkers for routine use in clinical stroke.
GLOSSARY
- BBB
= blood–brain barrier;
- BNP
= brain natriuretic peptide;
- CRP
= C-reactive protein;
- GFAP
= glial fibrillary acidic protein;
- HT
= hemorrhagic transformation;
- ICH
= intracerebral hemorrhage;
- IS
= ischemic stroke;
- MMP-9
= matrix metalloproteinase-9;
- NDKA
= nucleoside diphosphate kinase A;
- NMDA-R
= N-methyl-d-aspartate receptor;
- tPA
= tissue plasminogen activator;
- VLP-1
= visinin-like protein-1;
- vWF
= von Willebrand factor.
It is challenging to establish the diagnosis and to make therapeutic decisions in patients presenting with symptoms suspicious for acute stroke. Serum biomarker testing has the potential to serve as a valuable adjunct to routine clinical examination and imaging data. For decades, researchers have undertaken great efforts in investigating the relationship between serum markers and clinical findings in acute stroke. The aim of this mini-review is not to provide a comprehensive meta-analysis of this large field, but to highlight selected studies that appear to be of particular interest for the clinical neurologist. We focused on 3 clinical situations in which biomarker testing might be especially helpful: to distinguish ischemic stroke (IS) from intracerebral hemorrhage (ICH) and stroke mimics, to predict hemorrhagic complications after thrombolysis, and to identify patients at risk for developing massive cerebral edema.
EARLY DIAGNOSIS OF STROKE
Reviewing the past medical history and performing a physical examination are essential steps in the management of a patient with symptoms suspicious for acute stroke.1 Emergent imaging is required to distinguish between IS and ICH. For most cases and at most institutions, CT remains the most practical imaging modality.1 Unfortunately, less than a third of patients with IS show characteristic changes on CT scan within 3 hours of symptom onset.2 Thus, the combination of a neurologic deficit of sudden onset conforming to a vascular territory and a negative CT scan (thereby ruling out ICH) is routinely considered to be indicative of IS. Confirmatory imaging is not typically performed for the administration of thrombolysis. However, a number of other diseases may mimic acute cerebral ischemia, including migraine, epileptic seizures, and mass lesions. In prospective series, stroke mimics accounted for up to a third of patients presenting with stroke-like symptoms.3 MRI offers advantages for the assessment of acute stroke.4 However, within the first 3 hours of symptom onset, the sensitivity of MRI to detect a later clinically confirmed ischemic stroke is no higher than 73%.2 Therefore, a biomarker capable of identifying patients with IS might be a helpful diagnostic tool, particularly in the critical early time period. The ultimate goal would be using biomarkers in the prehospital setting to reliably identify and distinguish between the 2 major subtypes of stroke (i.e., IS, ICH). This would allow a cause-specific management and potentially facilitate the hyperacute delivery of stroke therapies.
To translate these ideas into practice, the diagnostic criteria required of a biomarker would depend on the specific clinical scenarios involved. For example, a test designed to differentiate IS from stroke mimics in the prehospital setting or the outpatient unit need to have a very high sensitivity in order to guarantee hospital admission and diagnostic evaluation for all true IS patients. But a somewhat lower specificity may be tolerable, as this would lead at worst to a false positive admission of a stroke mimic. Once the diagnosis of IS is established, deciding whether thrombolysis can be applied would require a second biomarker to identify any ICH with 100% sensitivity, which might be unrealistic for any blood assay. On the other hand, a biomarker test indicating ICH may still facilitate triage and the prehospital management of ICH patients. In this scenario, the sensitivity for diagnosing ICH needs to be high, but not necessarily 100%. Ultimately, the speed of an assay may be most critical within the compressed timeframes for decision making during acute stroke.
MARKERS FOR ISCHEMIC STROKE
A critical reduction in oxygen and glucose levels triggers the ischemic cascade, a series of reactions leading to excitotoxicity, oxidative stress, inflammation, and cell death. Some of these molecular and cellular alterations may be used for diagnosing IS, particularly when accompanied by biochemical changes in the peripheral blood (table).5,6 Dambinova et al.7 focused on the N-methyl-d-aspartate receptor (NMDA-R) as one of the key players mediating excitotoxicity, and speculated whether NMDA-R autoantibodies may be detectable in the bloodstream. Patients with IS of mostly atherothrombotic origin were found to have higher NMDA-R autoantibody concentrations than healthy controls. Test sensitivity and specificity for diagnosing IS within 3 hours of symptom onset was 97% and 98%. However, elevated NMDA-R autoantibody concentrations were found in individuals without stroke with hypertension and atherosclerosis and in patients with old ischemic lesions. Thus, certain risk factor constellations and previous ischemic episodes (either clinically apparent or not) may also contribute to autoantibody production and may therefore reduce the specificity of this measure. Furthermore, one may assume that the production of autoantibodies takes time, which may limit the clinical applications of this test, at least within the very early phase of IS. Ongoing research has now focused on directly measuring the NMDA-R fragments (and not the autoantibodies).
Table Overview of diagnostic accuracy measures for biomarkers and biomarker panels in potential fields of application
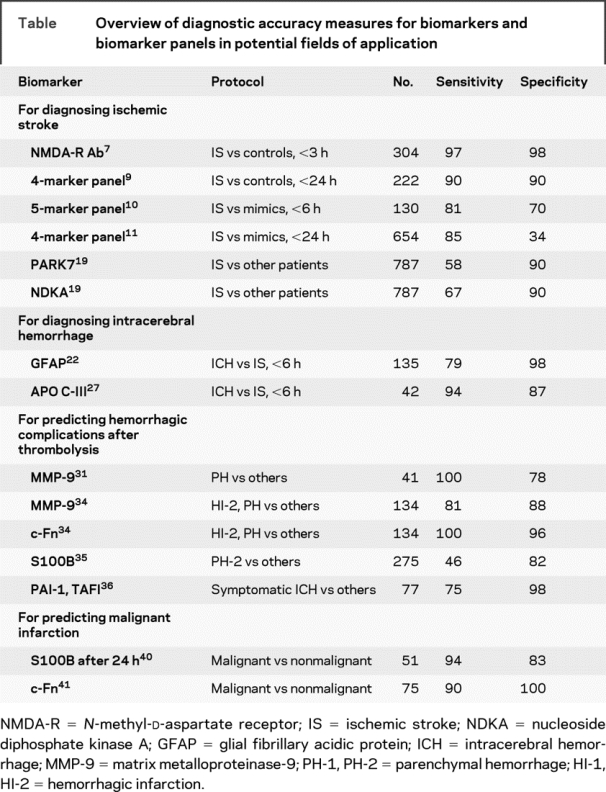
To better account for the molecular complexity of the ischemic cascade, the field has recently moved from the search for a single biomarker to evaluating multiple proteins simultaneously (biomarker panel). Reynolds et al.8 screened plasma samples from 223 stroke patients (including IS, ICH, and subarachnoid hemorrhage) and from 214 healthy individuals for more than 50 serum biomarkers. Univariate analysis revealed astroglial protein S100B, B-type neurotrophic growth factor, von Willebrand factor (vWF), matrix metalloproteinase-9 (MMP-9), and monocyte chemotactic protein-1 to be associated with stroke. The combined model had a test sensitivity of 91% and a specificity of 97% for the diagnosis of stroke within 12 hours after symptom onset. Shortly thereafter, the same group published data on 65 patients with suspected IS admitted within 24 hours of symptom onset and 157 healthy controls.9 Twenty-six bloodborne markers with relation to the ischemic cascade were analyzed. Protein S100B, MMP-9, vascular cell adhesion molecule, and vWF were identified to be associated with IS. The combined model revealed both a 90% sensitivity and specificity for predicting IS. Subsequently, Laskowitz et al.10 obtained data on 130 patients admitted with acute focal neurologic deficits within 6 hours of symptom onset. Forty-one patients were later on diagnosed with IS. The predictive model included brain natriuretic peptide (BNP), C-reactive protein (CRP), d-dimer, MMP-9, and S100B. A sensitivity of 81% and a specificity of 70% for diagnosing IS were reported.
Based on these encouraging results, the diagnostic accuracy of a biomarker panel including d-dimer, BNP, MMP-9, and S100B was evaluated in a prospective multicenter trial.11 Within a 3-year period, more than 1,100 patients presenting with symptoms suspicious for stroke were enrolled within 24 hours of symptom onset. The multivariate model was capable of only moderately differentiating between stroke patients (IS, ICH) and mimics. Setting the threshold of the model to the 25th percentile revealed a sensitivity of 86% and a specificity of 37% for discriminating stroke patients from mimics. The authors concluded that the diagnostic accuracy of this biomarker panel is clearly imperfect. Nevertheless, they claim that a point-of-care algorithm may be feasible to aid in the early management of patients with symptoms suspicious for stroke. Another study speculated on a reduced specificity of that panel, since changes in the selected biomarkers in marathon runners reflect rather a systemic inflammatory response to rhabdomyolysis than CNS damage.12
The lesson learned above perhaps points to the difficulty of taking a snapshot of a biomarker profile at one moment in time to predict events that would involve multiple genetic and environmental interactions over a lifetime, including genetic susceptibility to ischemic injury, comorbid conditions, and medications. Given the complexity of biologic events preceding and following IS, more accurate prediction of clinical variables likely depends on a more robust moment-to-moment physiologic profile, perhaps using the “omics” approach for both screening and discovery of newer biomarkers in the physiologically relevant pathway.
Initial findings have been reported for genomic analyses of circulating white blood cells, where distinct signatures of altered gene expression seem to reflect an activated inflammatory response after stroke in humans.13–15 A more recent study now suggests that some of these genomic profiles of peripheral blood cells might even begin to distinguish stroke subtypes, with intriguing differences between cardioembolic vs atherosclerotic strokes.16 In experimental stroke models in rodents, similar alterations in gene expression of circulating blood cells may involve large-scale adaptations in the immune system, with feedback loops between brain and blood.17
Whereas genomic signatures point toward how a biologic system reacts to stress, the actual pathophysiology of the acute stroke response surely requires a proteome analysis as well. This would provide insights into the spectrum of proteins present in a cell or an organism after extensive transcriptional and post-translational processing. In this regard, animal models are useful because they provide access to tissue. For example, using gene array analyses on a mouse model to screen for biomarkers that are both preferentially and abundantly produced in the brain, Laterza et al.18 identified visinin-like protein 1 (VLP-1) as a promising candidate. VLP-1 was then detected both in the plasma of IS patients and in the CSF of rats after middle cerebral artery occlusion. Allard et al.19 used human postmortem CSF (as a model of global brain insult) to identify PARK7 and nucleoside diphosphate kinase A (NDKA) as candidate biomarkers of stroke. Although ubiquitous, both proteins are expressed in neurons and are involved in pathophysiologic cascades following IS. The concentrations of both biomarkers were found to increase in serum in the early phase of IS. Test sensitivity for predicting IS vs controls was 58% for PARK7 and 67% for NDKA, and specificity was 90% for PARK7 and 90% for NDKA.
Although promising, these initial studies require rigorous validation. In proteomics, large datasets are often obtained and immediate connections to known stroke pathways may not be straightforward. Nevertheless, one might look at a recent proteomic analysis of MS as an example of how “discovered hits” might lead one back to mechanisms and novel.20 In this approach, analysis of tissue from active MS lesions revealed a surprising involvement of proteins from the coagulation cascade. Based on these hits, it was subsequently demonstrated that thrombin inhibitors and activated protein C proved efficacious in an animal model of experimental allergic encephalitis. Perhaps a similar approach can be envisioned in stroke, where promising hits from clinical blood samples can guide analyses in either brain autopsy samples in human strokes or excised tissue from animal models.21
MARKERS FOR INTRACEREBRAL HEMORRHAGE
Glial fibrillary acidic protein (GFAP) is a brain-specific intermediate filament protein found in astrocytes. It was recently identified as a biomarker candidate indicative of ICH in the acute phase of stroke.22 In their study, Foerch et al.22 prospectively included 93 patients with IS and 42 patients with ICH within 6 hours after symptom onset. GFAP was detectable in serum in 81% of ICH patients, but only in 5% of IS patients. Mean GFAP serum concentration was significantly higher in patients with ICH. A cutoff point of 2.9 ng/L was found to provide a sensitivity of 79% and a specificity of 98% for the differentiation of ICH from IS. The authors speculate that the more sudden disruption of astroglial cells and the blood–brain barrier (BBB) in ICH is responsible for the rapid occurrence of GFAP in serum, in comparison to a more delayed release of astroglial proteins in IS.23,24 Recently, GFAP was detected in the serum of patients with high-grade gliomas. Histologically, these tumors typically show both GFAP overproduction and BBB alteration, which may explain the GFAP release into serum. As high-grade gliomas may be considered as a potential albeit rare stroke mimic, this finding may reduce the specificity of GFAP for detecting ICH in the acute phase of stroke.25
Delgado et al.26 aimed to examine the diagnostic value of a panel of biomarkers to differentiate IS from ICH. A total of 776 patients with IS and 139 patients with ICH were included within 24 hours after symptom onset. CRP, d-dimer, RAGE, MMP-9, S100B, BNP, neurotrophin-3, caspase-3, chimerin, and secretagogin were used for biomarker testing. Within the first 6 hours after symptom onset, S100B and RAGE best differentiated ICH from IS. This study further strengthens astroglial proteins (i.e., GFAP, S100B) to be promising candidates for a biomarker able to differentiate IS and ICH in the acute phase of stroke.
Using proteomics, Allard et al.27 compared the protein profiles of 21 plasma samples obtained from stroke patients to 21 samples from healthy controls. Seven proteins were identified to be expressed differently, including apolipoprotein CI (ApoC-I) and apolipoprotein CIII (ApoC-III). When comparing ApoC-I and ApoC-III plasma concentrations between patients with IS (<6 hours after symptom onset, n = 16) and ICH (n = 15), significantly lower values of both lipoproteins were apparent in ICH. APoC-III was found to have a sensitivity of 94% and a specificity of 87% for differing ICH from IS (table).
PREDICTING HEMORRHAGIC COMPLICATIONS AFTER THROMBOLYTIC THERAPY
Recombinant tissue plasminogen activator (tPA) has been approved for therapeutic intervention in acute IS.28 However, the beneficial effect of tPA on vessel recanalization is hampered by an increased risk of symptomatic ICH. BBB disruption is a determinant of hemorrhagic transformation (HT) after thrombolysis.29,30 Thus, a rapidly diagnosable biomarker indicating early BBB damage may predict the individual risk for post-treatment HT, and may help in appropriately selecting patients to reap the maximum benefit while avoiding serious side effects of thrombolysis. The positive predictive value of such a test needs to be very high, in order to avoid withholding patients not at risk for HT from this evidence-based treatment. However, a slightly lower sensitivity may be tolerable. Even if not all patients developing HT after thrombolysis will be detected, there is still a chance to substantially reduce the number of affected patients. As not all forms of HT are detrimental, the ultimate goal should be to show an interaction between the level of a biomarker and the association between tPA and poor outcome, i.e., do patients with high levels of the biomarker fare better without tPA than with tPA after randomized treatment allocation? In this context, studies have assessed molecular BBB compounds (i.e., MMP-9, fibronectin), astroglial proteins, and coagulation markers (table). Most studies classified HT into petechial hemorrhagic infarction (HI-1, HI-2) and parenchymal hemorrhage (PH-1, PH-2).
MMP-9 is known to play a deleterious role in the acute phase of IS by degrading various compounds of the BBB, including laminin and fibronectin.31 Montaner et al.32 identified MMP-9 plasma levels determined prior to thrombolytic therapy to be a powerful predictor of post-treatment hemorrhagic complications. In their study, 41 patients underwent thrombolytic therapy due to an IS in the middle cerebral artery territory. Of these, 37% showed signs of HT on follow-up CT scans. A graded response was found between pretreatment MMP-9 blood levels and the degree of HT. Castellanos et al.33 reported MMP-9 to be an independent predictor of HT also in non-thrombolysed IS patients.
Fibronectins are adhesive dimeric glycoproteins that form cell–cell and cell–matrix interactions. Cellular fibronectin (c-Fn) is mainly synthesized by endothelial cells, and high plasma levels of this molecule might be indicative of endothelial damage. Castellanos et al.34 investigated pretreatment c-Fn levels in 87 IS patients who underwent thrombolytic therapy. Median c-Fn concentrations and the degree of blood in the follow-up scans increased in parallel, and c-Fn was independently associated with HT in a multivariate model. By means of a larger prospective study including 134 patients treated with tPA within 3 hours from symptom onset, Castellanos et al.35 reconfirmed the high predictive value of both pretreatment levels of c-Fn (at a cutoff value of 3.6 μg/mL) and MMP-9 (at a cutoff value of 140 ng/mL) for HT.
Foerch et al.36 described protein S100B to be predictive for hemorrhagic complications after thrombolytic therapy. At the very early stage of IS (i.e., within the first hours), S100B is likely to be a marker of BBB dysfunction rather than an indicator of infarct size, as the latter is not yet determined at this early time point. Protein S100B is much higher concentrated in CSF then in peripheral blood, and BBB opening may allow the protein to trespass into serum. Pretreatment S100B serum concentrations were analyzed in retrospect in 275 patients thrombolysed within 6 hours after symptom onset and emerged as an independent predictor of HT. However, diagnostic accuracy for individual S100B values was found to be low.
Other groups investigated whether members of the coagulation cascade may be useful as predictors of HT. Ribo et al.37 found pretreatment levels of the fibrinolysis inhibitors plasminogen activator inhibitor-1 (PAI-1) and thrombin activated fibrinolysis inhibitor (TAFI) to be predictive for symptomatic ICH after tPA therapy. The combination of admission PAI-1 <21.4 ng/mL and TAFI >180% had a sensitivity of 75% and a specificity of 98% predicting symptomatic ICH. However, Cocho et al.38 were not able to confirm these results when testing several hemostatic markers including TAFI and PAI-1 in a similar approach.
PREDICTING A MALIGNANT COURSE OF INFARCTION
Patients with a persisting occlusion of a large artery in the anterior circulation are at risk to develop extensive infarcts with space-occupying edema formation, incurring the risk of cerebral herniation.39 Recently, a randomized multicenter study revealed a benefit for early decompressive surgery in terms of improving both survival rate and functional outcome in these so-called malignant infarctions.40 A biomarker predictive of malignant cerebral edema might be helpful with the timely identification of patients at risk in order to transfer them to specialized units and identify candidates for early decompressive surgery. In this setting, a biomarker must have the ability to identify malignant edema formation rapidly (ideally within the first 24 hours). Sensitivity should be high, in order to identify all patients with malignant infarctions and—if treatment is indicated—to prevent them from cerebral herniation. On the other side, a slightly lower specificity may be tolerable, as the procedure does not necessarily harm patients with very large but nonmalignant infarctions. The design of a prospective study evaluating the diagnostic potential of biomarkers may be aligned with clinical studies in the field.40 Thus, either patients with large infarctions may be suitable, but also inclusion criteria based on vessel status appear to be justified.
In view of the underlying pathophysiology of malignant infarctions, biomarkers correlating with infarct volume (e.g., S100B) or BBB damage (e.g., c-Fn, MMP-9) appeared to be promising candidates (table). Foerch et al.41 studied 51 patients admitted within 6 hours after symptom onset with a proven occlusion of the proximal middle cerebral artery or the carotid T. As early as 12 hours after symptom onset, mean S100B serum concentrations were higher in patients who later developed a malignant cerebral edema as compared with those who did not. A 12-hour S100B value >0.35 μg/L predicted a malignant infarction with a sensitivity of 75% and a specificity of 80%. A 24-hour S100B value >1.03 μg/L provided a sensitivity of 94% and a specificity of 83%.
Serena et al.42 investigated whether molecular compounds of the BBB may be helpful in predicting malignant infarction, as severe BBB damage is supposed to occur in malignant infarctions coming along with extensive edema formation. The authors studied 40 patients with malignant infarctions and compared serum levels of various biomarkers to those obtained from 35 patients with large middle cerebral artery infarctions, but without signs of malignancy. c-Fn and MMP-9 levels were found to be higher in patients with malignant infarctions. c-Fn >16.6 μg/mL had a sensitivity of 90% and a specificity of 100% for the prediction of a malignant course of infarction.
DISCUSSION
Biomarkers can play many useful roles in medicine. Operationally, a biomarker may help one assess risk, diagnose, select treatments, quantitatively follow the efficacy of treatments, and ultimately, predict clinical outcomes. Such broad functional definitions suggest that different biomarkers will apply for different purposes. In this mini-review, we characterized 3 clinical scenarios in which stroke biomarker testing may facilitate the routine workup of patients presenting with stroke-like symptoms, by adding information on a molecular basis to data obtained from clinical examination and brain imaging. This selective approach gave us the possibility to discuss the pros and cons of many explorative biomarker studies in detail, particularly with regard to what appears to be of interest for the clinical neurologist. On the other hand, it is important to mention that we may have missed discussing a number of relevant publications. Particularly, our review did not focus on examining biomarkers of stroke risk, which is an entire field in itself.43 Regardless of the potential field of application, the clinical relevance of all candidate markers needs to be carefully validated in future controlled clinical trials with specific design and analysis geared toward focused clinical questions.
DISCLOSURE
Dr. Foerch is designated as an inventor in the European patent application #03021571.9: “Use of GFAP for identification of intracerebral haemorrhage.” Dr. Montaner has received speaker honoraria from Sanofi, Ferrer, Boehringer Ingelheim, and Genzyme; serves as a consultant for Biomerieux; receives research support from Biosite, Proteom Sciences, Ferrer, Uriach, and Boehringer Ingelheim; and has received public grants from the European, Spanish, and Catalan governments (EUSTROKE F2-2008-202213, EC07/90195, EC08/00137, MD07/00209, FIS08/361, RENEVAS RD06/026 and SGR614). Dr. Furie serves on advisory boards for GE Healthcare and Novartis. Dr. Ning receives research support from the NIH [NS051588 (PI) and NS052498 (PI)]. Dr. Lo serves as a consultant for Mitsubishi and has received research support from Boehringer Ingelheim, the NIH [R37-NS37074 (PI), R01-NS56458 (PI), P01-NS55104 (PI), and P50-NS51343 (Co-PI)], and the AHA.
Address correspondence and reprint requests to Dr. Christian Foerch, Department of Neurology, Goethe-University, Schleusenweg 2-16, 60528 Frankfurt am Main, Germany foerch@em.uni-frankfurt.de
Supported by the Deutsche Forschungsgemeinschaft (DFG), NIH grants R37-NS37074, R01-NS48422, R01-NS56458, P01-NS55104, NS051588-MMN, NS052498-MMN, and a Bugher award from the American Heart Association (AHA).
Disclosure: Author disclosures are provided at the end of the article.
Received October 30, 2008. Accepted in final form May 1, 2009.
REFERENCES
- 1.Adams HP, Jr., del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke. Stroke 2007;38:1655–1711. [DOI] [PubMed] [Google Scholar]
- 2.Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet 2007;369:293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hand PJ, Kwan J, Lindley RI, Dennis MS, Wardlaw JM. Distinguishing between stroke and mimic at the bedside: the brain attack study. Stroke 2006;37:769–775. [DOI] [PubMed] [Google Scholar]
- 4.Donnan GA, Davis SM. Neuroimaging, the ischaemic penumbra, and selection of patients for acute stroke therapy. Lancet Neurol 2002;1:417–425. [DOI] [PubMed] [Google Scholar]
- 5.Castellanos M, Serena J. Applicability of biomarkers in ischemic stroke. Cerebrovasc Dis 2007;24 suppl 1:7–15. [DOI] [PubMed] [Google Scholar]
- 6.Whiteley W, Tseng MC, Sandercock P. Blood biomarkers in the diagnosis of ischemic stroke: a systematic review. Stroke 2008;39:2902–2909. [DOI] [PubMed] [Google Scholar]
- 7.Dambinova SA, Khounteev GA, Izykenova GA, Zavolokov IG, Ilyukhina AY, Skoromets AA. Blood test detecting autoantibodies to N-methyl-D-aspartate neuroreceptors for evaluation of patients with transient ischemic attack and stroke. Clin Chem 2003;49:1752–1762. [DOI] [PubMed] [Google Scholar]
- 8.Reynolds MA, Kirchick HJ, Dahlen JR, et al. Early biomarkers of stroke. Clin Chem 2003;49:1733–1739. [DOI] [PubMed] [Google Scholar]
- 9.Lynch JR, Blessing R, White WD, Grocott HP, Newman MF, Laskowitz DT. Novel diagnostic test for acute stroke. Stroke 2004;35:57–63. [DOI] [PubMed] [Google Scholar]
- 10.Laskowitz DT, Blessing R, Floyd J, White WD, Lynch JR. Panel of biomarkers predicts stroke. Ann NY Acad Sci 2005;1053:30. [DOI] [PubMed] [Google Scholar]
- 11.Laskowitz DT, Kasner SE, Saver J, Remmel KS, Jauch EC. Clinical usefulness of a biomarker-based diagnostic test for acute stroke: the Biomarker Rapid Assessment in Ischemic Injury (BRAIN) study. Stroke 2009;40:77–85. [DOI] [PubMed] [Google Scholar]
- 12.Saenz AJ, Lee-Lewandrowski E, Wood MJ, et al. Measurement of a plasma stroke biomarker panel and cardiac troponin T in marathon runners before and after the 2005 Boston marathon. Am J Clin Pathol 2006;126:185–189. [DOI] [PubMed] [Google Scholar]
- 13.Baird AE. Blood genomics in human stroke. Stroke 2007;38:694–698. [DOI] [PubMed] [Google Scholar]
- 14.Moore DF, Li H, Jeffries N, et al. Using peripheral blood mononuclear cells to determine a gene expression profile of acute ischemic stroke: a pilot investigation. Circulation 2005;111:212–221. [DOI] [PubMed] [Google Scholar]
- 15.Tang Y, Xu H, Du X, et al. Gene expression in blood changes rapidly in neutrophils and monocytes after ischemic stroke in humans: a microarray study. J Cereb Blood Flow Metab 2006;26:1089–1102. [DOI] [PubMed] [Google Scholar]
- 16.Xu H, Tang Y, Liu DZ, et al. Gene expression in peripheral blood differs after cardioembolic compared with large-vessel atherosclerotic stroke: biomarkers for the etiology of ischemic stroke. J Cereb Blood Flow Metab 2008;28:1320–1328. [DOI] [PubMed] [Google Scholar]
- 17.Meisel C, Schwab JM, Prass K, Meisel A, Dirnagl U. Central nervous system injury-induced immune deficiency syndrome. Nat Rev Neurosci 2005;6:775–786. [DOI] [PubMed] [Google Scholar]
- 18.Laterza OF, Modur VR, Crimmins DL, et al. Identification of novel brain biomarkers. Clin Chem 2006;52:1713–1721. [DOI] [PubMed] [Google Scholar]
- 19.Allard L, Burkhard PR, Lescuyer P, et al. PARK7 and nucleoside diphosphate kinase A as plasma markers for the early diagnosis of stroke. Clin Chem 2005;51:2043–2051. [DOI] [PubMed] [Google Scholar]
- 20.Han MH, Hwang SI, Roy DB, et al. Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature 2008;451:1076–1081. [DOI] [PubMed] [Google Scholar]
- 21.Ning MM, Wang X, Lo EH. Reperfusion injury after stroke: neurovascular proteases and the blood-brain barrier. In: Fisher M, editor. Handbook of Clinical Neurology: Stroke. UK: Elsevier; 2008;92:117–136. [DOI] [PubMed] [Google Scholar]
- 22.Foerch C, Curdt I, Yan B, et al. Serum glial fibrillary acidic protein as a biomarker for intracerebral haemorrhage in patients with acute stroke. J Neurol Neurosurg Psychiatry 2006;77:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herrmann M, Vos P, Wunderlich MT, de Bruijn CH, Lamers KJ. Release of glial tissue-specific proteins after acute stroke: a comparative analysis of serum concentrations of protein S-100B and glial fibrillary acidic protein. Stroke 2000;31:2670–2677. [DOI] [PubMed] [Google Scholar]
- 24.Dvorak F, Haberer I, Sitzer M, Foerch C. Characterisation of the diagnostic window of serum glial fibrillary acidic protein for the differentiation of intracerebral haemorrhage and ischaemic stroke. Cerebrovasc Dis 2009;27: 37–41. [DOI] [PubMed] [Google Scholar]
- 25.Jung CS, Foerch C, Schanzer A, et al. Serum GFAP is a diagnostic marker for glioblastoma multiforme. Brain 2007;130:3336–3341. [DOI] [PubMed] [Google Scholar]
- 26.Delgado P, Alvarez-Sabin J, Ribó M, et al. Differentiating ischemic and hemorrhagic stroke by means of a panel of plasma biomarkers. Oral Presentation; 2005 European Stroke Conference; Bologna, Italy; 2005.
- 27.Allard L, Lescuyer P, Burgess J, et al. ApoC-I and ApoC-III as potential plasmatic markers to distinguish between ischemic and hemorrhagic stroke. Proteomics 2004;4:2242–2251. [DOI] [PubMed] [Google Scholar]
- 28.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–1587. [DOI] [PubMed] [Google Scholar]
- 29.Sumii T, Lo EH. Involvement of matrix metalloproteinase in thrombolysis-associated hemorrhagic transformation after embolic focal ischemia in rats. Stroke 2002;33:831–836. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Tsuji K, Lee SR, et al. Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke 2004;35:2726–2730. [DOI] [PubMed] [Google Scholar]
- 31.Ning MM, Furie KL, Koroshetz WJ, et al. Association between tPA therapy and raised early matrix metalloproteinase-9 in acute stroke. Neurology 2006;66:1550–1555. [DOI] [PubMed] [Google Scholar]
- 32.Montaner J, Molina CA, Monasterio J, et al. Matrix metalloproteinase-9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation 2003;107:598–603. [DOI] [PubMed] [Google Scholar]
- 33.Castellanos M, Leira R, Serena J, et al. Plasma metalloproteinase-9 concentration predicts hemorrhagic transformation in acute ischemic stroke. Stroke 2003;34:40–46. [PubMed] [Google Scholar]
- 34.Castellanos M, Leira R, Serena J, et al. Plasma cellular-fibronectin concentration predicts hemorrhagic transformation after thrombolytic therapy in acute ischemic stroke. Stroke 2004;35:1671–1676. [DOI] [PubMed] [Google Scholar]
- 35.Castellanos M, Sobrino T, Millan M, et al. Serum cellular fibronectin and matrix metalloproteinase-9 as screening biomarkers for the prediction of parenchymal hematoma after thrombolytic therapy in acute ischemic stroke: a multicenter confirmatory study. Stroke 2007;38:1855–1859. [DOI] [PubMed] [Google Scholar]
- 36.Foerch C, Wunderlich MT, Dvorak F, et al. Elevated serum S100B levels indicate a higher risk of hemorrhagic transformation after thrombolytic therapy in acute stroke. Stroke 2007;38:2491–2495. [DOI] [PubMed] [Google Scholar]
- 37.Ribo M, Montaner J, Molina CA, et al. Admission fibrinolytic profile is associated with symptomatic hemorrhagic transformation in stroke patients treated with tissue plasminogen activator. Stroke 2004;35:2123–2127. [DOI] [PubMed] [Google Scholar]
- 38.Cocho D, Borrell M, Marti-Fabregas J, et al. Pretreatment hemostatic markers of symptomatic intracerebral hemorrhage in patients treated with tissue plasminogen activator. Stroke 2006;37:996–999. [DOI] [PubMed] [Google Scholar]
- 39.Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R. ‘Malignant’ middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol 1996;53:309–315. [DOI] [PubMed] [Google Scholar]
- 40.Vahedi K, Hofmeijer J, Juettler E, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol 2007;6:215–222. [DOI] [PubMed] [Google Scholar]
- 41.Foerch C, Otto B, Singer OC, et al. Serum S100B predicts a malignant course of infarction in patients with acute middle cerebral artery occlusion. Stroke 2004;35:2160–2164. [DOI] [PubMed] [Google Scholar]
- 42.Serena J, Blanco M, Castellanos M, et al. The prediction of malignant cerebral infarction by molecular brain barrier disruption markers. Stroke 2005;36:1921–1926. [DOI] [PubMed] [Google Scholar]
- 43.Corson MA, Jones PH, Davidson MH. Review of the evidence for the clinical utility of lipoprotein-associated phospholipase A2 as a cardiovascular risk marker. Am J Cardiol 2008;101:41F–50F. [DOI] [PubMed] [Google Scholar]


