Abstract
Background:
In patients with active epilepsy, adverse medication effects and severity of depression are correlated with health-related quality of life, but seizure frequency is not. We sought to examine if the same pattern exists in patients with psychogenic nonepileptic seizures (PNES).
Methods:
We administered seizure calendars, quality of life (QOL) scales, depression scales, and symptom checklists to 49 patients with video EEG–confirmed PNES. Data analysis consisted of performing Pearson correlation coefficients, scatter plots, and t tests.
Results:
Depression and symptom scores significantly increased as health-related QOL scores decreased (partial correlation coefficient r = −0.73 for both comparisons), whereas seizure count was nonsignificant (partial correlation coefficient r = −0.19).
Conclusions:
As is seen in epilepsy, patients with psychogenic nonepileptic seizures demonstrate that higher depressive symptoms and somatic symptoms are independently related to worsening quality of life (QOL); however, seizure frequency is not. Seizure frequency is an important focus in patient care and treatment trials. The findings underscore the importance of, along with seizure counts, also examining QOL, depression, and somatic symptoms in patients with seizures.
GLOSSARY
- AED
= antiepileptic drug;
- AEP
= Adverse Events Profile;
- BDI
= Beck Depression Inventory;
- DSM-IV
= Diagnostic and Statistical Manual of Mental Disorders, 4th edition;
- HRQOL
= health-related QOL;
- NPE
= neuropsychiatric examination;
- PNES
= psychogenic nonepileptic seizures;
- QOL
= quality of life;
- QOLIE-31
= Quality of Life in Epilepsy–31;
- RIH
= Rhode Island Hospital;
- SCL-90
= Symptom Checklist–90;
- vEEG
= video EEG.
Despite the prevalence of psychogenic nonepileptic seizures (PNES), no formalized treatment for PNES has been found.1,2 Patients with PNES, presumed to have epileptic seizures, are prescribed antiepileptic drugs (AEDs), which do not treat PNES and which sometimes exacerbate the condition.3,4 All the while, patients with PNES are not receiving interventions such as psychotherapy and psychopharmacology that may treat the disorder.5
As attention to the prevalence and cost of PNES has increased,6 more research trials are being conducted to determine effective treatments.7–9 Most seizure trials present seizure count as a primary outcome. However, studies have suggested seizure remission alone may not be an adequate outcome in PNES studies, as patients with reduced seizures may continue to have psychiatric symptoms and remain disabled.10
The relationship between seizure frequency and quality of life (QOL) has been studied in patients with epilepsy, but not as extensively in patients with PNES. Gilliam11 studied 195 patients with epilepsy to determine the relationship of neuropsychiatric function and QOL. As patients’ mood symptoms and their symptoms/adverse events increased, QOL scores decreased (using the QOLIE-89 Global Scale12). Seizure frequency in patients with epilepsy, however, was found not to be related to QOL. We hypothesized that similar relationships to QOL would be found in the PNES population. In this study, we compared the relationship of QOL, depression, symptom scales, and seizure frequency in patients with PNES. We also sought to extend findings examining other contributors to QOL in PNES.
METHODS
Standard protocol approvals, registrations, and patient consents.
The protocol received approval from the Rhode Island Hospital (RIH) Institutional Review Board, an ethical standards committee on human experimentation. Written informed consent was obtained from all patients participating in the study.
Patients were referred to the RIH neuropsychiatry/behavioral neurology clinic after having their typical events captured on video EEG (vEEG) revealing PNES. Patients were diagnosed with PNES if they had stereotypic motor manifestations, with or without change in level of consciousness, on vEEG with no epileptiform activity immediately before, during, or after the event.
All patients underwent a neuropsychiatric examination (NPE) and clinical screening by a board-certified neurologist and psychiatrist (W.C.L.). The NPE consists of a complete neurologic history and examination and a complete psychiatric history and mental status examination.
Patients between the ages of 18 and 65, who met the inclusion/exclusion criteria, provided written informed consent and enrolled in the study. The inclusion criterion was vEEG diagnosis of PNES. Those with equivocal diagnoses on vEEG, such as simple partial-type PNES, were excluded. Patients with mixed epileptic seizures and PNES who could clearly distinguish between their events were included. Other exclusion criteria included the presence of a current psychosis (hallucinations or delusions), current suicidality (plan and intent of killing oneself), current substance dependence (DSM-IV criteria), mental retardation, pending litigation, or disability application.
After enrolling, patients completed seizure frequency, psychosocial functioning, and symptom scales. Self-report scales included Quality of Life in Epilepsy (QOLIE-31),13 Beck Depression Inventory (BDI),14 and the Symptom Checklist–90 (SCL-90).15 The SCL-90 is a 90-item self-report clinical rating scale oriented toward symptoms of outpatients. The checklist assesses 9 primary symptom dimensions and is a valid and reliable scale.16 The SCL-90 was substituted for the Adverse Events Profile (AEP),17 as the AEP used in the Gilliam study11 assesses the severity of common AED side effects. While not all patients with PNES are taking AEDs, they have many somatic complaints, and the SCL-90 captures somatic complaints, independent of medication usage. The SCL-90 has been tested previously in the PNES population and patients with PNES are found to have higher SCL scores than controls.18 Medical and historical information was collected from chart review and patient query. Seizure frequency in the 2 weeks prior to enrolling was obtained from the patient. Two-week seizure frequency also was collected prospectively in weekly intervals.
Statistical analysis.
Analysis of the data was performed using the SAS System, version 9.1 (Cary, NC). Correlations were calculated between the QOLIE-31 and seizure frequency, BDI, and the SCL-90 survey scores, controlling for age and gender, given the potential for bias with the known demographic distribution of the PNES population (80% women, 80% between the ages of 16 and 40).19 Pearson correlation coefficients were calculated for all correlations. An a priori p value less than or equal to 0.05 was considered significant. Comparisons using t tests of SCL-90 means between subjects taking and not taking AEDs were calculated.
RESULTS
This population of patients with PNES is similar to populations found in the literature in sociodemographic characteristics, psychiatric diagnoses, neurologic findings, and clinical factors (table). Three patients had mixed epilepsy/PNES. At enrollment, AED usage was found in 25 study patients. Seizure frequency data were available for 49 patients with PNES. The comparison of retrospectively collected (biweekly count prior to enrollment) and prospective seizure counts (2 weeks after enrollment) revealed similar correlation calculations.
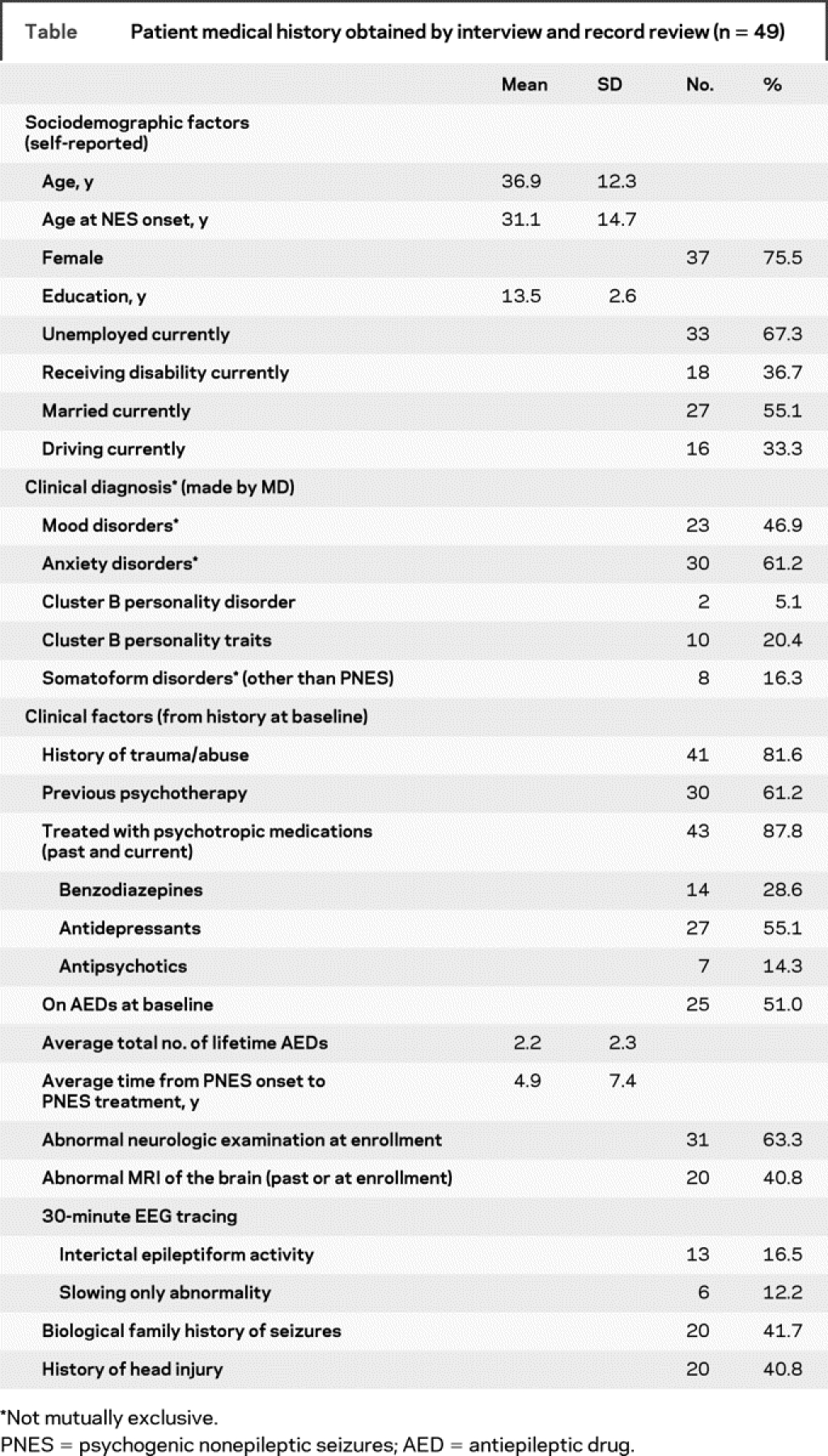
Table Patient medical history obtained by interview and record review (n = 49)
After controlling for age and gender, as SCL-90 scores increased, health-related QOL (HRQOL) decreased (partial correlation coefficient r = −0.73, p = 0.0001), as shown in figure 1.
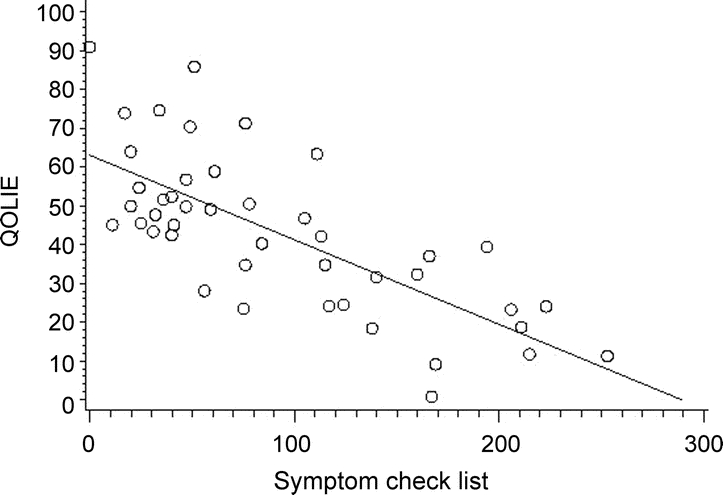
Figure 1 Scatterplot of the correlation of health-related quality of life (Quality of Life in Epilepsy–31 [QOLIE-31]) with somatic symptoms (Symptom Checklist–90 [SCL-90]) (r = −0.73, p = 0.0001)
QOLIE-31: the higher the score, the better the perceived quality of life. SCL-90: the higher the score, the worse the number of reported symptoms.
As severity of depression increased, determined by the BDI scores, HRQOL decreased (partial correlation coefficient r = −0.73, p = 0.0001), displayed in figure 2.
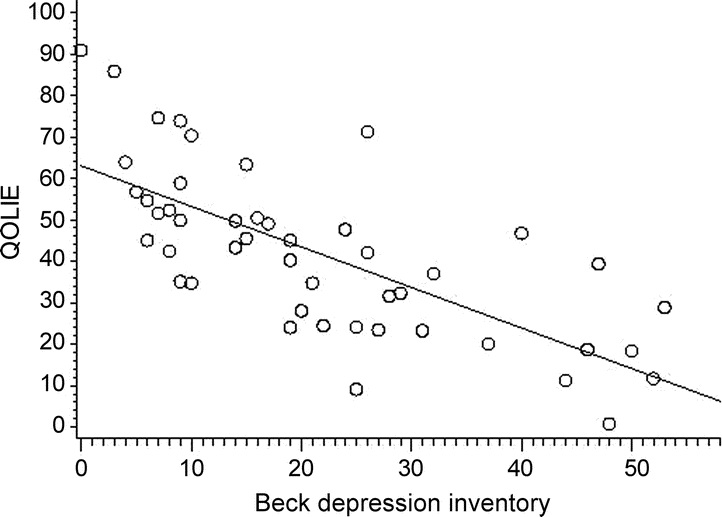
Figure 2 Scatterplot of the correlation of health-related quality of life (Quality of Life in Epilepsy–31 [QOLIE-31]) with depression scale scores (Beck Depression Inventory–II [BDI-II]) (r = −0.73, p = 0.0001)
QOLIE-31: the higher the score, the better the perceived quality of life. BDI-II: the higher the score, the worse the number of reported depressive symptoms.
QOLIE scores and seizure count, however, were not related (partial correlation coefficient r = −0.19, p = 0.21), as shown in figure 3.
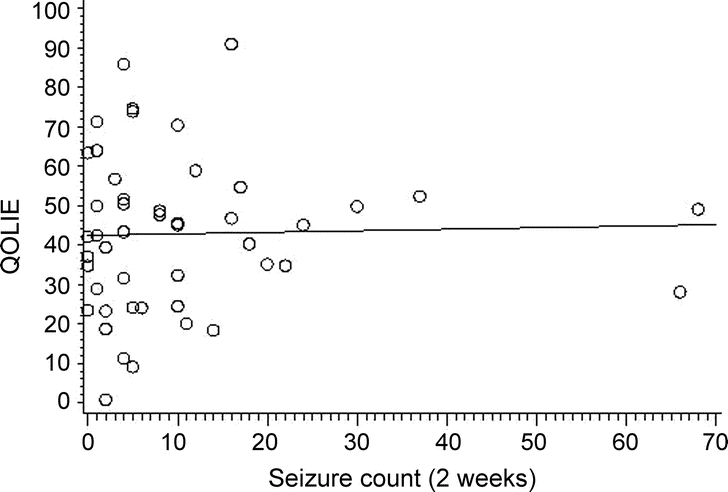
Figure 3 Scatterplot of the correlation of health-related quality of life (Quality of Life in Epilepsy [QOLIE-31]) with seizure count (r = −0.19, p = 0.21)
QOLIE-31: the higher the score, the better the perceived quality of life.
Seizure frequency and depression scores showed no relationship (partial correlation coefficient r = −0.03, p = 0.84).
Paired t test of SCL-90 means between patients with PNES taking AEDs (n = 25; mean = 100.1; SD 66.96) and those not (n = 24; mean = 94.27; SD 76.72) revealed no difference (t = −0.274 [df 44], p = 0.785). Patients who took AEDs and those not taking AEDs both reported symptoms including headaches, insomnia, memory problems, and neurovegetative symptoms of depression and of anxiety, without group differences of endorsement (t = −0.003 [df 47], p = 0.998).
DISCUSSION
In our study of patients with PNES, as depression and somatic symptoms increased, HRQOL decreased, while seizure frequency was not correlated with HRQOL. The patterns of findings in patients with PNES are the same as those that have been found in the epilepsy population.11 In both populations of patients with epilepsy or with PNES, clinicians typically focus on seizures, and seizure treatment trials typically use seizure counts as the primary outcome measure. These findings underscore the importance of also examining secondary outcomes, such as QOL and other psychiatric measures, along with seizures, in our patients and in seizure trials.
Mood symptoms play a significant role in HRQOL in patients with seizures. PNES improvement or even cessation does not necessarily result in improved psychiatric condition. For example, in one study, nearly half of the patients remained disabled because of psychiatric symptoms even though they no longer had PNES.10 Depression contributes to poor QOL in patients with PNES and ES. In patients with epilepsy, depression symptomology is a strong predictor of a worse QOL.20 Neuropsychological batteries administered to patients with PNES or with epilepsy do not distinguish between the 2 groups, rather reveal that their scores are worse than those of healthy controls in both groups. However, patients with PNES report worse QOL in comparison to both epilepsy and depression norms.21 These findings underscore the importance of evaluating and treating mood status in patients with PNES with the hopes of improving QOL. Though it is important to make a diagnosis of PNES, merely presenting the diagnosis of PNES alone is not enough, as post-diagnosis frequency decreases are not maintained long term.22 Diagnosing comorbid psychiatric conditions and discontinuing AEDs, thereby mitigating side effects, may be warranted in patients with PNES.
Patients with PNES are a neuropsychiatric population in that they not only have the psychiatric diagnosis but many times have neurologic comorbidities. Brain abnormalities identified in epileptiform EEG changes, MRI abnormalities, and neuropsychological deficits are common in the PNES population.23 Our neurologic study findings were consistent with the literature, and neurologic findings on examination included nystagmus, tremors, hypesthesia, hyperreflexia, and ataxia on tandem gait, many of which could be attributable to the presence of AEDs. Of note, AED toxicity, which can be manifested with some of these neurologic signs, has been proposed as an exacerbating factor in PNES.4
Slightly differing from the literature, however, is that 50% of patients in our study were on AEDs. The lower percentage may be explained by the manner the AED variable was captured, which was at enrollment in a well-defined time period, usually just after video EEG, when many patients may have been tapered off AEDs when lone PNES was identified. Consistent with the literature,24 our population had received a lifetime average of 2.2 (±2.3) AEDs, indicating that the reported 50% currently taking AEDs may have been a time-sampling issue. Patients taking AEDs in this study reported no more or less side effects compared to those not taking AEDs. We are not able to comment directly on the side effects due specifically to AEDs because patients were taking other CNS-active agents. The similar rates of endorsement of side effects between the 2 groups could be a manifestation of the somatic nature of the population or to other CNS-active medications.
Limitations of this study include the inclusion of only adults age 18 to 65 with PNES. This study was restricted to those over the age of 18 to minimize potential age effect bias. PNES does occur in children but may have a different mechanism, often resolving with better outcomes.25 PNES does occur in the elderly, however, studies reporting outcome measures in the elderly are limited.26–28 Another potential limitation is that the SCL-90 was used as a surrogate for patient symptoms, instead of the AEP used in the Gilliam study.11 We elected to use the SCL-90 because not all patients with PNES were taking AEDs, the symptoms of which the AEP scale targets specifically. By using the SCL-90 instead of the AEP, however, we were able to broadly survey somatic symptoms, allowing us to assess the impact of symptoms on QOL. Another limitation is that in modeling the study after the comparisons made in the Gilliam study to replicate the finding in the PNES population, we did not explore the contributions of other psychiatric disorders, which are known to occur in the PNES population.29 Thus, we are not equating depression with QOL, given the other psychopathologies in this population. We are merely looking at select potential contributors to QOL. A potential critique is that a 2-week seizure frequency may not be an adequate measure of burden in PNES, acknowledging that other studies in epilepsy have represented seizures per month or over a year. We would argue that the 2-week cross-section is more precise than the retrospective “seizures per month/year” window used in some studies, in that it more accurately captured seizure frequency. Secondly, our study builds on a recent study that explored the HRQOL-PNES relationship using follow-up mailed surveys,30 in that the QOL, depression, and symptoms scales in our study were collected at the time of assessment and were time-linked with the 2 weeks related to those seizure frequencies.
As in epilepsy, both somatic symptoms and depression have a major impact on subjective health status in patients with PNES. Though epilepsy and PNES have different etiologies, both patient populations have a similar pattern of findings, i.e., higher depression and adverse events/symptoms are associated with worse QOL, but seizure frequency does not correlate with QOL. Furthermore, seizure frequency was not related to depression severity in our study. Based on reports of patients who have enrolled in our PNES clinical trials, we speculate that the lack of PNES frequency to QOL correlation may be due to the fact that infrequent seizures yield the same results as many PNES, that is, loss of driving privileges, loss of job, loss of income, and loss of family stability. So, 2 seizures can be just as socially devastating as 20 seizures. The attention given by caretakers also needs to be considered in the potential for secondary gain in PNES. These factors may impact QOL equally and independent of seizure frequency.
For this reason, the primary outcome in our PNES clinical trials and most epilepsy clinical trials remains some form of seizure count, whether a frequency or proportion reduction. As clinicians who treat patients with seizures, one of our aims is to reduce seizures, as they account for significant social restrictions in our patients’ lives. The findings from this study are not intended to shift therapeutic outcome targets from seizures to QOL scores. Rather, we emphasize that secondary measures, such as QOL and mood scales, may be important adjunctive components to clinical care and clinical trials.
In point of fact, day-to-day functioning also may be related with how depressed and how many other symptoms the patient experiences, possibly explaining the relationship between QOL and depression/somatic symptom severity. On the other hand, though the disorders present similarly clinically and share QOL determinant characteristics, the experience of patients with epilepsy and PNES is very different. Patients with PNES, when compared to patients with epilepsy, often have a more external locus of control and use their emotions to cope with situations.31 Some authors have proposed that patients with PNES have a tendency to over-report somatic and social functioning difficulties and may minimize self-reported mood disorders.32
Research examining the potential causal pathways linking seizures, QOL, depressive and somatic symptoms, and health outcomes would be of great benefit. Just as in symptomatic epilepsies where an underlying etiology produces a seizure, many times, the seizures of PNES are a sign of an underlying process. Focusing only on the PNES without attending to the underlying process would be as egregious as only addressing the patient with epilepsy with AEDs while overlooking the underlying neoplasm causing the seizures. Acknowledging the complexity of the 2 disorders, further exploration in the similarities and differences between patients with epilepsy and PNES could be warranted to inform prognosis and treatment.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by W. Curt LaFrance, Jr., MD, MPH.
ACKNOWLEDGMENT
The authors thank Drs. Christine Ryan and Gabor Keitner for comments on the manuscript and Joan Kelly for assistance with database management.
DISCLOSURE
Portions of this study were funded by NINDS 1K23 NS45902. Dr. LaFrance Jr. has received speaker honorarium from the Epilepsy Foundation; receives research support from the NIH NINDS [#1K23NS45902 (PI)], the Rhode Island Hospital (PI), the Epilepsy Foundation (PI), and the Siravo Foundation (PI); and has served as a consultant for Disability Services. S. Syc reports no disclosures.
Address correspondence and reprint requests to Dr. W. Curt LaFrance, Jr., Rhode Island Hospital, 593 Eddy Street, Potter 3, Providence, RI 02903 William_LaFrance_Jr@Brown.edu
Disclosure: Author disclosures are provided at the end of the article.
Received February 12, 2009. Accepted in final form April 17, 2009.
REFERENCES
- 1.LaFrance WC Jr, Devinsky O. The treatment of nonepileptic seizures: historical perspectives and future directions. Epilepsia 2004;45(suppl 2):15–21. [DOI] [PubMed] [Google Scholar]
- 2.LaFrance WC Jr, Barry JJ. Update on treatments of psychological nonepileptic seizures. Epilepsy Behav 2005;7:364–374. [DOI] [PubMed] [Google Scholar]
- 3.Oto M, Espie C, Pelosi A, Selkirk M, Duncan R. The safety of antiepileptic drug withdrawal in patients with non-epileptic seizures. J Neurol Neurosurg Psychiatry 2005;76:1682–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niedermeyer E, Blumer D, Holscher E, Walker BA. Classical hysterical seizures facilitated by anticonvulsant toxicity. Psychiatr Clin (Basel) 1970;3:71–84. [DOI] [PubMed] [Google Scholar]
- 5.LaFrance WC Jr, Devinsky O. Treatment of nonepileptic seizures. Epilepsy Behav 2002;3(5 suppl 1):S19–S23. [DOI] [PubMed] [Google Scholar]
- 6.LaFrance WC Jr, Benbadis SR. Avoiding the costs of unrecognized psychological nonepileptic seizures. Neurology 2006;66:1620–1621. [DOI] [PubMed] [Google Scholar]
- 7.Baker GA, Brooks JL, Goodfellow L, Bodde N, Aldenkamp A. Treatments for non-epileptic attack disorder. Cochrane Database Syst Rev 2007(1):CD006370. [DOI] [PubMed] [Google Scholar]
- 8.LaFrance WC Jr, Blum AS, Miller IW, Ryan CE, Keitner GI. Methodological issues in conducting treatment trials for psychological nonepileptic seizures. J Neuropsychiatry Clin Neurosci 2007;19:391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LaFrance WC Jr, Miller IW, Ryan CE, et al. Cognitive behavioral therapy for psychogenic nonepileptic seizures. Epilepsy Behav 2009;14:591–596. [DOI] [PubMed] [Google Scholar]
- 10.Reuber M, Mitchell AJ, Howlett S, Elger CE. Measuring outcome in psychogenic nonepileptic seizures: how relevant is seizure remission? Epilepsia 2005;46:1788–1795. [DOI] [PubMed] [Google Scholar]
- 11.Gilliam F. Optimizing health outcomes in active epilepsy. Neurology 2002;58(suppl 5):S9–S20. [DOI] [PubMed] [Google Scholar]
- 12.Devinsky O, Vickrey BG, Cramer J, et al. Development of the quality of life in epilepsy inventory. Epilepsia 1995;36:1089–1104. [DOI] [PubMed] [Google Scholar]
- 13.Vickrey BG, Perrine KR, Hays RD, et al., eds. Quality of Life in Epilepsy. QOLIE-31. Scoring Manual. Version 1.0 ed. Santa Monica, CA: RAND; 1993. [Google Scholar]
- 14.Beck AT, Steer RA, Brown GK. Manual for The Beck Depression Inventory–Second Edition (BDI-II). San Antonio: Psychological Corporation; 1996. [Google Scholar]
- 15.Derogatis LR. Symptom Checklist 90-R: Administration, Scoring, and Procedures Manual. 3rd ed. Minneapolis, MN: National Computer Systems, Inc.; 1994. [Google Scholar]
- 16.Uhlenhuth EH, Balter MB, Mellinger GD, Cisin IH, Clinthorne J. Symptom checklist syndromes in the general population: correlations with psychotherapeutic drug use. Arch Gen Psychiatry 1983;40:1167–1173. [DOI] [PubMed] [Google Scholar]
- 17.Baker GA, Frances P, Middleton E. Initial development, reliability and validity of a patient-based adverse events scale. Epilepsia 1994;35(suppl 7):80. [Google Scholar]
- 18.Prueter C, Schultz-Venrath U, Rimpau W. Dissociative and associated psychopathological symptoms in patients with epilepsy, pseudoseizures, and both seizure forms. Epilepsia 2002;43:188–192. [DOI] [PubMed] [Google Scholar]
- 19.van Merode T, de Krom MC, Knottnerus JA. Gender-related differences in non-epileptic attacks: a study of patients’ cases in the literature. Seizure 1997;6:311–316. [DOI] [PubMed] [Google Scholar]
- 20.Loring DW, Meador KJ, Lee GP. Determinants of quality of life in epilepsy. Epilepsy Behav 2004;5:976–980. [DOI] [PubMed] [Google Scholar]
- 21.Szaflarski JP, Szaflarski M. Seizure disorders, depression, and health-related quality of life. Epilepsy Behav 2004;5:50–57. [DOI] [PubMed] [Google Scholar]
- 22.Wilder C, Marquez AV, Farias ST, et al. Long-term follow-up study of patients with PNES. Epilepsia 2004;45(suppl 7):349. Abstract 2.469.
- 23.Reuber M, Fernandez G, Helmstaedter C, Qurishi A, Elger CE. Evidence of brain abnormality in patients with psychogenic nonepileptic seizures. Epilepsy Behav 2002;3:249–254. [DOI] [PubMed] [Google Scholar]
- 24.Reeves AL, McAuley JW, Moore JL, Capestany J. Medication use, self-reported drug allergies, and estimated medication cost in patients with epileptic versus nonepileptic seizures. J Epilepsy 1998;11:191–194. [Google Scholar]
- 25.Wyllie E, Friedman D, Luders H, Morris H, Rothner D, Turnbull J. Outcome of psychogenic seizures in children and adolescents compared with adults. Neurology 1991;41:742–744. [DOI] [PubMed] [Google Scholar]
- 26.Shulman KI, Silver IL. Hysterical seizures as a manifestation of “depression” in old age. Can J Psychiatry 1985;30:278–280. [DOI] [PubMed] [Google Scholar]
- 27.Kellinghaus C, Loddenkemper T, Dinner DS, Lachhwani D, Luders HO. Non-epileptic seizures of the elderly. J Neurol 2004;251:704–709. [DOI] [PubMed] [Google Scholar]
- 28.Duncan R, Oto M, Martin E, Pelosi A. Late onset psychogenic nonepileptic attacks. Neurology 2006;66:1644–1647. [DOI] [PubMed] [Google Scholar]
- 29.Bowman ES, Markand ON. Psychodynamics and psychiatric diagnoses of pseudoseizure subjects. Am J Psychiatry 1996;153:57–63. [DOI] [PubMed] [Google Scholar]
- 30.Lawton G, Mayor RJ, Howlett S, Reuber M. Psychogenic nonepileptic seizures and health-related quality of life: The relationship with psychological distress and other physical symptoms. Epilepsy Behav 2009;14:167–171. [DOI] [PubMed] [Google Scholar]
- 31.Stone J, Binzer M, Sharpe M. Illness beliefs and locus of control. A comparison of patients with pseudoseizures and epilepsy. J Psychosom Res 2004;57:541–547. [DOI] [PubMed] [Google Scholar]
- 32.Donofrio N, Perrine K, Alper K, Devinsky O. Chapter 11. Depression and anxiety in patients with non-epileptic versus epileptic seizures. In: Gates JR, Rowan AJ, eds. Non-Epileptic Seizures. 2nd ed. Boston, MA: Butterworth-Heinemann; 2000:151–157. [Google Scholar]


