Abstract
We compared 8,144 Salmonella isolates collected from meat imported to or produced in Denmark, as well as from Danish patients. Isolates from imported meat showed a higher rate of antimicrobial drug resistance, including multidrug resistance, than did isolates from domestic meat. Isolates from humans showed resistance rates lower than those found in imported meat but higher than in domestic meat. These findings indicate that programs for controlling resistant Salmonella spp. are a global issue.
Keywords: Salmonella, zoonoses, multidrug resistance, antimicrobial drug resistance, meat products, imported meat, humans, dispatch
Salmonella spp. are among the most common causes of human bacterial gastroenteritis worldwide, and food animals are important reservoirs of the bacteria (1). In recent years, an increase in the occurrence of antimicrobial drug–resistant Salmonella spp. has been observed in several countries (2–5). Fatality rates are higher for patients with infections caused by drug-resistant Salmonella spp., and these patients are more likely to require hospitalization and to be hospitalized for longer periods than are patients with infections caused by antimicrobial drug–susceptible Salmonella spp. (6,7).
Antimicrobial drug resistance of Salmonella spp. isolated from food animals in Denmark has so far been relatively low (8). However, an estimated 30% of all poultry, 10% of all pork, and 50% of all beef sold in Denmark is imported (9). Imported meat is therefore an important potential source of human infection with drug-resistant Salmonella spp. We compared antimicrobial drug resistance of Salmonella isolates from both imported meat and meat produced within Denmark (domestic meat), as well as from outpatients with diarrhea.
Salmonella isolates from humans and meat were obtained from July 1998 through June 2002. Isolates from domestic poultry, pork, and beef were obtained through the national Salmonella control program (10), and isolates from imported poultry, pork, and beef were obtained from the Denmark import control and from the regional food control units. Human salmonellosis is a notifiable disease in Denmark, and all human Salmonella spp. isolates are collected at the Statens Serum Institute. The serovars included were restricted to S. Typhimurium, S. Hadar, S. Dublin, S. Saintpaul, S. Enteritidis, S. Virchow, and S. Newport because these were the serovars of which a sufficient number of isolates had been tested for antimicrobial drug susceptibility. Data on 4,081 Salmonella isolates from humans were included in the study.
Identification, serotyping, phage typing, and susceptibility testing were done as described (8,11,12). Susceptibility to the following antimicrobial agents was determined: ampicillin, ceftiofur, chloramphenicol, ciprofloxacin, co-amoxiclav, colistin, florphenicol, gentamicin, nalidixic acid, neomycin, streptomycin, sulfamethoxazole, tetracycline, and trimethoprim.
Statistical analyses were performed using S-PLUS version 6.2 (Insightful Corp., Seattle, WA, USA). The trend in the occurrence of resistant isolates over time, the occurrence of multidrug-resistant isolates over time, and the occurrence of nalidixic acid–resistant isolates were investigated by fitting a logistic regression model with origin (domestic/imported), time (year), product type (beef, pork, poultry), and all 2-way interactions as explanatory variables. The regression models were reduced by using a likelihood ratio test. Significance in all 2-by-2 tables (only tables with minimum 30 domestic and 30 imported samples) was tested by a Pearson χ2 test with continuity correction; if the number in any cell in the contingency table was <5, Fisher exact test was applied. All tests were done on a 5% significance level (p<0.05). No correction for multiple testing was done. An isolate was considered multidrug resistant if the isolate was resistant to >4 antimicrobial agents.
Salmonella spp. were isolated from 1,078 (11.8%) of 9,135 samples from imported poultry, pork, and beef and 2,985 (1.4%) of 213,214 samples from domestic poultry, pork, and beef. Among the isolates from domestic meat, the serovars S. Typhimurium, S. Infantis, and S. Derby were the 3 most frequently isolated; in imported meat, the 3 most frequently isolated serovars were S. Heidelberg, S. Typhimurium, and S. Hadar (Table 1). In isolates from domestic meat originating from pigs and poultry, S. Typhimurium was the most frequently isolated serovar; in beef isolates, S. Dublin was most common. Among isolates from imported meat, S. Typhimurium was the most frequently isolated serovar from pork and beef, while S. Heidelberg was the most frequently isolated serovar from poultry.
Table 1. Number and proportion of susceptible (S), resistant (R), multidrug-resistant (M), and nalidixic acid–resistant (Nal) Salmonella spp. isolates within different serotypes isolated from meat and humans, Denmark, July 1998–July 2002*.
| Serotype | Domestic meat |
Imported meat |
Humans† |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. tested | % |
No. tested | % |
No. tested | % |
||||||||||
| S | R | M | Nal | S | R | M | Nal | S | R | M | Nal | ||||
| Typhimurium‡ |
1,508 |
73 |
21 |
6 |
1 |
138 |
34 |
24 |
42§ |
9§ |
1,886 |
61 |
20 |
19 |
3 |
| Infantis‡ |
184 |
94 |
4 |
2 |
2 |
50 |
84 |
10 |
6 |
8§ |
|
|
|
|
|
| Derby‡ |
163 |
55 |
44 |
1 |
1 |
34 |
32 |
59 |
9§ |
3 |
|
|
|
|
|
| Heidelberg |
6 |
67 |
33 |
0 |
17 |
157 |
49 |
13 |
38 |
4 |
|
|
|
|
|
| Hadar‡ |
38 |
74 |
26 |
0 |
11 |
113 |
1 |
53§ |
46§ |
81§ |
189 |
26 |
71 |
3 |
58 |
| Enteritidis‡ |
91 |
90 |
9 |
1 |
4 |
50 |
84 |
16 |
0 |
10 |
1,706 |
92 |
7 |
0 |
4 |
| Indiana‡ |
94 |
95 |
4 |
1 |
0 |
40 |
45 |
43§ |
13§ |
3 |
|
|
|
|
|
| Newport |
2 |
0 |
100 |
0 |
100 |
78 |
28 |
51 |
21 |
60 |
59 |
88 |
7 |
5 |
5 |
| Kottbus |
26 |
81 |
19 |
0 |
15 |
49 |
6 |
90 |
4 |
92 |
|
|
|
|
|
| Dublin |
71 |
99 |
1 |
0 |
1 |
4 |
100 |
0 |
0 |
0 |
88 |
95 |
5 |
0 |
2 |
| Anatum |
50 |
100 |
0 |
0 |
0 |
12 |
75 |
8 |
17 |
0 |
|
|
|
|
|
| Saintpaul |
9 |
11 |
0 |
89 |
22 |
39 |
31 |
8 |
62 |
15 |
58 |
72 |
9 |
19 |
7 |
| Regent |
47 |
0 |
100 |
0 |
100 |
1 |
0 |
100 |
0 |
0 |
|
|
|
|
|
| Virchow |
3 |
100 |
0 |
0 |
0 |
39 |
44 |
36 |
21 |
49 |
95 |
35 |
56 |
9 |
62 |
| Bredeney |
3 |
100 |
0 |
0 |
0 |
38 |
34 |
0 |
66 |
11 |
|
|
|
|
|
| Other‡ |
690 |
71 |
24 |
5 |
5 |
256 |
56 |
24 |
20 |
17 |
|
|
|
|
|
| Total | 2,985 | 74 | 22 | 4 | 4 | 1,078 | 42 | 30 | 28 | 26 | 4,081 | 73 | 17 | 9 | 7 |
*Only serotypes with ≥40 isolates are included. †Only subsets of selected serovar are routinely tested for susceptibility to antimicrobial agents. ‡Indicates serotypes with >30 samples from Danish produced meat and >30 samples from imported meat, which were statistically tested. §Indicates clinical significance.
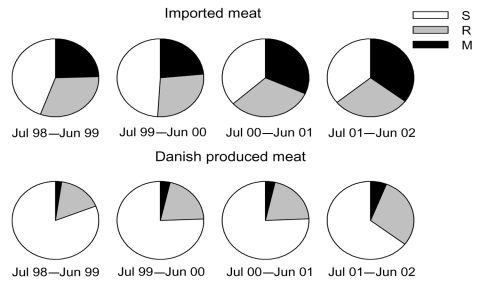
A significantly higher (χ2, p<0.001) proportion of the Salmonella spp. isolates from imported meat (58%) were resistant to ≥1 antimicrobial agents compared with isolates from domestic meat (26%) (Table 1). A significant difference (χ2, p<0.001) was also observed between the proportions of multidrug-resistant isolates from domestic (4%) compared with imported (28%) poultry, pork, and beef.
The regression results (Table 2) showed a significant increase in the proportion of resistant (p<0.001) and multidrug-resistant (p = 0.015) isolates over time and an increase in odds per year of 27% (corresponding to an increase in probability of 5% per year) and 14% (corresponding to an increase in probability of 3% per year), respectively (Figure 1). Furthermore, the probability for isolating a resistant and a multidrug-resistant isolate from imported meat compared with domestic meat was significant, with an odds ratio of ≈5. The probability of isolating a resistant isolate differed between product types; pork had the highest probability, followed by poultry and beef.
Table 2. Results from the reduced logistic regression models*.
| Variable |
OR (95% CI) |
Estimate (95% CI) |
SE (Est.) |
p value |
|---|---|---|---|---|
| Resistance vs. nonresistance | ||||
| Intercept |
0.164 (0.129 to 0.207) |
–1.81 (–2.05 to –1.57) |
0.121 |
|
| Origin |
5.08 (4.19 to 6.18) |
1.62 (1.43 to 1.82) |
0.0988 |
<0.00001 |
| Year |
1.27 (1.19 to 1.35) |
0.235 (0.174 to 0.297) |
0.0313 |
<0.00001 |
| Cattle vs. poultry |
0.400 (0.230 to 0.662) |
–0.917 (–1.47 to –0.413) |
0.268 |
<0.00001 |
| Pork vs poultry |
1.26 (1.06 to 1.51) |
0.233 (0.0553 to 0.414) |
0.0916 |
|
| Multidrug resistance vs. resistance | ||||
| Intercept μ |
0.141 (0.0976 to 0.201) |
–1.96 (–2.33 to –1.60) |
0.185 |
|
| Origin |
4.98 (3.87 to 6.44) |
1.61 (1.35 to 1.86) |
0.129 |
<0.00001 |
| Year |
1.14 (1.03 to 1.27) |
0.133 (0.0259 to 0.240) |
0.0547 |
0.0148 |
| Nalidixic acid resistance vs. non–nalidixic acid resistance | ||||
| Intercept |
0.0611 (0.0333 to 0.107) |
–2.80 (–3.40 to –2.24) |
0.296 |
|
| Origin |
6.54 (3.45 to 12.8) |
1.88 (1.24 to 2.55) |
0.334 |
|
| Year |
1.41 (1.18 to 1.69) |
0.342 (0.167 to 0.526) |
0.0914 |
|
| Origin and year |
0.732 (0.587 to 0.909) |
–0.311 (–0.532 to –0.0956) |
0.111 |
0.00448 |
| Cattle vs. poultry |
0.0404 (0.00229 to 0.182) |
–3.21 (–6.08 to –1.70) |
1.01 |
<0.00001 |
| Pork vs. poultry | 0.0668 (0.0425 to 0.101) | –2.71 (–3.16 to –2.29) | 0.220 | |
*OR, odds ratio; CI, confidence interval; SE, standard error; Est., estimated.
Figure 1.
Proportion of susceptible (S), resistant (R), and multidrug-resistant (M) Salmonella isolates from domestic and imported meat, Denmark, July 1998–July 2002.
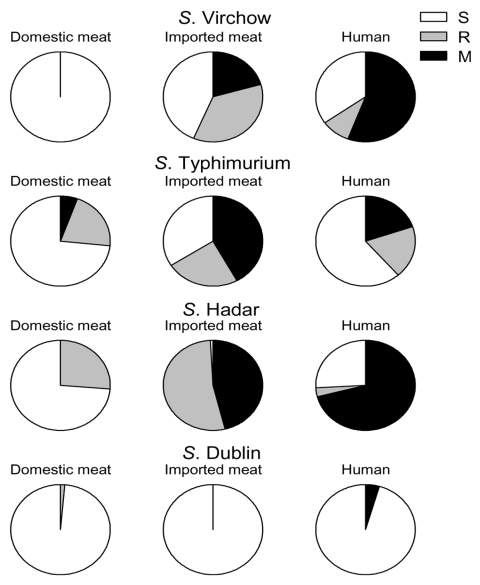
A high proportion of resistant and multidrug-resistant isolates was found among S. Hadar, S. Newport, S. Typhimurium, and S. Heidelberg in imported meat (Table 1). Among S. Typhimurium, antimicrobial drug resistance was particularly prominent in the phage types DT104, DT170, DT193, DT120, DT208, DT107, U302, and DT135 (Table 3). Multidrug-resistant DT104, DT120, and DT193 were found in both domestic and imported poultry, pork, and beef, whereas multidrug-resistant DT107, DT170, and DT208 were more common in domestic meat, and multidrug-resistant U302 was more common in imported meat (Table 3).
Table 3. Number and proportion of susceptible (S), resistant (R), and multidrug-resistant (M) meat isolates within Salmonella Typhimurium phage types, Denmark, July 1998–July 2002*.
| Serovar/phage type | Domestic meat |
Imported meat |
Total no. | ||||||
|---|---|---|---|---|---|---|---|---|---|
| M, % | R, % | S, % | Total no. | M, % | R, % | S, % | Total no. | ||
| All S. Typhimurium isolates |
6 |
21 |
73 |
1,508 |
42 |
24 |
34 |
138 |
1,646 |
| DT104 |
70 |
13 |
17 |
23 |
88 |
7 |
5 |
43 |
66 |
| DT170 |
3 |
68 |
29 |
97 |
0 |
0 |
0 |
0 |
97 |
| DT193 |
13 |
37 |
51 |
63 |
50 |
17 |
33 |
6 |
69 |
| DT120 |
16 |
29 |
55 |
38 |
57 |
43 |
0 |
7 |
45 |
| DT208 |
57 |
40 |
3 |
30 |
0 |
57 |
43 |
7 |
37 |
| DT107 |
5 |
55 |
41 |
22 |
0 |
0 |
0 |
0 |
22 |
| U302 |
0 |
33 |
67 |
6 |
38 |
31 |
31 |
13 |
19 |
| DT135 |
6 |
56 |
38 |
16 |
0 |
100 |
0 |
2 |
18 |
| Other S. Typhimurium |
5 |
21 |
74 |
1,213 |
21 |
32 |
47 |
60 |
1,273 |
|
Salmonella minus Typhimurium |
3 |
22 |
75 |
1,477 |
26 |
31 |
43 |
940 |
2,417 |
| Total | 4 | 22 | 74 | 2,985 | 28 | 30 | 42 | 1,078 | 4,063 |
*Not all S. Typhimurium isolates from humans were phage typed.
Resistance to nalidixic acid was higher among isolates from imported meat (26%) compared with isolates from domestic meat (4%) (χ2, p<0.001, odds ratio = 6.54, Table 3), with an increase over time in the proportion of domestic nalidixic acid–resistant isolates (p = 0.004, data not shown). Furthermore, the probability of isolating a nalidixic acid–resistant isolate differed between product types; poultry (domestic 14%, imported 30%) had the highest probability, followed by pork (domestic 1%, imported 3.2%) and beef (domestic 1%, imported 0%). Nalidixic acid resistance among Salmonella spp. from imported products was highest among S. Hadar, S. Newport, S. Kottbus, and S. Virchow (Table 1).
For S. Typhimurium, S. Hadar, and S. Virchow, the proportion of resistant and multidrug-resistant isolates was much higher among isolates from humans than among isolates from domestic meat (Table 1, Figure 2). For S. Dublin and S. Enteritidis, the proportion of resistant and multidrug-resistant isolate did not differ between the meat sources and the human isolates, whereas for S. Saintpaul and S. Newport the rates of resistance and multidrug resistance were lower for isolates from humans than from both domestic and imported meat.
Figure 2.
Proportion of susceptible (S), resistant (R), and multidrug-resistant (M) isolates among different Salmonella serotypes in isolates from domestic meat, imported meat, and humans, Denmark, July 1998–July 2002.
S. Hadar, S. Virchow, S. Newport, and S. Heidelberg were frequently found in imported products but rarely found in domestic products. Isolates that belong to these serovars are common causes of human salmonellosis in Denmark (13). Overall, a significantly higher number of resistant and multidrug-resistant Salmonella isolates were found among isolates from imported poultry, pork, and beef compared with domestic products. This finding implies that consumers in Denmark are more likely to be exposed to drug-resistant Salmonella spp. when eating imported compared with domestic meat. An increase in the occurrence of resistance over time was also observed among isolates from both domestic and imported meat; this is in agreement with observations worldwide (2–5). Antimicrobial agents might not be essential for treatment of gastroenteritis caused by Salmonella spp., but they are essential for treatment of patients with invasive infections. In particular, the frequent occurrence of resistance to quinolones is a matter of concern because these compounds are often used for first treatment of serious human infections, before the results of susceptibility testing are available.
International trade of food products is expected to increase in the future. Thus, endeavors to improve food safety must take into account the importance of resistant Salmonella spp. in imported food products and, through international agreements, limit contamination with antimicrobial drug–resistant Salmonella spp. at the primary production site.
Acknowledgement
We thank the Danish Veterinary and Food Administration for providing import control data.
This study was supported by the Danish Institute for Food and Veterinary Research.
Biography
Dr Skov is senior researcher in the Research Unit for Clinical Microbiology at the University of Southern Denmark. Her main research interests are the epidemiology and genotyping of foodborne Salmonella spp.
Footnotes
Suggested citation for this article: Skov MN, Andersen JS, Aabo S, Ethelberg S, Aarestrup FM, Sørensen AH, et al. Antimicrobial drug resistance of Salmonella isolates from meat and humans, Denmark. Emerg Infect Dis [serial on the Internet]. 2007 Apr [date cited]. Available from http://www.cdc.gov/eid/content/13/4/638.htm
References
- 1.Humphrey TJ. Public-health aspects of Salmonella infections. In: Wray C, Wray A, editors. Salmonella in domestic animals. Wallingford (England): CABI Publishing; 2000. [Google Scholar]
- 2.Cailhol J, Lailler R, Bouvet P, La Vieille S, Gauchard F, Sanders P, et al. Trends in antimicrobial resistance phenotypes in non-typhoid salmonellae from human and poultry origins in France. Epidemiol Infect. 2006;134:171–8. 10.1017/S0950268805004863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoge CW, Gambel JM, Srijan A, Pitarangsi C, Echeverria P. Trends in antibiotic resistance among diarrheal pathogens isolated in Thailand over 15 years. Clin Infect Dis. 1998;26:341–5. 10.1086/516303 [DOI] [PubMed] [Google Scholar]
- 4.Davis MA, Hancock DD, Besser TE, Rice DH, Gay JM, Gay C, et al. Changes in antimicrobial resistance among Salmonella enterica serovar Typhimurium isolates from humans and cattle in the Northwestern United States, 1982–1997. Emerg Infect Dis. 1999;5:802–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Duijkeren E, Wannet WJ, Houwers DJ, van Pelt W. Antimicrobial susceptibilities of salmonella strains isolated from humans, cattle, pigs, and chickens in the Netherlands from 1984 to 2001. J Clin Microbiol. 2003;41:3574–8. 10.1128/JCM.41.8.3574-3578.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helms M, Vastrup P, Gerner-Smidt P, Mølbak K. Excess mortality associated with antimicrobial drug–resistant Salmonella typhimurium. Emerg Infect Dis. 2002;8:490–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varma JK, Molbak K, Barrett TJ, Beebe JL, Jones TF, Rabatsky-Ehr T, et al. Antimicrobial-resistant nontyphoidal Salmonella is associated with excess bloodstream infections and hospitalizations. J Infect Dis. 2005;191:554–61. 10.1086/427263 [DOI] [PubMed] [Google Scholar]
- 8.Aarestrup FM, Bager F, Jensen NE, Madsen M, Meyling A, Wegener HC. Resistance to antimicrobial agents used for animal therapy in pathogenic-, zoonotic- and indicator bacteria isolated from different food animals in Denmark: a baseline study for the Danish Integrated Antimicrobial Resistance Monitoring Programme (DANMAP). APMIS. 1998;106:745–70. [DOI] [PubMed] [Google Scholar]
- 9.Alban L, Olsen A-M, Granly Koch A. Food safety risk assessment for imports of meat. In: Proceedings of the 18th International Pig Veterinary Society Congress, 27 June–1 July, 2004, Hamburg, Germany. Hamburg: The Society; 2004. p. 667. [Google Scholar]
- 10.Wegener HC, Hald T, Lo Fo Wong D, Madsen M, Korsgaard H, Bager F, et al. Salmonella control programs in Denmark. Emerg Infect Dis. 2003;9:774–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aarestrup FM, Lertworapreecha M, Evans MC, Bangtrakulnonth A, Chalermchaikit T, Hendriksen RS, et al. Antimicrobial susceptibility and occurrence of resistance genes among Salmonella enterica serovar Weltevreden from different countries. J Antimicrob Chemother. 2003;52:715–8. 10.1093/jac/dkg426 [DOI] [PubMed] [Google Scholar]
- 12.Baggesen DL, Sandvang D, Aarestrup FM. Characterization of Salmonella enterica serovar typhimurium DT104 isolated from Denmark and comparison with isolates from Europe and the United States. J Clin Microbiol. 2000;38:1581–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Annual reports on zoonoses in Denmark, 2000–2003. Copenhagen: Ministry of Agriculture, Food and Fisheries; 2001–2004. [cited 15 Feb 2007]. Available from http://www.dfvf.dk/Default.aspx?ID=9606