Synthetic biology is an emerging field with large potential for research and development, and future benefits for economy and society. The European Union (EU) has started measures to structure and develop the field, such as a high-level expert group. However, research activities are still scattered across Europe and scientific disciplines, and are concentrated in a relatively small number of working groups. Further integration is also hampered because there is no common understanding of synthetic biology, no clear description of its status quo and no comprehensive assessment of its potential. The situation is similar in the USA, although the field seems to be more advanced there in terms of activity and networking within the scientific community. Accordingly, to strengthen European competitiveness in synthetic biology, it is necessary to integrate the various activities and to draft a comprehensive strategy for the field.
Further integration is hampered because there is no common understanding of synthetic biology, no clear description of its status quo and no comprehensive assessment of its potential
We therefore developed a ‘roadmap' for synthetic biology in Europe, defining the essential steps to be taken in regard to regulation, funding, public-sector integration and scientific research. Our study, which was supported as part of the New and Emerging Science and Technology (NEST) programme (European Commission, Brussels, Belgium), involved an expert committee and the broader scientific community in an attempt to develop a common understanding of synthetic biology. This process was intended to generate awareness of the field among researchers, funding agencies and research organizations such as the National Academies of Science (Washington, DC, USA), the Max-Planck-Society (Munich, Germany) and the Centre National de la Recherche Scientifique (CNRS; Paris, France).
Without public support and understanding of research into synthetic biology, both funding and regulation are unlikely to support significant scientific advances
The mapping process consisted of three phases. The first phase involved coordinating roadmap committee workshops with representatives from ongoing synthetic biology projects and funding agencies in the UK, France, Spain, Germany and Italy. The second phase comprised fact-finding workshops with representatives from European research projects in synthetic biology, in which we discussed milestones and possible scientific and/or political measures that would represent or facilitate progress. Finally, once the two workshop series were completed and a draft roadmap was written, the third phase involved an online survey of the broader scientific community designed to involve as many persons with an interest in synthetic biology as possible. We initially invited 588 people from a diverse range of backgrounds, in terms of scientific discipline and institutional affiliation, to participate in the survey, and then asked each of them to invite colleagues who they felt would have an interest in contributing to our research. The survey was also promoted at two international conferences in 2007: the Synthetic Biology 3.0 conference held in Zurich (Switzerland) and the European Conference on Synthetic Biology held in Sant Feliu de Guixols (Spain). To attract the attention of a broad spectrum of the scientific community, the survey used the draft version of the roadmap to stimulate critical feedback on the timing and relevance of the measures and milestones that our committees had identified, and encouraged participants to identify additional topics.
In total, 37.6% of the people asked to complete the survey did so and we analysed 176 answers, giving an overall response rate of 29.7%. The answers to our survey revealed a distinct set of opinions within the community that we were able to access in regard to the appropriate ways in which to foster the growth of synthetic biology. In general, our respondents saw a clear need for various activities to be undertaken, such as interdisciplinary training, more funding, the development of clear guidelines and a code of conduct, and the regulation of intellectual property; however, they had divergent views about when each individual activity would become necessary.
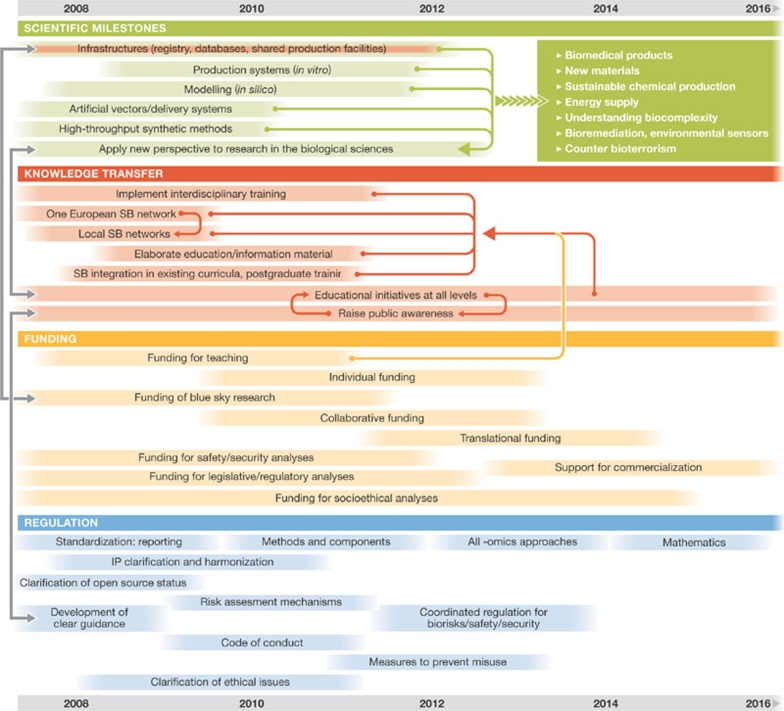
On the basis of the workshops and the results of the online survey, the final roadmap is structured into four fields of activities that represent different groups of actors and milestones: regulation, funding, knowledge transfer and scientific milestones (Fig 1). These fields are connected to each other: progress in one—scientific milestones, for example—can be achieved only with complementary advances or changes in the others and vice versa. Given the interconnectedness of the four fields, and the potential impact of synthetic biology on society, knowledge transfer will have an important role in its development in Europe. Without public support and understanding of research into synthetic biology, both funding and regulation are unlikely to support significant scientific advances. Knowledge-transfer activities will therefore include creating national networks of synthetic biologists to facilitate communication, ultimately linking each of these into a European-wide network, as well as the integration of synthetic biology into existing school and university curricula, partly through the development of educational materials. All of the experts whom we consulted emphasized the need for increased interdisciplinarity involving more extensive collaborations among natural scientists, engineers, information and communication experts, chemists and physicists, not only at the research and training levels, but also within research programmes and funding agencies. Moreover, integrated knowledge-transfer activities among all stakeholder groups, such as educational and informational activities, are important for raising public awareness and encouraging dialogue, as well as for addressing public fears or correcting misunderstandings about these new technologies in Europe.
Figure 1.
Roadmap of measures and milestones towards a successful European synthetic biology. IP, intellectual property; SB, synthetic biology.
Funding for synthetic biology projects was identified as one of the main bottlenecks to progress during the mapping process. Because synthetic biology is still in its infancy as a defined research field, funding activities should predominantly support so-called ‘blue sky' research or basic research projects. Too strong a focus on specific themes with defined milestones and goals could hinder explorative and creative research, and the exploitation of non-intended and/or non-predicable results. The challenge is to avoid lock-in effects on the one hand and to enable effective research on the other hand.
We also suggest the introduction of an additional two-step ‘evolutionary' funding scheme, which, although it is a more unconventional approach, might be more appropriate for this emerging field. In the initial step, more projects would be supported, based on their expected contribution to a specific outcome, in order to—speaking in evolutionary terms—support the creation of variety. This stage would not require strict selection procedures. After a defined period, all projects would be reviewed by an expert panel with respect to their actual contribution to the desired outcome, and a selection of projects for further funding would be made accordingly. Such a scheme would allow the pursuit and testing of unconventional and creative ideas at the initial stage, so all projects would have a chance to demonstrate their quality. Obviously, the crucial part of such a scheme is defining and applying the right selection criteria. A combination of established review criteria with measures of the actual, or even potential, contribution of the project to the defined outcome could be suitable, as it would allow the inclusion of projects with good performance, even if their progress as measured by ‘classical' criteria would not be wholly convincing.
...many researchers are worried that too much bureaucracy could slow down the development of synthetic biology
According to the first European Report on Science and Technology Indicators (European Commission, 1994), Europe has a leading role in terms of scientific excellence and the provision of highly skilled human resources. However, Europe lags behind when it comes to converting science-based findings into wealth-generating innovations. This assessment is underlined by data on scientific publications and patents showing that after the mid-1990s, the EU-15 became the largest producer of scientific literature worldwide in absolute terms as well as in world shares (36.4% compared with 31.4% for the USA in 2002), but lagged behind the USA and Japan in generating patents with high economic value and with respect to its share of patents in biotechnology. To avoid such a ‘European paradox', it is necessary to raise awareness of the commercial potential of synthetic biology now, while the field is still in an early stage of development, perhaps by funding and supporting joint projects between industry and academia. According to our roadmap, we predict that funding for translational research will be required by 2011, whereas further support for commercialization is likely to be required from 2013 onwards.
The expert committee outlined other measures in addition to funding research, such as a thorough analysis of biosafety, biosecurity, and legislative and regulatory concerns, as well as social and ethical studies and education. As the results of such studies are likely to influence the regulation of synthetic biological research, this work should begin immediately. The funding for such ‘context activities' ought to be allocated between 5% and 10% of total funding.
It is difficult to determine the actual amount of funding that is needed to nurture systems biology successfully; however, the funding provided during the infancy of other research fields can provide some clues. The development of systems biology in Germany, for example, was supported with €50 million over 5 years. At the European level, systems biology was supported with €25 million as a joint activity of six individual countries within the European Research Area (ERA)-Net scheme of the ERA for Systems Biology (ERASysBio) initiative (http://www.erasysbio.net). Experts have estimated that synthetic biology will require a minimum initial investment for research activities of between €10 million and €25 million over the next 2–3 years at the EU level. The actual amount needed is difficult to estimate as technologies such as DNA sequencing and synthesis are rapidly improving, which drives down costs.
From the point of view of industrial applications, it will be important to achieve a certain level of standardization of parts and devices, similarly to that in the electronics industry. However, researchers are concerned that early standardization could limit scientific research and development, and that more time is needed to explore the possibilities before committing to standards. The roadmap therefore proposes a stepwise standardization process over the next 10 years, beginning with standards for reporting—for instance, in the context of repositories and databases—followed by standards for methods and components in 2010. Moreover, the roadmap indicates that standardization should be developed by the research community itself, rather than by a top-down approach. Taking a broader perspective, it was also proposed that standards for all ‘-omics' approaches and the underlying mathematics should eventually be developed during the remaining years.
From a commercial point of view, the roadmap proposes that it will also be necessary to catalogue parts and devices in public repositories, as well as ensuring that the associated intellectual property is open source. As this will involve different national jurisdictions, it will require the discussion and harmonization of national and international patent laws, mainly in the USA and Europe. Generally, further progression of synthetic biology will strongly rely on formal and informal regulation and regulatory activities. In order to come up with coordinated regulations for biosafety and biosecurity, the roadmap proposes the development of clear guidance documents, risk-assessment mechanisms and a code of conduct for researchers within the next 4 years.
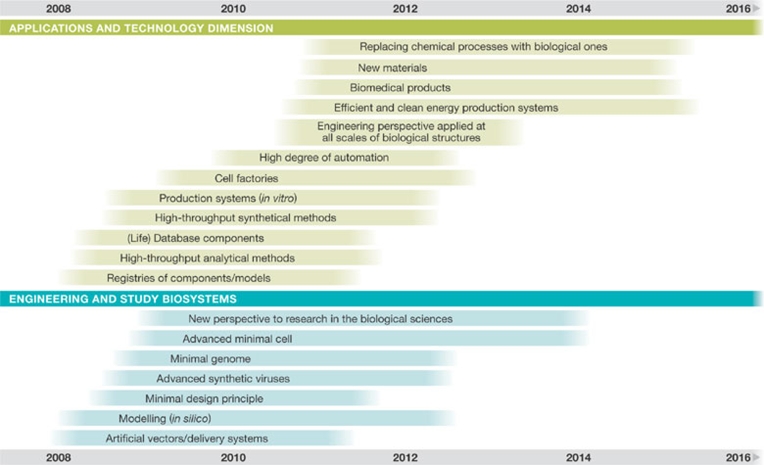
Our development of a roadmap for synthetic biology has revealed a clear chronology of scientific milestones in which basic technologies, such as high-throughput analytical and synthetic methods, are among the initial steps (Fig 2). Further down the road are activities that will increase automation and apply an engineering perspective to biological structures at all scales. Milestones in the field of engineering and biosystem studies will be the perfection of in silico modelling, the establishment of minimal design principles and the creation of a minimal genome. Notably, the expert committee emphasized that scientific advancements are moving targets, as research in the private sector could accelerate scientific progress; accordingly, the scientific roadmap should be updated regularly.
Figure 2.
Chronological order of scientific milestones as projected in the online survey of December 2007.
The establishment of appropriate infrastructures, such as a parts registry, databases and shared production facilities, at the European level will be necessary for the future development of synthetic biology, and to close the gap between scientific progress and knowledge transfer. A European consortium could establish and maintain the required facilities through an interdisciplinary network of competence that would link engineering, computer sciences, chemistry and life science. Most experts actually argued against a central institution dedicated to ‘pure' synthetic biology as the field is not yet mature enough. However, shared DNA analysis and synthesis centres, computational facilities and a validated registry could be run by European research institutes—such as the European Molecular Biology Laboratory in Heidelberg, Germany—and would provide a strong impetus for synthetic biology in Europe.
The process of drafting and refining the roadmap provided us with a distinct evaluation of milestones, requirements and supporting factors that could strengthen and improve the status, funding and progress of synthetic biology in Europe. Comments from the online survey indicated that our project was regarded as helpful for the creation of a strong synthetic biology research field in Europe. It also revealed that many researchers are worried that too much bureaucracy could slow down the development of synthetic biology. The roadmap demonstrates that synthetic biology can only progress if research is done in close connection with its social context, which will require continuous dialogue and interaction between the different stakeholders: natural and social scientists, engineers, representatives of industry, non-governmental organizations, the public, decision-makers and funding agencies. Online platforms, such as the home page of the Towards a European Strategy for Synthetic Biology (TESSY) project (Karlsruhe, Germany; http://www.tessy-europe.eu), the Synthetic Biology inventory (http://www.synthetic-biology.info), and the SYNBIOSAFE web page on safety and ethical aspects of synthetic biology (http://www.synbiosafe.eu) will help to disseminate information among scientists and to the public, and will also help with the establishment of research networks. Although national funding agencies are crucial to support this highly interdisciplinary field, international networking and collaboration between both funders and scientists across international borders will remain a strong driving principle in synthetic biology. It therefore seems that future activities at the European level, rather than a national level, will be a crucial factor for the success of synthetic biology.
References
- European Commission (1994) The European Report on Science and Technology Indicators 1994. Luxembourg: Office for Official Publications of the European Community