Abstract
This report provides a detailed description of the surgical repair of perineal hypospadias in a Boston terrier. A 2-year patient follow-up, including diagnostic data demonstrating urethral patency and resolution of recurrent urocystitis and urine skin scald are reported.
Résumé
Réparation d’un hypospadias périnéal important chez un Boston terrier en utilisant l’urétroplastie à lame gravée tubulaire. Ce rapport présente une description détaillée de la réparation chirurgicale d’un hypospadias périnéal chez un Boston terrier. Un rapport est présenté sur un suivi de 2 ans chez un patient qui inclut des données diagnostiques démontrant la perméabilité urétrale et la résolution des rechutes de cystites et de taches dermiques causées par l’urine.
(Traduit par Isabelle Vallières)
A 7-week-old, intact, male Boston terrier was presented to the referring veterinarian for evaluation of a penile and perineal defect present since birth. Physical examination revealed incomplete fusion of the entire urethra distal to the pelvic urethra (perineal hypospadias) and no preputial covering of the glans penis. The urethral opening was 8 mm ventral to the anus (Figure 1). An underdeveloped prepuce, ventrally deviated blunt penis, and retained left testicle were also noted (Figure 2). No other abnormalities were noted on physical examination except for moderate superficial pyoderma extending from the perineum to the ventral abdomen. Chlorhexidine gluconate shampoo (Chlorhexiderm Shampoo; DVM Pharmaceuticals, Miami, Florida, USA), 2 to 3 times weekly for 14 d and clinda-mycin hydrochloride (Clinsol; Virbac, Fort Worth, Texas, USA), 11 mg/kg body weight (BW), orally (PO), q24h for 10 d were initially used to treat the staphylococcal pyoderma diagnosed by skin culture.
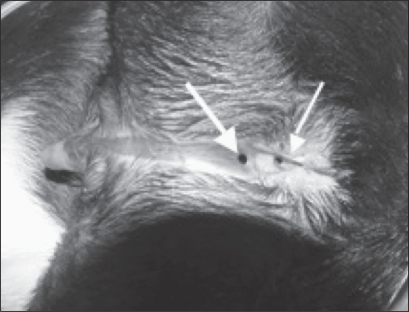
Figure 1.
Perineal hypospadias in a 7-month-old, intact male Boston terrier. Image shows dog in dorsal recumbancy, cranial is to the left; location of the urethral meatus (thick arrow) in relation to the anus (thin arrow).
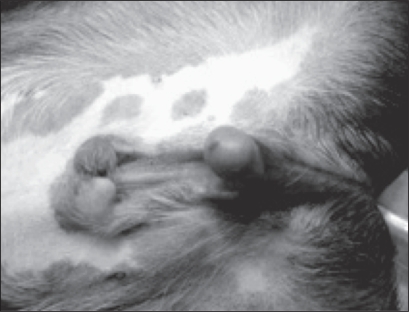
Figure 2.
Failure of fusion of the urethra, prepuce, and scrotum resulting in a nonfunctional, caudoventrally deviated and exposed penis with a nonfused prepuce.
Case description
At 7 months of age, the dog was referred to The Ohio State University for surgical correction of his congenital defects. The owner noted that the dog dribbled urine only during voluntary urination, and was unable to form a consistent stream of urine. Increased frequency and urgency of urination and misdirection of the urine stream causing urine scald dermatitis and penile tissue irritation were also observed by the owner.
On physical examination, mild local dermatitis and penile erythema and inflammation were noted. No additional abnormalities were observed at that time and both testicles had descended into the scrotum appropriately. A negative urine culture captured via cystocentesis was confirmed prior to surgery. Additionally, a complete blood (cell) count (CBC) and serum biochemical profile were evaluated preoperatively and were within normal limits. Surgical correction was pursued due to the increased risk of the dog acquiring chronic recurrent urinary tract infections, chronic urine scald dermatitis, and penile irritation. Urethral reconstruction, bilateral orchiectomy, and penile and preputial amputation were planned.
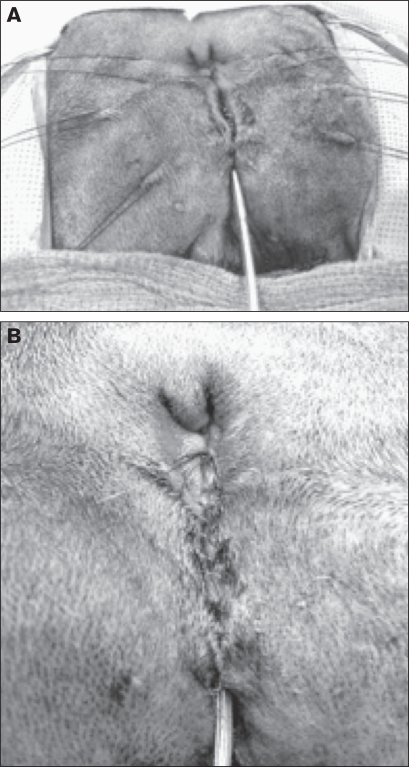
The patient was sedated with acepromazine maleate (Acepromazine Maleate; Vetco, St. Joseph, Missouri, USA), 0.1 mg/kg BW, intramuscularly (IM) and morphine sulfate (Morphine Sulfate; Baxter Healthcare, Deerfield, Illinois, USA), 0.125 mg/kg BW, IM and induced with propofol (Propofol; Abbott Animal Health, Chicago, Illinois, USA), 4.7 mg/kg BW, intravenously (IV). A purse-string suture was placed in the anus and the dog was prepared aseptically for surgery. The urethral reconstruction was performed first, with the dog in ventral recumbancy. An 8-French urinary catheter (Kendall Sovereign Feeding Tube and Urethral Catheter; Tyco Healthcare Group LP, Mansfield, Massachusetts, USA) was placed in the urethra to maintain a patent lumen during urethral repair (Figure 3). A full-thickness incision was made with a #15 scalpel blade at the urethral mucosa-dermal junction around the urethral opening just ventral to the anus and down the perineal urethra on both sides delineating the urethral plate. The incision extended to the level of the scrotum. Connective tissue surrounding the penile shaft in the perineal area was undermined bluntly using tenotomy scissors to free up regional skin for a tension-free closure. The incised urethral plate was then tubularized by using 6-0 polyglactin 910 (Vicryl; Ethicon, Somerville, New Jersey, USA) to suture together the urethral edges to form an inverted tube starting from just ventral to the anus and continuing to the scrotum (4 cm ventral to the anus) with a simple continuous pattern (Figure 4). Anticipating temporary urethral swelling and possible partial urethral obstruction from surgically induced inflammation, an 8-French urinary catheter was replaced with a 6-French Foley urinary catheter (Kendall Dover Silicone-Treated Latex Foley Catheter; Tyco Healthcare Group LP). The softer catheter was advanced into the urinary bladder and remained until the patient was discharged. The subcutaneous tissues were closed over the urethra with 5-0 poliglecaprone 25 (Monocryl; Ethicon) in a simple continuous pattern. The skin was closed with 4-0 polybutester (Novafil; Tyco Healthcare Group LP, Syneture, Norwalk, Connecticut, USA) in a simple continuous pattern (Figures 5A, 5B).
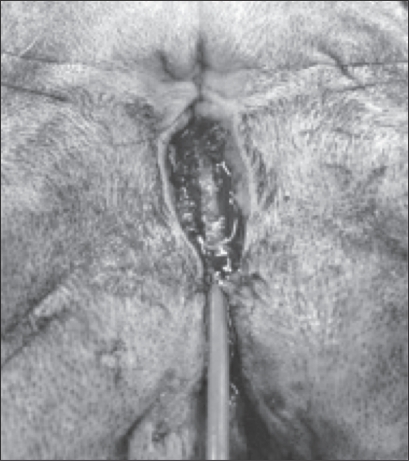
Figure 3.
Location of the urethral meatus immediately preoperatively (catheter is shown exiting the urethral meatus).
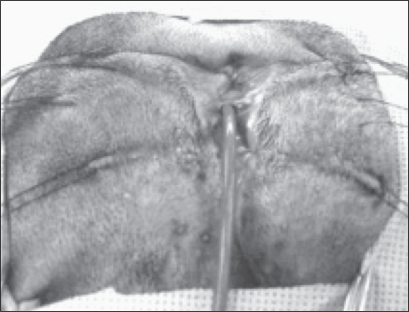
Figure 4.
Intraoperative image showing incised urethral edges apposed with suture creating an inverted urethral tube over a urinary catheter.
Figure 5.
Subcutaneous closure over the urethral reconstruction (A) and final perineal skin closure (B).
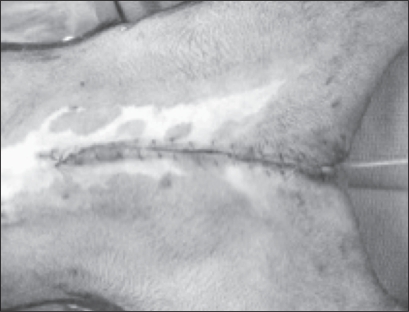
The patient was then positioned in dorsal recumbancy and aseptically prepared for penile amputation and castration. An elliptical skin incision was created around the penis and prepuce to the level of the scrotum. The penis was dissected from the body wall in a cranial to caudal direction. A closed castration was preformed routinely through the existing incision, and the penis was amputated at the proximal end of the os penis in a wedge fashion (1). The penis and prepuce were removed. Edges of the tunica albuginea at the amputation site were apposed with 3-0 poliglecaprone 25 using a simple interrupted pattern. The penile stump was sutured to the ventral abdominal fascia with one simple interrupted 3-0 poliglecaprone 25 suture to help hold the penile stump flush with the abdominal wall. Subcutaneous tissue and skin were closed routinely (Figure 6).
Figure 6.
View of the ventral abdomen showing final skin closure after preputial and penile amputation.
Postoperative analgesia included hydromorphone (Hydromorphone; Baxter Healthcare, Deerfield, Illinois, USA), 0.045 mg/kg BW, IV, plus 0.045 mg/kg BW, IM, once, then 0.05 mg/kg BW, IV, q4h or when necessary (PRN) for 2 d and meloxicam (Metacam; Boehringer Ingelheim Vetmedica, St. Joseph, Missouri, USA), 0.2 mg/kg BW, PO, q24h once, then 0.1 mg/kg BW, PO, q24h for 7 d. The urinary catheter was removed 96 h postoperatively. The patient was voluntarily urinating and had no signs of stranguria. Both testicles were submitted for histopathology and were reported as histologically normal.
Two weeks postoperatively, the owner estimated that the increased frequency and urgency of urination had improved by 50% and the dog still had no evidence of stranguria. However, similar to preoperatively, the urine stream did not always appear continuous during voluntary urination. The incision was healed and sutures were removed. At this time, a urine culture and sensitivity was performed. Results were consistent with an active mixed Escherichia coli and Enterococcus urocystitis, both bacteria showing susceptibility to amoxicillin/clavulanate (Clavamox; Pfizer Animal Health, Exton, Pennsylvania, USA). The dog was treated with this antibiotic for 14 d at 13.75 mg/kg BW, PO, q12h.
Four weeks postoperatively, the surgery site appeared healthy and the urethrostomy showed no evidence of stricture. A repeat urine culture showed resolution of the urinary tract infection. The owner reported improvement of all clinical signs but the dog still produced an inconsistent urine stream. There was no evidence of stranguria.
Two months postoperatively, the owner reported that the patient began straining to urinate and still could not produce a consistent stream of urine. Additionally, the patient had not been eating well for a few days and the owner witnessed the dog rubbing his perineum on the carpet. Upon examination, erythema and inflammation around the urethral stoma were seen; however, the dog’s urinary bladder was not considered obstructed since the bladder was small on abdominal palpation. Mupirocin ointment (Bactoderm Ointment; Pfizer Animal Health) that was prescribed at this visit alleviated the perineal dermatitis. No bacteria were isolated upon culturing urine collected via cystocentesis. The owner was instructed to monitor for signs of a possible urinary obstruction, including straining to urinate, vocalizing during urination, decreased volume of urine, and sudden onset vomiting or lethargy.
One year following surgery, the urine scald dermatitis had resolved and the skin around the surgery site and urethral stoma had healed. There was a decrease in the frequency and urgency of urination compared with that before surgery. A urine culture was performed and showed a mixed Escherichia coli and Enterococcus infection. The infection was treated again with amoxicillin/clavulanate, 13.75 mg/kg BW, PO, q12h for 14 d, on the basis of a susceptibility test. The owner noted that the dog still had a thin stream of urine and occasional inconsistent streams, no stranguria was noted, and the dog was active and healthy otherwise.
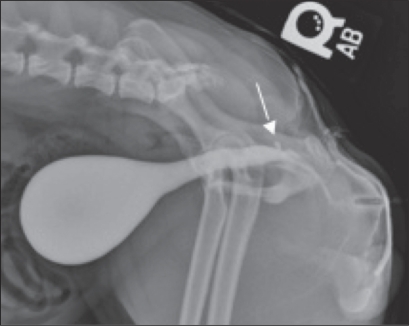
Two years after surgery, the owner reported that the dog still had a thin inconsistent urine stream; however, there was still no evidence of stranguria. In regards to the frequency and urgency of urination, the owner stated that these signs had not changed from the 1-year postoperative evaluation. A urine culture and positive contrast cystourethrogram were performed to ascertain the cause of the thin, inconsistent stream and recurrent urocystitis. Escherichia coli and Enterococcus were isolated and again; they were susceptible to amoxicillin/clavulanate. The dog was sedated with butorphanol tartrate (Torphaject; Butler Animal Health, Dublin, Ohio, USA), 15 mg/kg BW, IV and medetomidine (Domitor; Pfizer Animal Health), 15 μg/kg BW, IV to perform the cystourethrogram. A 6-French Foley urinary catheter was also used in this study. The cystourethrogram revealed a 2–3 mm ovoid structure projecting from the dorsal wall of the urethra in the area of the ischiatic arch. This defect appeared to be a small urethral diverticulum (Figure 7). No urethral strictures were present on this study and the repaired urethra was considered to be an adequate diameter for full evacuation of the bladder in a male small breed dog.
Figure 7.
Cystourethrogram showing a urethral diverticulum highlighted by arrow.
The dog was treated with a 4-week course of amoxicillin/clavulanate, 13.75 mg/kg BW, PO, q12h and the urine was re-cultured 3 d after the last dose of antibiotic. The urine culture had no growth. After this course of antibiotics, the dog had no evidence of pollakiuria or stranguria. He was also able to form a consistent urine stream. Also, during this follow-up appointment, the residual urine volume in the urinary bladder after a voluntary voiding was assessed by catheterizing the urinary bladder just after voiding. The purpose of this was to investigate if the recurrent urinary tract infections were initiated by the inability of the urinary bladder to be fully emptied after voiding. The dog was allowed to voluntarily urinate and then was immediately sedated with butorphanol tartrate, 15 mg/kg BW, IV and medetomidine, 15 μg/kg BW, IV. A 5-French polypropylene catheter (Polypropylene Catheter; Sherwood Medical, St. Louis, Missouri, USA) was placed in the urinary bladder and negative pressure was applied to the catheter with a 3 cc syringe, and 0.1 cc of urine was obtained. It was concluded that there was no residual urine left in the urinary bladder after voluntary urination. Therefore, the dog was able to empty his urinary bladder fully and the urinary tract infections were likely not due to incomplete bladder emptying and residual urine in the urinary bladder. The dog was given a 2-week course of amoxicillin/clavulanate, 13.75 mg/kg BW, PO, q12h, prophylactically after the catheterization.
Recommendations to the owner regarding the chronic urinary tract infections were to repeat a urine culture again 3–5 d post-antibiotic therapy, then every mo for 2 mo, and then every 2 mo thereafter for 1 y. If the urine culture is positive in less than 2 to 3 mo, surgical removal of the urethral diverticulum should be considered. If this is not an option, then long-term pulse dose or low dose antibiotic treatment may be indicated.
Discussion
Hypospadias is an uncommon developmental defect resulting in the failure of fusion of urogenital folds during fetal development, causing incomplete closure of the penile urethra (2). The urethral meatus then opens on the ventral surface of the penile shaft proximal to its normal location (3,4). Failure of ventral midline fusion of the prepuce and/or scrotum may be seen in these patients as well, similar to this reported case. The penis itself may be hypoplastic and nonfunctional. It may also have a ventral deviation due to the lack of support from the prepuce (5). Affected animals may also have other urogenital anomalies like cryptorchidism and an underdeveloped prepuce and penis (4,6). Other congenital or developmental abnormalities have been seen in affected animals, which include, but are not limited to, hermaphrodism or pseudohermaphrodism and renal aplasia (6,7). The dog in this report had no evidence of these associated conditions based on testicular biopsy following castration, although there was a history of delayed testicular descent. Clinical signs associated with hypospadias include urine scald dermatitis, misdirection of urine stream, pollakiuria, stranguria, and penile tissue irritation (4).
The etiology of hypospadias remains elusive, though several hypotheses have been presented in the human and animal literature (6,8). Many factors affect penile development, one of which is a defect in androgen metabolism or androgen receptors (8). However, this may only explain a small subset of cases implying that other factors are responsible for hypospadias (8). Other theories include the lack of urethral fusion due to the failure of epithelial to mesenchymal transformation of the urethra (8). Endocrine disrupters, such as estrogenic contaminants in the environment may be responsible for this maltransformation (8). Additionally, the fact that Boston terriers have a disproportionately higher prevalence of hypospadias suggests that genetics may be a contributing factor. Since these genetic predispositions have been identified, castration is recommended in all dogs with hypospadias (3,9).
Hypospadias is classified by its location along the urethra; examples of which are glandular, penile, scrotal, perineal, and anal. Treatment of these cases, whether medical or surgical, depends on the location and severity of the defect. Surgery is aimed at correcting the anatomical abnormality to minimize the risks of recurrent urinary tract infections, urethral stricture, urine scald dermatitis, or penile and urethral irritation. Mild cases of glandular or penile hypospadias usually do not require surgical correction and may be medically managed with frequent bathing and application of water-impermeable ointment around the urethral meatus and on the penile mucosa to prevent urine scald and desiccation (4). Minor defects that are not successfully managed medically have been surgically managed with urethral reconstruction and closure of the preputial meatus (4). Also, minor defects that do not increase the risk of urinary tract infections and have no clinical signs associated with them do not have to be treated. For more severe cases or cases that are scrotal, perineal, or anal, surgical correction is indicated if there is a history of recurrent urinary tract infection, urine scald dermatitis, misdirection of the urine stream, or chronic irritation of exposed tissue. Surgical correction for severe cases such as the dog reported herein may include urethral reconstruction, a urethrostomy and external genitalia excision (4).
The surgical treatment for this patient was designed to eliminate the source of inflammation and irritation from the exposed penis, help focus the urine stream to reduce urine scald and increase the length of the urethra (move the urethral meatus well away from the anus). In humans, the length of the urethra and the distance between the urethral meatus and the anus have been linked to acquiring urinary tract infections (10). In dogs, it is known that females, which have a shorter urethra and a urethral meatus closer to the anus than males, have a higher prevalence of urinary tract infections than males (11). Since the urethral meatus of this dog was 8 mm from the anus, and the functional urethral length was short, the risk of recurrent infection from this congenital defect was substantial. Excision of the penis and prepuce was performed to eliminate the persistent irritation and to allow for cosmetic repair. Castration was also performed due to the possible heritable nature of this condition.
We planned the surgical repair with the following surgical principles of successful urethral repair/reconstruction careful approximation of the incised urethral mucosal edges, no tension on the repair and gentle tissue handling to ensure adequate blood supply to the healing tissue (12). These same principles apply to human hypospadias repair as well (13); although in human medicine the indications and techniques for reconstruction of the penile urethra differ due to anatomical differences and the psychological consequences involved with penile amputation (14).
The complications of hypospadias repair are likely the same in human and veterinary medicine, but there is little information available in the veterinary literature. We found one published case study of a German shepherd dog that had a similar congenital perineal hypospadias (15). It was corrected in a similar manner to the dog in this study using an inverse tubed bipedicle flap (15). However, the German shepherd developed a urethrocutaneous fistula in addition to cystitis post-operatively (15). No cystourethrogram was performed post-operatively to identify stricture or urethral diverticula. The urethrocutaneous fistula may be related to surgical technique or to the fact that the dog had a urinary catheter in place for only 24 h after surgery. The fistula was resected along its borders and closed successfully (15). In human medicine, post-operative complications following hypospadias repair include urethral diverticula, urethral stricture, and urethrocutaneous fistulas as the most prevalent (16,17).
Human pediatric urologists have found that a well-vascularized second layer coverage over the primary urethroplasty decreases the incidence of fistula formation (16,17,19). The tissue most commonly used for the second layer closure is the dartos or tunica vaginalis (20). It has been documented that incorporation of the dartos into the closure overlying the neourethra prevents urethrocutaneous fistulas 90% of the time (17). Regardless of the surgical technique of hypospadias repair, providing a second layer closure with a de-epithelialized, well-vascularized pedicle flap has clearly been shown in humans to decrease substantially fistula formation and it should be a part of any hypospadias repair when possible (20).
The second layer closer may also be helpful in preventing urethral diverticula from forming. The reported incidence of diverticula formation after hypospadias repair is 0% to 21% (21). It was found that the most common cause of acquired urethral diverticula in children was previous hypospadias repair (21). The factors increasing the risk of diverticula include a proximal defect, use of poorly supported tissue for interposition grafting (for example, bladder mucosa or tubularized pedicle grafts), and the presence of a distal stricture resulting in relatively high outlet pressures (22). Strictures, however, were not found to be a common cause (21). Urethral diverticula may lead to stone formation, incomplete voiding, infection, post-void dribbling, hematuria, and other problems (21). Because a cystourethrogram was not performed pre-operatively we do not know if the urethral diverticulum was present before surgery or was acquired after the repair. However, its approximate location at the junction of the urethra and the neourethra suggests that it may have been acquired from surgery, due to difficulty transitioning from the pelvic urethra to the neourethra when suturing. In addition, there was evidence of urethral narrowing distal to the diverticulum on the cystourethrogram, supporting an acquired etiology. This narrowing could have caused higher outlet pressures that weakened the urethroplasty and lead to diverticulum formation.
This diverticulum may have been a factor contributing to the recurrent urinary tract infections in this dog. We were concerned that long-term treatment with antibiotics may eventually cause an antibiotic-resistant urinary tract infection (23). For this reason, a urethral diverticulectomy was suggested to the owner. However, the owner opted against surgical correction due to financial constraints, since there was no guarantee this would resolve the urinary tract infections.
A urethral diverticulum of the size found in this dog would not necessarily require surgical correction, if the dog remained asymptomatic. In humans, urethral diverticulae are not corrected provided there is no evidence of stone formation, incomplete voiding, infection and signs of, post-void dribbling, hematuria, incontinence, or any other functional abnormality (24). The inherent risks of urethral surgery must always be considered, as well, when discussing a urethral diverticulectomy. The most notable long-term complication is urethral stenosis (16).
The presence of recurrent urinary tract infections may have been associated with the close proximity of the urethral meatus to the anus (although not as close as before surgery) and the short length of the urethra itself. Another contributing factor could also have been that the duration of antibiotic therapies were perhaps too short and the infection was never fully cleared despite the negative urine cultures. In any event, the urinary tract infections were at least partially responsible for the thin inconsistent stream of urine and the pollakiuria. However, the role of the urethral diverticulum plays in this is still unknown.
The diameter of the neourethra was considered to be adequate for complete voiding in this dog. We found no excess residual urine in the bladder after voiding. However, there was evidence of urethral narrowing just distal to the pelvic urethra. This could explain the inconsistent thin urine stream after surgery. A urethral pressure profile could have helped determine if this narrowing was causing a partial urethral obstruction (25). Normal urethral diameters in male beagles have been studied, but this information may not apply to other breeds (26). Perhaps the neourethral diameter was just adequate for voiding, but additional narrowing from urethral spasm or inflammation from infection exacerbated the dog’s clinical signs.
To our knowledge, this case report documents the second successful repair of an extensive hypospadias in a dog. Urethral reconstruction is recommended for severe perineal hypospadias causing clinical signs that are unresponsive to medical therapy. The surgery is designed to move the urethral stoma to a more cranioventral location away from the anus. A bilateral orchiectomy is recommended in all dogs with hypospadias due the potential heritability of this condition. Excision of the nonfunctional exposed external genitalia to prevent irritation is also advised. Postoperative complications following urethral reconstruction include urethral fistula and diverticulum. Preparation of a well-vascularized layer of soft tissue over the urethral closure, and creation of an adequate urethral diameter help prevent these problems.
Acknowledgment
The author thanks Dr. Carlos Aragon. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office ( hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Hobson HP. Penis and prepuce. In: Bojrab MJ, editor. Current Techniques in Small Animal Surgery. 4th ed. Philadelphia: Williams and Wilkins; 1998. p. 528. [Google Scholar]
- 2.Boothe HW. Penis, prepuce, and scrotum. In: Slatter D, editor. Textbook of Small Animal Surgery. 3rd ed. Philadelphia: WB Saunders; 2003. pp. 1535–1536.pp. 1640–1641. [Google Scholar]
- 3.Hayes HM, Wilson GP. Hospital incidence of hypospadias in dogs in North America. Vet Rec. 1986;118:605–606. doi: 10.1136/vr.118.22.605. [DOI] [PubMed] [Google Scholar]
- 4.Hedlund CS. Surgery of the male reproductive tract. In: Fossum TW, editor. Small Animal Surgery. 2nd ed. St. Louis: Mosby; 2002. pp. 662–665. [Google Scholar]
- 5.Croshaw JE, Jr, Brodey RS. Failure of preputial closure in a dog. J Am Vet Med Assoc. 1960;136:450–452. [PubMed] [Google Scholar]
- 6.Hardy RM, Kustritz MV. Theriogenology question of the month. Hypospadias. J Am Vet Med Assoc. 2005;227:887–888. doi: 10.2460/javma.2005.227.887. [DOI] [PubMed] [Google Scholar]
- 7.McFarland LZ, Deniz E. Unilateral renal agenesis with ipsilateral cryptorchidism and perineal hypospadias in a dog. J Am Vet Med Assoc. 1961;139:1099–1100. [Google Scholar]
- 8.Baskin LS. Hypospadias and urethral development. J Urol. 2000;163:951–956. [PubMed] [Google Scholar]
- 9.Ader PL, Hobson HP. Hypospadias: A review of the veterinary literature and a report of three cases in the dog. J Am Anim Hops Assoc. 1978;14:721–727. [Google Scholar]
- 10.Lipsky BA. Urinary tract infections in men. Epidemiology, pathophysiology, diagnosis, and treatment. Ann Intern Med. 1989;110:138–150. doi: 10.7326/0003-4819-110-2-138. [DOI] [PubMed] [Google Scholar]
- 11.Kogika MM, Fortunato VAB, Mamizuka EM, et al. Etiologic study of urinary tract infection in dogs. Braz J Vet Res Anim Sci. 1995;32:31–36. [Google Scholar]
- 12.Cameron J. William Stewart Halstead: Our surgical heritage. Annals of Surgery. 1997;225:445–458. doi: 10.1097/00000658-199705000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cimador M, Castagnetti M, Grazia EDE. Urethrocutaneous fistula repair after hypospadias surgery. BJU Int. 2003;92:621–623. doi: 10.1046/j.1464-410x.2003.04437.x. [DOI] [PubMed] [Google Scholar]
- 14.de Jong TP. Hypospadias and congenital curvature of the penis in children and their surgical treatment. Ned Tijdschr Geneeskd. 2006;150:2072–2077. [PubMed] [Google Scholar]
- 15.Pavletic MM. Reconstruction of the urethra by use of an inverse tubed bipedicle flap in a dog with hypospadias. J Am Vet Med Assoc. 2007;231:71–73. doi: 10.2460/javma.231.1.71. [DOI] [PubMed] [Google Scholar]
- 16.Radojicic ZI, Perovic SV, Djordjevic MLJ, Vukadinovic VM, Djakovic N. ‘Pseudospongioplasty’ in the repair of a urethral diverticulum. BJU Int. 2004;94:126–130. doi: 10.1111/j.1464-410X.2003.04913.x. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi Y, Kojima Y, Nakane A, Kurokawa S, Tozawa K, Kohri K. Ventral based dartos flap for the prevention of the urethrocutaneous fistula urethroplasty. Int J Urol. 2007;14:725–728. doi: 10.1111/j.1442-2042.2007.01811.x. [DOI] [PubMed] [Google Scholar]
- 18.Borer JG, Retik AB. Current trends in hypospadias repair. Urol Clin North Am. 1999;26:15–37. doi: 10.1016/s0094-0143(99)80004-4. [DOI] [PubMed] [Google Scholar]
- 19.Ulman I, Erikci V, Avanoğlu A, Gökdemir A. The effect of suturing technique and material on complication rate following hypospadias repair. Eur J Pediatr Surg. 1997;7:156–157. doi: 10.1055/s-2008-1071079. [DOI] [PubMed] [Google Scholar]
- 20.Borer JG, Bauer SB, Peters CA, et al. Tubularized incised plate urethroplasty: Expanded use in primary and repeat surgery for hypospadias. J Urol. 2001;165:581–585. doi: 10.1097/00005392-200102000-00075. [DOI] [PubMed] [Google Scholar]
- 21.Synder CL, Evangelidis A, Snyder RP, Ostlie DJ, Gatti JM, Murphy JP. Management of urethral diverticulum complicating hypospadias repair. J Pediatr Urol. 2005;1:81–83. doi: 10.1016/j.jpurol.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Horton CE, Jr, Horton CE. Complications of hypospadias surgery. Clin Plast Surg. 1988;15:371–379. [PubMed] [Google Scholar]
- 23.Homer J, Ritchie-Dunham J, Rabino H, et al. Toward a dynamic theory of antibiotic resistance. System Dynamics Review. 2000;16:287–319. [Google Scholar]
- 24.Elbakry A. Complications of the preputial island flap-tube urethroplasty. BJU International. 1999;84:89–94. doi: 10.1046/j.1464-410x.1999.00097.x. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan MP, Comiter CV, Yalla SV. Micturitional urethral pressure profilometry. Urol Clin North Am. 1996;23:263–278. doi: 10.1016/s0094-0143(05)70310-4. [DOI] [PubMed] [Google Scholar]
- 26.Feeney DA, Johnston GR, Osborne CA, Tomlinson MJ. Dimensions of the prostatic and membranous urethra in normal male dogs during maximum distension retrograde urethrocystography. Vet Radiol. 1984;25:249–253. [Google Scholar]