Abstract
An 8-year-old, castrated male, miniature wire-haired dachshund was presented with a 4-month history of intermittent facial twitching (myoclonus). The myoclonic episodes progressed over a 16-month period. Generalized seizure activity was infrequent. Clinical examination revealed visually stimulated myoclonus. Response to therapy with antiepileptic drugs was equivocal. Genetic testing identified the dog as being affected by Lafora disease.
Résumé
Maladie de Lafora comme cause d’attaques myocloniques exacerbées chez un chien. Un Dachsund miniature à poils raides mâle castré âgé de 8 ans a été présenté avec une anamnèse de 4 mois de secousses musculaires faciales intermittentes (myoclonie). Les épisodes myocloniques ont progressé pendant une période de 16 mois. L’activité épileptique généralisée était peu fréquente. Un examen clinique a révélé la myoclonie à stimulation visuelle. La réponse à la thérapie à l’aide de médicaments antiépileptiques a été équivoque. Un dépistage génétique a identifié le chien comme étant atteint par la maladie de Lafora.
(Traduit par Isabelle Vallières)
In veterinary medicine, movement disorders can be categorized as being either hyperkinetic (excessive muscular activity) or hypokinetic (reduced muscle activity) (1). Myoclonus, a form of hyperkinesia, is characterized by sudden, rapid, and involuntary muscular twitches affecting a muscle or group of muscles. Positive myoclonus refers to twitching resulting from repeated bouts of muscle contraction while negative myoclonus refers to sudden and frequent relaxations in tonic muscle contraction (2). Sporadic myoclonus can be benign or a form of seizure disorder (3). Repetitive myoclonus can be classified according to when the myoclonus occurs (constant, action-related, postural, episodic, or resting) (3). In human medicine, however, myoclonus can be categorized according to clinical appearance, anatomic origin, or etiology (4). Anatomically, myoclonus is classified as being cortical, thalamic, brainstem (reticular), or spinal (4). With respect to etiologic classification, myoclonus is classified as being physiologic, essential, epileptic, or symptomatic (4). Herein, we describe the clinical features, and response to therapy, of a dog with progressive myoclonus and epilepsy due to Lafora disease, a condition rarely reported in North America.
Case description
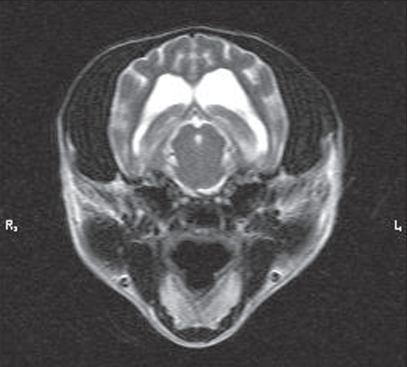
An 8-year-old, castrated male, miniature wire-haired dachshund was presented to the surgical service at Western Veterinary Specialist Centre (WVSC) with a 4-month history of progressively worsening facial twitching that was exacerbated by visual stimulation, in particular, sudden movements near the dog’s face. Distant, physical and neurological examinations were unremarkable. The animal had received all the recommended vaccines. Results of complete blood (cell) count (CBC), serum biochemistry, urinalysis, blood pressure, and electrocardiogram performed by the referring veterinarian 3 mo previously were unremarkable. The owners declined repeating any of these tests. The owners agreed to magnetic resonance (MR) imaging but declined cerebrospinal fluid collection and analysis. The MR imaging was performed using a 1.5 Tesla Siemens Symphony MR scanner. The sequences performed, in various directional planes, included T1-weighted with and without gadolinium enhancement, T2-weighted, fluid attenuated inversion recovery (FLAIR), and diffusion-weighted imaging including apparent diffusion coefficient (ADC) mapping. Magnetic resonance imaging revealed moderately dilated lateral ventricles (Figure 1). There was no evidence of any neoplastic, cerebrovascular, or inflammatory processes affecting the brain. A differential diagnosis of probable symptomatic epilepsy was made. The dog was started on potassium bromide (KBr) therapy (loading dose — 100 mg/kg PO, SID for 5 d; then maintained at 22 mg/kg PO, SID) for a presumptive diagnosis of a focal seizure disorder of undetermined origin.
Figure 1.
Cross section T2-weighted MR image of the dog’s brain. Note the dilatation of the lateral ventricles.
Nearly 6 wk after beginning KBr therapy, the dog was presented for re-evaluation to the internal medicine service at the WVSC. The presenting complaint was continued myoclonic events. These events occurred randomly and seemed to be most prominent when the patient was startled. The previously prescribed KBr therapy had been discontinued at the discretion of the owners, who felt that there was no notable improvement. Potassium bromide levels had not been assessed following the loading doses. Complete physical, neurologic, and fundic examination revealed no abnormalities. The myoclonic episodes reported by the owner were not observed at the time of initial evaluation and instructions to obtain a video of the described events were recommended. The KBr was reinstituted (22 mg/kg PO, SID). Video obtained from the dog’s owners revealed subtle intermittent myoclonus of the dog at rest.
Five months following instituting KBr therapy for the 2nd time, the patient was again presented to WVSC, the owners reported worsening symptoms. In addition to the myoclonic episodes, the owners reported 1 generalized seizure characterized by whole body twitching that lasted 20 to 30 s with hypersalivation and an associated postictal phase characterized by disorientation and ataxia. Physical examination and neurological examination were unchanged; however, myoclonic episodes originally described by the owners were now evident. These episodes involved rapid myoclonic movements involving the head, neck, and forelimbs. Complete blood (cell) count, serum biochemistry, and urinalysis were performed in light of the onset of generalized seizure activity. There was a mild elevation in serum alanine transferase (ALT) (306 IU/L; reference range: 0–113 IU/L), but blood work was otherwise unremarkable. The KBr levels were measured and found to be 13.8 mmol/L (therapeutic range for KBr monotherapy: 16.8–25.2 mmol/L) (5). Although KBr was not in the range for monotherapy for canine epileptic disorders, the dosage was not increased; adjunctive therapy with gabapentin (50 mg/kg PO, TID) was instituted instead. Therapy with phenobarbitol was not instituted, as seizures did not appear to be a predominant component of the clinical picture and because of the newly detected hepatic enzyme changes.
One month following gabapentin therapy, the owners reported an equivocal response in the frequency and severity of the dog’s resting myoclonus to anticonvulsant drug therapy. Importantly, the types of stimuli that evoked these myoclonic attacks were increased. Specifically, bright light, television, lightning, and stroboscopic light (for example, light and shade alternation when driving alongside a row of trees with sunlight peering through) resulted in stimulation of the myoclonic attacks. No changes in physical or neurological examinations were noted. Re-evaluation of serum biochemistry in the subsequent 5 mo did not reveal any additional hepatic enzyme changes, excluding the consistently mild elevation in serum ALT (range: 149–207 IU/L). Therapy with gabapentin was discontinued due to equivocal clinical improvement and because the owners were having difficulties administering the drug. Consultation with the neurology/electrodiagnostic service at WVSC was agreed upon by the owners.
Potential neuroanatomic lesion localizations that were considered for the dog’s myoclonus included brain, spinal cord, or muscle. Given the dog’s signalment, clinical history, the clinical examination findings, especially the lack of any apparent muscular hypertrophy or atrophy, and lack of clinical laboratory evidence of any pre-existing systemic condition, disease processes affecting muscle (such as Cushing’s-related myotonia) were considered highly unlikely. Instead, given the signalment, history, clinical and laboratory findings, the progressive nature of this condition, the age of onset, and the generalized seizures, a central neurodegenerative disease process was suspected, most likely affecting the forebrain (storage diseases or demyelinating diseases). In particular, Lafora disease as the underlying etiology for the myoclonic events was suspected, though conditions such as neuronal ceroid lipofuscinosis have been reported in other breeds of dogs, and were considered. The patient returned for laboratory tests to confirm this suspected disease.
Neurologic and complete ophthalmic examinations were unremarkable aside from visually stimulated myoclonus of the musculature of the face, head, and neck during completion of the menace response. Blood was submitted to the Hospital for Sick Children for testing of the EPM2B mutation previously reported in miniature wire-haired dachshunds and basset hounds affected with Lafora disease, as this test is not commercially available (6). Results of the polymerase chain reaction (PCR) assay revealed that this dog was homozygous for the mutation responsible for Lafora disease. Electrodiagnostic testing was used to help identify any involvement of central neural structures. Electroretinography was not performed as it was deemed potentially unsafe to stimulate the dog with stroboscopic light. Brainstem auditory evoked response (BAER) testing was performed to evaluate involvement of brainstem auditory pathways, although clinical signs of hearing loss were not present. Given our high suspicion of a neurodegenerative or demyelinating disease process, BAER testing, which is relatively non-invasive was performed using a Cadwell Sierra Wedge II electrodiagnostic unit. Two thousand 100 μs multi-tonal alternating clicks were delivered to each ear at 21.1 Hz at 80 dB nHL using insert ear phones to obtain the BAER. Each ear was tested separately with a masking noise of 30 dB nHL being delivered to the ear not being tested. Performing the BAER examination did not stimulate myoclonus. The BAER examination revealed that all 5 major waveforms were present. Latencies for peaks I–V were within previously reported reference ranges. The owners were counselled regarding the fact that there is no cure or proven therapy for dogs with Lafora disease and that the dog’s condition would inevitably worsen over time. Given preliminary evidence suggesting that the myoclonus may improve with feeding a commercially available diet (Hills b/d) high in antioxidants, it was recommended to the owners that they institute an exclusive diet of Hills b/d (7).
Discussion
Physiological myoclonus describes those myoclonic episodes that occur naturally in unaffected individuals (for example, hiccup — diaphragmatic myoclonus; possibly reverse sneezing in dogs). Essential myoclonus, although described as an idiopathic form of myoclonus in humans (4), has not been described in domesticated animals. Epileptic myoclonus is that form of myoclonus where epilepsy is the primary disorder (4). Finally, and most commonly reported in the human and veterinary literature, symptomatic myoclonus includes those disease entities in which myoclonus occurs as a feature of some primary underlying disorder (4). Symptomatic myoclonus has been reported as a clinical feature of a variety of diseases in cats (8–10), dogs (11–18), cattle (19–23), horses (24,25), pigs (26,27), and birds (28). Symptomatic myoclonus can result from infectious diseases such as distemper and pseudorabies, various idiopathic meningoencephalomyelitides, or may be the result of drug reactions. Inherited conditions causing myoclonus include inherited myoclonus of polled Hereford cattle, familial reflex myoclonus in Labrador retrievers, and various cellular storage diseases, including Lafora disease. It should be noted that epileptic and symptomatic myoclonus are not mutually exclusive of each other. That is, myoclonic epilepsies are a form of symptomatic myoclonus.
In humans, 6 main diseases make up the inherited progressive myoclonic epilepsies. These include neuronal ceroid lipofuscinoses, sialidosis, Unverridcht-Lundbord disease, myoclonic epilepsy with ragged red fibers, dentatorubral-pallidoluysian atrophy, and Lafora disease (29,30). Neuronal ceroid lipofuscinosis (31) and Lafora disease (6,32) have so far been identified as causes of progressive myoclonic epilepsy in dogs. Lafora disease has been uncommonly reported in a variety of breeds; however, it has been most notably reported as an inherited trait in the beagle, basset hound, and miniature wire-haired dachshund (6).
Lafora disease in humans is caused by a mutation of EPM2A or EPM2B genes (33), that code for the proteins laforin and malin, respectively. Laforin is a glycogen phosphatase while malin is an E3 ubiquitin ligase (34). The function of these proteins is poorly understood. Recently, however, it has been shown that laforin deficiency leads to elevated phosphorylation of glycogen which is thought to result in poorly branched glycogen-like polysaccharides (polyglucosan) intracellularly (34) throughout a wide variety of tissue including nervous, skin, heart, muscle, spleen, lymph nodes, retina, and liver (35–37). These intracellular inclusions are periodic acid-Schiff (PAS) positive and are known as Lafora bodies, hence the name Lafora disease.
Lafora disease in the miniature wire-haired dachshund is clinically recognized between 6 and 9 years of age and is caused by an expanded repeat mutation in the EPM2B gene (6). Affected animals appear physically and neurologically normal except for progressive myoclonus that can be triggered by audio or, in our instance, visual stimulation (6). Atonic attacks resulting in the animal collapsing on the ground and generalized seizures are reported (6). Interestingly, visually stimulated seizures with or without myoclonus, but not associated with Lafora disease, are reported with increasing frequency in humans (38). These photosensitive epilepsies are known as video game epilepsies, Pokemon shock, photic and pattern-induced seizures (39). In these epilepsies, the most common precipitating factor is strobic light conditions or specific alternating patterns of different colors (38). Photosensitive seizures can be controlled by antiepileptic drugs or avoidance of the photic stimulation. In advancing stages of Lafora disease, however, animals may become ataxic and/or blind, and increased frequency of seizures refractory to antiepileptic drugs will be noted (6,32).
Although Lafora bodies may occur in the retina, specifically within the retinal ganglion cells (37), we failed to identify any abnormalities after performing a complete ophthalmic examination. It may have been interesting to complete an electroretinogram, but we did not believe it was in the best interest of the patient given his photic myoclonic epileptic attacks.
Similar to the dog in the current report, MR imaging may reveal generalized ventricular dilatation, and cortical atrophy may be present in some cases (6). Whether these imaging findings represent features unique to the disease or are simply a manifestation associated with aging in the dog remain unknown (40). Imaging studies of age- and breed-matched controls have not been compared to dogs with Lafora disease.
Results from electrodiagnostic testing of dogs with Lafora disease have been limited to electroencephalography (EEG) and electromyography (EMG) (6,32). The EEG from dogs with Lafora disease is characterized by bilateral synchronous polyspike wave paroxysms and erratic myoclonus without EEG correlation (32). Electromyograms from 1 dog with Lafora disease revealed spontaneous muscular discharges, including fibrillation potentials and positive sharp waves, indicative of neurogenic or primary muscle disease (41). Given that Lafora disease represents a primarily gray matter (neuronal) disease, and that BAER recordings have not been reported in cases of canine Lafora disease, we hypothesized that abnormalities may be detected by evaluating neuronal function using BAER testing. Each of the waveforms represents different regions of the neural hearing pathway (for review see reference 42). Waves I–II represent activity in the vestibulocochlear nerve, wave III is generated by 2nd order neurons in and around the cochlear nucleus, wave IV is generated possibly by 2nd and 3rd order neurons found within the superior olivary complex and from neurons in the cochlear and lateral lemniscus nuclei, and wave V is thought to be generated by lateral lemniscal fibers synapsing in the caudal colliculus. We did not find any evidence of neuronal involvement of brainstem pathways involved in hearing, consistent with what has been reported in humans with Lafora disease (43).
There is no known curative treatment for Lafora disease. In humans with Lafora disease, clinical symptoms begin in the teenage years and progress to intractable myoclonic and cortical seizures, hallucinations, and dementia (44). The disease is invariably fatal with affected people dying within 10 y of developing clinical signs (45). In dogs, many of the affected animals are euthanized when their quality of life is deemed unacceptable (6). Although gabapentin has synergistic anti-epileptic effects in combination with other anti-epileptic drugs in some dogs with refractory epilepsy (46), this adjunctive therapeutic had no additional benefit in controlling myoclonic attacks and, in fact, the clinical signs progressed.
Given the pathophysiology of Lafora disease, dietary management has been considered. Eating foods that are ketogenic (low in carbohydrates) may slow the progression of Lafora disease, although large prospective studies are required to support this hypothesis (47). Preliminary evidence suggests that a diet high in anti-oxidants will also slow the progression of clinical signs (7). As Hills b/d has been shown to have no detrimental effects on health and to halt or even improve cognitive decline associated with aging (48), and it may be useful at halting the progression of clinical signs associated with Lafora disease in dogs (7), we prescribed this diet as final adjunctive therapy for the dog presented herein.
The present case highlights that Lafora disease should be considered when presented with a dog with progressive visually stimulated myoclonus and epilepsy. This case also highlights that the genetic mutation for Lafora disease exists within the miniature wire-haired dachshund population in Canada. It is important that a detailed description of the clinical signs be obtained from the animal’s owners in the early stages of the disease. Obtaining video recordings of the myoclonus and seizures is important. Although therapy for Lafora disease is purely palliative, it is possible to identify carrier and affected animals and to thereby eliminate the disease from the breeding population. Genetic testing also allows identification of individuals with the disease, so that creation of an experimental breeding colony can be developed to evaluate various therapies for use in dogs and humans.
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office ( hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Podell M. Tremor, fasciculations, and movement disorders. Vet Clin North Am Small Anim Pract. 2004;34:1435–1452. doi: 10.1016/j.cvsm.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 2.Faught E. Clinical presentations and phenomenology of myoclonus. Epilepsia. 2003;44 (Suppl 11):7–12. doi: 10.1046/j.1528-1157.44.s11.3.x. [DOI] [PubMed] [Google Scholar]
- 3.de Lahunta A, Glass E. Upper motor neuron. In: de Lahunta A, Glass E, editors. Veterinary Neuroanatomy and Clinical Neurology. St Louis: Saunders/Elsevier; 2009. pp. 192–220. [Google Scholar]
- 4.Fahn S. Overview, history, and classification of myoclonus. Adv Neurol. 2002;89:13–17. [PubMed] [Google Scholar]
- 5.Trepanier LA, Van SA, Schwark WS, Carrillo J. Therapeutic serum drug concentrations in epileptic dogs treated with potassium bromide alone or in combination with other anticonvulsants: 122 cases (1992–1996) J Am Vet Med Assoc. 1998;213:1449–1453. [PubMed] [Google Scholar]
- 6.Lohi H, Young EJ, Fitzmaurice SN, et al. Expanded repeat in canine epilepsy. Science. 2005;307:81. doi: 10.1126/science.1102832. [DOI] [PubMed] [Google Scholar]
- 7.Rusbridge C, Fitzmaurice SN, Lohi H, Young EJ, Minassian BA. Treatment of Lafora disease (inherited myoclonic epilepsy) in dogs. J Vet Intern Med. 2005;19:289. [Google Scholar]
- 8.Benitah N, de Lorimier LP, Gaspar M, Kitchell BE. Chlorambucil-induced myoclonus in a cat with lymphoma. J Am Anim Hosp Assoc. 2003;39:283–287. doi: 10.5326/0390283. [DOI] [PubMed] [Google Scholar]
- 9.Weissenbock H, Rossel C. Neuronal ceroid-lipofuscinosis in a domestic cat: Clinical, morphological and immunohistochemical findings. J Comp Pathol. 1997;117:17–24. doi: 10.1016/s0021-9975(97)80063-1. [DOI] [PubMed] [Google Scholar]
- 10.Blythe LL, Schmitz JA, Roelke M, Skinner S. Chronic encephalomyelitis caused by canine distemper virus in a Bengal tiger. J Am Vet Med Assoc. 1983;183:1159–1162. [PubMed] [Google Scholar]
- 11.Roux FA, Deschamps JY. Inadvertent intrathecal administration of ionic contrast medium to a dog. Vet Radiol Ultrasound. 2007;48:414–417. doi: 10.1111/j.1740-8261.2007.00269.x. [DOI] [PubMed] [Google Scholar]
- 12.da Cunha AF, Carter JE, Grafinger M, et al. Intrathecal morphine overdose in a dog. J Am Vet Med Assoc. 2007;230:1665–1668. doi: 10.2460/javma.230.11.1665. [DOI] [PubMed] [Google Scholar]
- 13.Kona-Boun JJ, Pibarot P, Quesnel A. Myoclonus and urinary retention following subarachnoid morphine injection in a dog. Vet Anaesth Analg. 2003;30:257–264. doi: 10.1046/j.1467-2995.2003.00076.x. [DOI] [PubMed] [Google Scholar]
- 14.Koutinas AF, Polizopoulou ZS, Baumgaertner W, Lekkas S, Kontos V. Relation of clinical signs to pathological changes in 19 cases of canine distemper encephalomyelitis. J Comp Pathol. 2002;126:47–56. doi: 10.1053/jcpa.2001.0521. [DOI] [PubMed] [Google Scholar]
- 15.Tipold A. Diagnosis of inflammatory and infectious diseases of the central nervous system in dogs: A retrospective study. J Vet Intern Med. 1995;9:304–314. doi: 10.1111/j.1939-1676.1995.tb01089.x. [DOI] [PubMed] [Google Scholar]
- 16.Inada S. Electromyographic analysis of canine distemper myoclonus. Electromyogr Clin Neurophysiol. 1989;29:323–331. [PubMed] [Google Scholar]
- 17.Fox JG, Averill DR, Hallett M, Schunk K. Familial reflex myoclonus in Labrador retrievers. Am J Vet Res. 1984;45:2367–2370. [PubMed] [Google Scholar]
- 18.Richards RB, Kakulas BA. Spongiform leucoencephalopathy associated with congenital myoclonia syndrome in the dog. J Comp Pathol. 1978;88:317–320. doi: 10.1016/0021-9975(78)90034-8. [DOI] [PubMed] [Google Scholar]
- 19.Heal PJ, Dennis JA, Windsor PA, Pierce KD, Schofield PA. Genotyping cattle for inherited congenital myoclonus and maple syrup urine disease. Aust Vet J. 2002;80:695–697. doi: 10.1111/j.1751-0813.2002.tb11301.x. [DOI] [PubMed] [Google Scholar]
- 20.Gundlach AL. Disorder of the inhibitory glycine receptor: Inherited myoclonus in Poll Hereford calves. FASEB J. 1990;4:2761–2766. doi: 10.1096/fasebj.4.10.2165010. [DOI] [PubMed] [Google Scholar]
- 21.Scott PR, Aldridge BM, Clarke M, Will R. Bovine spongiform encephalopathy in a cow in the United Kingdom. J Am Vet Med Assoc. 1989;195:1745–1747. [PubMed] [Google Scholar]
- 22.Duffell SJ, Harper PA, Healy PJ, Dennis JA. Congenital hypomyelinogenesis of Hereford calves. Vet Rec. 1988;123:423–424. doi: 10.1136/vr.123.16.423. [DOI] [PubMed] [Google Scholar]
- 23.Harper PA, Healy PJ, Dennis JA. Inherited congenital myoclonus of polled Hereford calves (so-called neuraxial oedema): A clinical, pathological and biochemical study. Vet Rec. 1986;119:59–62. doi: 10.1136/vr.119.3.59. [DOI] [PubMed] [Google Scholar]
- 24.Heath SE, Peter AT, Janovitz EB, Selvakumar R, Sandusky GE. Ependymoma of the neurohypophysis and hypernatremia in a horse. J Am Vet Med Assoc. 1995;207:738–741. [PubMed] [Google Scholar]
- 25.Gundlach AL, Kortz G, Burazin TC, Madigan J, Higgins RJ. Deficit of inhibitory glycine receptors in spinal cord from Peruvian Pasos: Evidence for an equine form of inherited myoclonus. Brain Res. 1993;628:263–270. doi: 10.1016/0006-8993(93)90963-n. [DOI] [PubMed] [Google Scholar]
- 26.Mare CJ, Kluge JP. Pseudorabies virus and myoclonia congenita in pigs. J Am Vet Med Assoc. 1974;164:309–310. [PubMed] [Google Scholar]
- 27.Berry JF, Fletcher TF, Bovis M. Phospholipid class and fatty acid composition of developing spinal cord in normal pigs and in congenital tremor (Myoclonia congenita) Lipids. 1969;4:623–627. doi: 10.1007/BF02531053. [DOI] [PubMed] [Google Scholar]
- 28.Smith EL, Evans JE, Parraga CA. Myoclonus induced by cathode ray tube screens and low-frequency lighting in the European starling (Sturnus vulgaris) Vet Rec. 2005;157:148–150. doi: 10.1136/vr.157.5.148. [DOI] [PubMed] [Google Scholar]
- 29.Minassian BA. Progressive myoclonus epilepsy with polyglucosan bodies: Lafora disease. Adv Neurol. 2002;89:199–210. [PubMed] [Google Scholar]
- 30.Shahwan A, Farrell M, Delanty N. Progressive myoclonic epilepsies: A review of genetic and therapeutic aspects. Lancet Neurol. 2005;4:239–248. doi: 10.1016/S1474-4422(05)70043-0. [DOI] [PubMed] [Google Scholar]
- 31.Awano T, Katz ML, O’Brien DP, et al. A frame shift mutation in canine TPP1 (the ortholog of human CLN2) in a juvenile Dachshund with neuronal ceroid lipofuscinosis. Mol Genet Metab. 2006;89:254–260. doi: 10.1016/j.ymgme.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 32.Gredal H, Berendt M, Leifsson PS. Progressive myoclonus epilepsy in a beagle. J Small Anim Pract. 2003;44:511–514. doi: 10.1111/j.1748-5827.2003.tb00113.x. [DOI] [PubMed] [Google Scholar]
- 33.Ianzano L, Zhang J, Chan EM, et al. Lafora progressive Myoclonus Epilepsy mutation database-EPM2A and NHLRC1 (EPM2B) genes. Hum Mutat. 2005;26:397. doi: 10.1002/humu.9376. [DOI] [PubMed] [Google Scholar]
- 34.Tagliabracci VS, Turnbull J, Wang W, et al. Laforin is a glycogen phosphatase, deficiency of which leads to elevated phosphorylation of glycogen in vivo. Proc Natl Acad Sci U S A. 2007;104:19262–19266. doi: 10.1073/pnas.0707952104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berard-Badier M, Pellissier JF, Gambarelli D, et al. The retina in Lafora disease: Light and electron microscopy. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1980;212:285–294. doi: 10.1007/BF00410522. [DOI] [PubMed] [Google Scholar]
- 36.Hegreberg GA, Padgett GA. Inherited progressive epilepsy of the dog with comparisons to Lafora’s disease of man. Fed Proc. 1976;35:1202–1205. [PubMed] [Google Scholar]
- 37.Holland JM, Davis WC, Prieur DJ, Collins GH. Lafora’s disease in the dog. A comparative study. Am J Pathol. 1970;58:509–530. [PMC free article] [PubMed] [Google Scholar]
- 38.Fisher RS, Harding G, Erba G, Barkley GL, Wilkins A. Photic- and pattern-induced seizures: A review for the Epilepsy Foundation of America Working Group. Epilepsia. 2005;46:1426–1441. doi: 10.1111/j.1528-1167.2005.31405.x. [DOI] [PubMed] [Google Scholar]
- 39.Shoja MM, Tubbs RS, Malekian A, et al. Video game epilepsy in the twentieth century: A review. Childs Nerv Syst. 2007;23:265–267. doi: 10.1007/s00381-006-0285-2. [DOI] [PubMed] [Google Scholar]
- 40.Su MY, Head E, Brooks WM, et al. Magnetic resonance imaging of anatomic and vascular characteristics in a canine model of human aging. Neurobiol Aging. 1998;19:479–485. doi: 10.1016/s0197-4580(98)00081-5. [DOI] [PubMed] [Google Scholar]
- 41.Schoeman T, Williams J, van WE. Polyglucosan storage disease in a dog resembling Lafora’s disease. J Vet Intern Med. 2002;16:201–207. doi: 10.1892/0891-6640(2002)016<0201:psdiad>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 42.Wilson WJ, Mills PC. Brainstem auditory-evoked response in dogs. Am J Vet Res. 2005;66:2177–2187. doi: 10.2460/ajvr.2005.66.2177. [DOI] [PubMed] [Google Scholar]
- 43.Aguglia U, Farnarier G, Tinuper P, Quattrone A. Brainstem auditory evoked responses in Lafora disease. Clin Electroencephalogr. 1985;16:202–207. doi: 10.1177/155005948501600407. [DOI] [PubMed] [Google Scholar]
- 44.Shahwan A, Farrell M, Delanty N. Progressive myoclonic epilepsies: A review of genetic and therapeutic aspects. Lancet Neurol. 2005;4:239–248. doi: 10.1016/S1474-4422(05)70043-0. [DOI] [PubMed] [Google Scholar]
- 45.Shahwan A, Farrell M, Delanty N. Progressive myoclonic epilepsies: A review of genetic and therapeutic aspects. Lancet Neurol. 2005;4:239–248. doi: 10.1016/S1474-4422(05)70043-0. [DOI] [PubMed] [Google Scholar]
- 46.Platt SR, Adams V, Garosi LS, et al. Treatment with gabapentin of 11 dogs with refractory idiopathic epilepsy. Vet Rec. 2006;159:881–884. [PubMed] [Google Scholar]
- 47.Cardinali S, Canafoglia L, Bertoli S, et al. A pilot study of a ketogenic diet in patients with Lafora body disease. Epilepsy Res. 2006;69:129–134. doi: 10.1016/j.eplepsyres.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 48.Landsberg G. Therapeutic agents for the treatment of cognitive dysfunction syndrome in senior dogs. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:471–479. doi: 10.1016/j.pnpbp.2004.12.012. [DOI] [PubMed] [Google Scholar]