Abstract
Objectives
Possessing the ε4 allele of apolipoprotein E (APOE-ε4) genotype is associated with cognitive impairment in non-demented older adults. We hypothesized that we might find a subtype of depression related to impaired cognitive performance associated with the APOE-ε4 allele.
Design
A survey conducted between 2001-2003 with APOE genotyping.
Setting
Primary care offices in the Baltimore, Maryland area.
Participants
The study sample consisted of 305 adults aged 65 or older with complete information on APOE genotyping and covariates.
Measurements
We used the latent class model to classify respondents according to symptom criteria of the American Psychiatric Association's Diagnostic and Statistical Manual as assessed in the Composite International Diagnostic Interview and the following four measures of cognitive function: the Mini-Mental State Exam, Hopkins Verbal Learning Test, Controlled Oral Word Association Test, and the Brief Test of Attention. We examined the relationship between class membership and APOE genotype.
Results
The latent class model yielded three classes: a non-depressed class, a class with depressive symptoms and average cognitive functioning, and a class with depressive symptoms (particularly thoughts of death and suicide) and impaired cognitive functioning. Possessing at least one APOE-ε4 allele was not predictive of class membership.
Conclusion
A subgroup of elderly patients with depressive symptoms, cognitive impairment, and a high likelihood of experiencing thoughts of death or suicide may exist that may not be related to APOE-ε4. Subgroups of older patients with depressive symptoms may be important to identify because of the association with thoughts of death or suicide and cognitive impairment (249 words).
Keywords: aged, depressive symptoms, cognition, genetic testing, apolipoprotein E, primary care
Objectives
A significant percentage of older depressed adults also have cognitive impairment [1]. Studies based on diagnostic criteria for major and minor depression may miss important associations between depression symptoms and cognitive impairment among older adults. Subtypes of depression in which symptoms are present intermittently or in which sadness and anhedonia are denied may be as important as major depression in older adults because of the association with cognitive impairment. For example, Blazer and colleagues, employing grade-of-membership analysis to analyze the Durham-Piedmont ECA data, found a symptom profile tending to occur in older persons in which cognitive impairment was prominent and accompanied by sleep and appetite disturbance, hopelessness, and thoughts of death [2]. In addition, proneness to psychological distress has been found to increase risk of Alzheimer's disease [3]. Futhermore, recent findings by Woo et. al, suggest that subsyndromal depressive symptoms in late-life may impair physiologic processes underlying memory [4].
Behavioral genetics may provide information regarding the overlap between depression and cognition. Possessing the ε4 allele of apolipoprotein E (APOE-ε4) has been associated with an increased risk for a range of neuropsychiatric conditions in older adults, perhaps the most widely known of which is Alzheimer's disease [5, 6]. The APOE-ε4 allele has also been associated with cognitive impairment in non-demented older adults. A review by Savitz et al. [7] found such a relationship in 32 out of 45 studies examined, associating APOE-ε4 with impaired cognitive functioning both cross-sectionally and longitudinally. APOE-ε4 has been shown to increase risk of cognitive impairment in specific domains of cognition such as memory [8, 9], attention [10, 11], and implicit learning [12]. Specifically, the presence of the APOE-ε4 allele has been shown to increase risk of cognitive impairment when brain reserve is low [13]. Depression is another neuropsychiatric condition that has been explored for a potential association with APOE-ε4. A similar association between APOE-ε4 and depression has not been clearly established. Initial work done by Krishnan et al. [14] suggested that APOE-ε4 was associated with late-onset depression in older adults. More recently, an analysis conducted by Yung-Chieh et. al found that APOE-ε4 may be correlated with severe depression in the elderly [15]. Other investigators found similar associations [15, 16] although some studies failed to replicate these findings (e.g. [17, 18]).
Recent studies examining the collective relationships between APOE-ε4, depression and cognitive function have led to new insights. Geda et al. [19] examined 840 cognitively normal, non-depressed older adults at baseline and then at a 3.5-year follow-up. The authors noted that respondents who developed depression were at a higher risk for developing mild cognitive impairment, but also found an additive interaction in which respondents who developed depression and possessed APOE-ε4 were at a greater risk of developing mild cognitive impairment than could be accounted for by the separate effects of depression and APOE-ε4. Similarly, Hwang et al. [20] found that depressed older adults with APOE-ε4 showed significantly lower scores on the Mini-Mental State Exam (MMSE) and an increased risk for suicide compared to depressed respondents who did not possess APOE-ε4. These results suggest that a separate class of depression may exist related to the presence of the APOE-ε4 allele and decreased cognitive functioning.
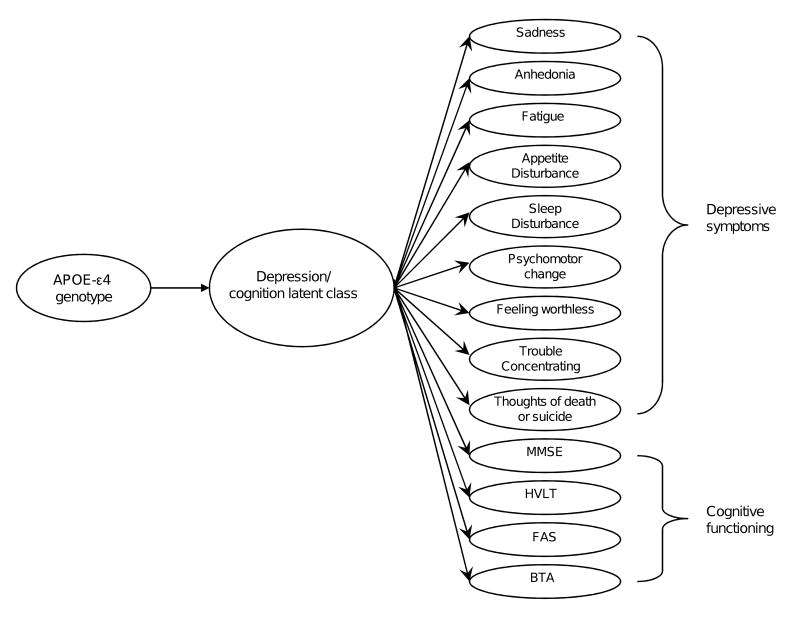
To our knowledge, this is the first investigation to examine the relationship between depressive symptoms, specific domains of cognition, and APOE-ε4 using latent class statistical methods. The underlying principle of latent class analysis is to search for an unobserved latent categorical variable that explains the association among a set of observed variables, in our study depression symptoms and cognitive functioning (Figure 1). The latent class model has two advantages. First, the model does not demand that we assign patients to a specific class, as each patient receives an estimated probability of class membership. In other words, misclassification error is minimized. Second, the latent class model also lets us, simultaneously, take into account baseline covariates other than the key variables under study in assessing their independent relationship to depressive symptoms and cognitive function, namely APOE-ε4 status.
Figure 1.
Latent class analysis of depressive symptoms and cognitive functioning. Note: Data were gathered from the Spectrum Survey, 2001-2003. APOE-ε4 = ε4 allele of apolipoprotein E, MMSE = Mini-Mental State Examination, HVLT = Hopkins Verbal Learning Test, FAS = Controlled Oral Word Association Test, BTA = Brief Test of Attention.
Our purpose was to conduct analysis through the use of latent class models to employ a new way to look at the relationship between patterns of depressive symptoms and specific domains of cognition, and how these patterns might be related to the APOE-ε4 allele. In addition, because APOE genotype was a part of our analysis, we wanted to examine a range of depressive symptoms and not assume the genotype would be associated with meeting the diagnostic criteria for major or minor depression. We recognize the exploratory nature of our analyses but took the opportunity afforded by a primary care study which included genotyping of patients. We hypothesized that we might find a class of depression related to impaired cognitive performance that is associated with the APOE-ε4 allele. Previous work by Yen et al. [15] used the latent class model to identify potential subtypes of depression in older adults in relation to the APOE-ε4 allele but defined their latent classes using depressive symptoms alone, while we used measures of both depressive symptoms and specific domains of cognition. Our study employed both the Centers for Epidemiologic Studies Depression (CES-D) scale and the Composite International Diagnostic Interview (CIDI) Depression Section, along with four different measures of specific domains of cognition, thereby providing an especially broad picture of respondent symptoms of depression and cognitive functioning. We were able to use the CIDI to define the latent classes while using the CES-D to aid with clinical interpretation of the latent classes. In summary, we carried out a latent class analysis to examine the heterogeneity of the depressive syndrome in late life that pertains to cognitive impairment and how these latent classes might be related to the APOE-ε4 allele.
Methods
The Spectrum Study
The Spectrum Survey was an observational study designed to characterize how depression presents among older primary care patients. Details of the Spectrum Study have been described elsewhere [21]. In brief, primary care practices recruited from the community provided the venue for sampling older patients. Trained lay interviewers were instructed in screening and study interviews by the study investigators working with Battelle Memorial Institute's Center for Public Health Research and Evaluation, Baltimore, Maryland. Screened patients were invited to participate with the following sampling probabilities: (1) 100% identified as depressed according to the CES-D; (2) 50% without significant depression but who were currently taking medications for sleep, pain, or an emotional problem; and (3) 10% of the remaining patients. In-home interviews were obtained for 357 people between the years 2001 and 2003, but 2 persons broke off the interview before it was completed, leaving a sample of 355 persons. In all, 50 of these respondents declined APOE genotyping [21], leaving a sample size of 305 persons for this analysis. The study protocols were approved by the Institutional Review Board of the University Of Pennsylvania School Of Medicine. A federal Certificate of Confidentiality was obtained from the Department of Health and Human Services to further safeguard the confidentiality of the survey and the genetic data.
Measurements
Sociodemographic Characteristics
Sociodemographic characteristics were obtained using standard self-reported questions on age, gender, ethnicity, and education level. Questions from the 36-item Short-Form Health Survey (SF-36) were used to assess functional status. The SF-36 has been employed in studies of outcomes of patient care and appears to be reliable and valid even in frail elders [22]. Medical conditions were assessed by self-report. Participants were asked if they had ever been told by a doctor that they had a heart attack, bypass surgery or angioplasty (e.g., balloon procedure), congestive heart failure, atrial fibrillation, stroke, diabetes, and cancer or leukemia assessed in separate questions for each condition. Cardiovascular conditions included heart attack, bypass surgery or angioplasty (e.g. balloon procedure), congestive heart failure, atrial fibrillation, or stroke.
Depression Measures
Symptoms of depression were evaluated using the both the Composite International Diagnostic Interview (CIDI) Depression Section and the Centers for Epidemiologic Studies Depression (CES-D) scale. The CIDI Depression Section is an interview used by trained lay interviewers in evaluating mental disturbances according to the International Classification of Diseases (ICD) and the American Psychiatric Association's Diagnostic and Statistical Manual (DSM-IV). The items of the CIDI are designed to evaluate the criteria for Major Depression, which include sadness, anhedonia, fatigue, appetite disturbance, sleep disturbance, psychomotor change, trouble concentrating, thoughts of death or suicide, and feelings of worthlessness, shame, or guilt [23]. Response options for each of these symptoms were yes or no. The Centers for Epidemiologic Studies Depression (CES-D) scale was developed by the Center for Epidemiologic Studies at the National Institute of Mental Health for use in studies of depression in community samples. The CES-D contains 20 items and has been employed in studies of older adults [24].
Measures of Cognitive Functioning
Cognitive functioning was assessed using four scales: the Mini-Mental State Exam (MMSE), Controlled Oral Word Association Test (FAS), Hopkins Verbal Learning Test (HVLT), and the Brief Test of Attention (BTA). The Mini-Mental State Examination (MMSE) is a brief global measure of cognition that has been employed for clinical and research purposes [25]. The MMSE utilizes a continuous scale to assesses orientation to time and place, registration, memory, attention and concentration, praxis, and constructional and language capacity. The Controlled Oral Word Association Test (FAS) serves as a test of verbal production and access to semantic knowledge and language [26]. The respondent is asked to generate as many words as possible that begin with a certain letter (F, A, and S) in one minute. The score is the sum of all words produced in the three one-minute trials. The Hopkins Verbal Learning Task (HVLT) evaluates new verbal learning and memory. On each of three trials participants listened to an audio-taped list of 12 words (4 from each of 3 different semantic categories), read at 2-second intervals, and were asked to recall as many words as possible [27]. Scores were recorded as the total number of words correctly recalled over three learning trials and analyzed as a continuous variable [28]. FAS and HVLT improve assessment of tasks not well mapped by the MMSE alone (namely, executive function and memory). The BTA is a perception task that measures divided attention in the verbal-linguistic system providing a rapid, bedside assessment of attentional impairment among nonaphasic hearing adults. The BTA has high short, and long term reliability [29, 30].
Genotyping Procedures
Apolipoprotein E genotyping was performed as described by Addya, Wang, and Leonard [31], with modification using buccal cell DNA instead of DNA from blood. Biosamples were obtained from study participants using a buccal swab protocol. The interviewers were trained to remove the buccal swab from its sterile container, and the participants were trained to swirl the brush on the inside of their cheek for 30 seconds and to replace the brush into the storage tube. Consistent with standards for genotyping, two samples were obtained from each participant to ensure an adequate sample was available for extraction of DNA. The APOE-ε4 variable was categorized as 0 if APOE-ε4 was not present and 1 if APOE-ε4 was present.
Analytic Strategy
Our analytic strategy was divided into two phases. The first phase involved an examination of means and proportions in a descriptive analysis of the study sample. In phase 2, we estimated a latent class model to identify potential subtypes of depression and cognitive functioning in older adults. APOE genotype among the potential subtypes was compared using the regression component of the model.
The aim of latent class analysis is to find individuals who are similar using categorical latent variables. The latent class model does not demand that we assign patients to a specific class, as each patient receives an estimated probability of class membership. Class probabilities represent estimates of the percentage of respondents who belong to each class. Item probabilities are the probabilities of endorsing an item given membership in a particular class. Latent class analysis of depressive symptoms using Composite International Diagnostic Interview (CIDI) Depression Section and cognitive functioning using the MMSE, HVLT, FAS, and the brief test of attention was applied to identify potential subtypes of depression and cognitive functioning. Binary indicators (1,0) were obtained from the depression symptom-level data from the CIDI [32]. Participants responded with yes or no answers to indicate the presence or absence of anhedonia, fatigue, appetite disturbance, sleep disturbance, psychomotor change, trouble concentrating, thoughts of death or suicide, and feelings of worthlessness, shame, or guilt. If respondents indicated that they had the symptom (an answer of yes) they were assigned code 1; if they indicated that they did not have the symptom (an answer of no) they were assigned code 0. The four cognitive variables were standardized and modeled continuously. The within-class means represent the mean difference between the cognitive functioning score of that particular class as compared to the overall mean for the entire sample. Simultaneously, we estimated a regression model predicting latent class membership. We examined whether APOE genotype was predictive of latent class membership. Odds ratios with 95% confidence intervals are reported comparing APOE genotype among the classes. Confidence intervals were Wald-based with 1 degree of freedom. In our final model we adjusted for physical functioning, cardiovascular conditions, and sociodemographic characteristics.
The latent class model with covariates yields three sets of parameters. The first set of parameters estimate the mean latent class prevalences. The second set estimates the depression characteristic probabilities or mean cognitive characteristic conditional on unobserved latent class membership. Lastly, the regression coefficients yield the relative probability of class membership associated with the covariate, APOE genotype.
Data analysis was performed using Mplus [33], which utilizes an efficient estimation-maximization (EM) algorithm for maximum likelihood estimation. Model choice, in terms of the number of classes was determined through examination of fit indices as well as for clinically interpretable results. Akaike Information Criteria (AIC) [34] and Bayesian Information Criteria (BIC) [35] were used to asses the model goodness of fit. Both measures assess improvements in fit while adjusting for the number of parameters. Results are reported in terms of probability of depressive symptoms and mean cognitive impairment conditional on class membership.
Results
The study sample included 305 persons in the Spectrum Study whose APOE genotype was available. In all, 8 persons (2.6% of the study sample) met criteria for major depression and 13 persons (4.3% of the study sample) met criteria for minor depression. The sociodemographic characteristics, APOE genotype, depression symptoms and cognitive functioning scores of the sample are presented in Table 1. The mean age in our sample was 75.0 years with a standard deviation of 5.8 years. The age range was 65 to 92 years. Two hundred and thirty (75%) of the participants were women, and 96 (32%) participants were African-American. One hundred and twenty-four (41%) participants had less than a high school education.
Table 1.
Depressive symptoms, APOE Genotype, Sociodemographic Characteristics, Cognitive Status, and Medical Comorbidity (n = 305)
| Patient characteristics | N (%) |
|---|---|
| Sadness | 63 (21%) |
| Anhedonia | 67 (22%) |
| Fatigue | 102 (33%) |
| Appetite disturbance | 35 (12%) |
| Sleep disturbance | 58 (19%) |
| Psychomotor change | 64 (21%) |
| Worthlessness/shameful/guilty | 20 (7%) |
| Trouble concentrating | 39 (13%) |
| Thoughts of death or suicide | 113 (37%) |
| APOE-ε4 (at least 1 allele) | 80 (23%) |
| Female | 230 (75%) |
| African American | 96 (32%) |
| Education less than high school | 124 (41%) |
| Mean ± SD | |
|
| |
| MMSE | 27.0 ± 2.8 |
| HVLT | 18.0 ± 6.1 |
| FAS | 26.5 ± 12.6 |
| Attention | 5.5 ± 2.5 |
| SF-36 physical functioning | 58.4 ± 29.1 |
| Total cardiovascular conditions | 2.6 ± 1.5 |
| Total medical conditions | 7.3 ± 3.5 |
Note: Data were gathered from the Spectrum Survey, 2001-2003. APOE-ε4 = ε4 allele of apolipoprotein E, MMSE = Mini-Mental State Examination, HVLT = Hopkins Verbal Learning Test, FAS = Controlled Oral Word Association Test, SF-36 = 36-item Short-Form Health Survey.
A series of latent class models was fitted to the depression symptoms. The three-class model presented in Table 2 improved the fit over the two- and four-class models. A three class model also yielded the most clinically relevant results. Persons in class 1 (estimated latent prevalence of 62%, “non-depressed class”) infrequently reported depressive symptoms, and had cognitive functioning slightly higher than the overall average. Persons in class 2 (estimated latent prevalence of 26%, “depressive symptoms class”) frequently reported depressive symptoms, but had cognitive functioning around the overall average. Persons in class 3 (estimated latent prevalence of 12%, “depressive symptoms and impaired cognitive functioning class”) reported some depressive symptoms (particularly thoughts of death and suicide) along with decreased cognitive functioning when compared to the overall average. The class labels were further validated by comparing the conditional class specific means of CES-D scores using posterior probability-based pseudoclasses [36]. Average CES-D scores for class 2 (“depressive symptoms class” mean=21.1, se=1.4) were significantly higher than CES-D scores for class 1 (“non-depressed class” mean=10.8, se=0.7, χ2(df=1) = 40.3, p<0.001). Average CES-D scores for class 3 (“depressive symptoms and impaired cognitive functioning class” mean=17.3, se=1.9) were also significantly higher than CES-D scores for class 1 (χ2(df=1) = 10.0, p=0.002).
Table 2.
Estimated conditional probabilities for binary variables representing depression symptoms and estimated within-class means and standard errors for continuous variables representing standardized cognitive function measures from the 3-class model (n=305)
| Variable | Class 1 | Class 2 | Class 3 |
|---|---|---|---|
| Non-depressed | Depressive Symptoms | Depressive symptoms/Cognitive Impairment | |
| Depressive symptoms | Conditional probabilities | ||
|
| |||
| Sadness | 0.061 | 0.541 | 0.209 |
| Anhedonia | 0.034 | 0.632 | 0.261 |
| Fatigue | 0.157 | 0.769 | 0.312 |
| Appetite disturbance | 0.055 | 0.264 | 0.104 |
| Sleep disturbance | 0.039 | 0.539 | 0.219 |
| Psychomotor change | 0.118 | 0.409 | 0.288 |
| Worthless/shameful/guilty | 0.000 | 0.248 | 0.036 |
| Trouble concentrating | 0.001 | 0.433 | 0.123 |
| Thoughts of death or suicide | 0.150 | 0.803 | 0.582 |
| Cognitive function measures | Estimated within-class means*(standard error) | ||
|
| |||
| MMSE | 0.293 (0.073) | 0.349 (0.133) | -1.978 (0.360) |
| HVLT | 0.244 (0.095) | 0.122 (0.192) | -1.212 (0.239) |
| FAS | 0.191 (0.096) | -0.045 (0.176) | -0.844 (0.099) |
| Attention | 0.234 (0.080) | 0.076 (0.172) | -1.181 (0.197) |
| Latent prevalence | 0.62 | 0.26 | 0.12 |
Note: Data were gathered from the Spectrum Survey, 2001-2003. MMSE = Mini-Mental State Examination, HVLT = Hopkins Verbal Learning Test, FAS = Controlled Oral Word Association Test.
Estimated within-class means represent the mean difference between the cognitive functioning score of that particular class as compared to the overall mean. Standard errors are in parentheses.
Latent class analysis is adjusted for physical functioning, cardiovascular conditions, and sociodemographic characteristics.
Possessing at least one APOE-ε4 allele was not predictive of class membership in the “depressive symptoms class” compared to the “non-depressed class” (unadjusted OR = 1.02, p=0.96, 95% CI [0.5, 2.08]; adjusted OR = 0.96, p=0.80, 95% CI [0.48, 1.91]) or in the “depressive symptoms and impaired cognitive functioning class” compared to the “non-depressed class” (unadjusted OR = 0.94, p=0.91, 95% CI [0.36, 2.5]; adjusted OR = 0.65, p=0.35, 95% CI [0.25, 1.67]). The likelihood ratio test comparing the adjusted model with APOE-e4 allele to the adjusted model without APOE-e4 allele was not statistically significant (LR χ2 (df=2) =0.51, p=0.77). The final model was adjusted for physical functioning, cardiovascular conditions, and sociodemographic characteristics.
Conclusions
The latent class analysis yielded three classes: a non-depressed class, a depressive symptoms class, and a depressive symptoms and impaired cognitive functioning class. No statistically significant relationship was observed between the APOE-ε4 allele and class membership. Among older adults with depressive symptoms, a subgroup with cognitive impairment may exist that is not related to the APOE-ε4 allele. The potential existence of the subgroup suggests that such individuals may or may not meet criteria for major depression but have impaired cognitive functioning and a high likelihood of experiencing thoughts of death or suicide.
Before discussing our findings, the limitations of this study must be considered. First, our sample was drawn from primary care offices in the Baltimore, Maryland area so our results may not be representative of all practices. Second, our study is subject to biases associated with self-report data, including imperfect recall and response bias. We attempted to minimize these potential effects by using validated mental health, cognitive and physical health measures that have been widely used in geriatric mental health research. Third, bias may have arisen due to exclusion of respondents who declined genotyping. However, previous research indicates that this bias may be minimal [21]. Fourth, our small sample size of respondents having two APOE-ε4 alleles prevented an analysis of the possible dose-dependent effect of the number of ε4 alleles. Fifth, we realize that standardized measures of global cognition, memory, and attention and may tap constructs that were not intended by the developers or implied by the labels given to them. Sixth, the small number of participants meeting diagnostic criteria on the CIDI for major and minor depression was small (8 with major depression and 13 with minor depression). This may limit the usefulness of our findings for work focusing specifically on diagnostic criteria for major and minor depression. Lastly, we also acknowledge we must be careful in our conclusions since the results are based on cross-sectional analyses [37].
Despite limitations, our results deserve attention because we attempted to characterize patterns of depressive symptoms and cognitive impairment and the association of the APOE-ε4 allele with those patterns. Numerous studies have examined the relationship between depressive symptoms and cognitive functioning indicating that an association may exist between these conditions (e.g. [1, 38]). Findings also indicate that depression may be related to the presence of APOE-ε4 (e.g. [17, 18]) and that the presence of APOE-ε4 increases the risk of cognitive impairment (e.g. [7, 12]). Only a few studies have evaluated the complex interplay between all of these factors (depression, cognitive, APOE-ε4) (e.g. [19, 20]). No known studies have used latent class analysis to evaluate depressive symptoms in persons who may or may not meet criteria for major or minor depression in relation to cognitive functioning and the distribution of APOE-ε4.
Of note are the lower CES-D scores in the depressive symptoms class in comparison with the depressive symptoms and impaired cognitive functioning class. Our data are consistent with previous work indicating distressed patients with cognitive impairment are at greater risk for suicidality [39, 40]. The potential existence of the above subgroup suggests that while these individuals may or may not meet criteria for major depression, they do have a high likelihood of experiencing thoughts of death or suicide and may require additional screening and services in primary care. However, our results are not wholly consistent with our hypotheses because we failed to find a relationship between APOE-ε4 and class membership. Our study is consistent with other studies reporting evidence that the APOE-ε4 was not associated with depression and cognitive functioning (e.g. [17, 18]).
We believe our findings have both clinical and methodological implications. One implication may be that cognitive functioning, depressive symptoms, and suicidal ideation should not be assessed in isolation. Interventions to improve treatment of depression in primary care settings, particularly depressive symptoms that do not meet standard criteria for major depression among older adults, may need to include more of a focus on cognition and suicidal ideation. From a methodological viewpoint, we employed latent class models to employ a new way to look at the relationship between patterns of depressive symptoms and specific domains of cognition, and how these patterns might be related to the APOE-ε4 allele. Latent class analysis appears to be an informative model for examining subtypes using both depressive symptoms and cognitive profiles. Class membership was not related to APOE-ε4 allele distribution in our sample. Future studies might employ a longitudinal analysis in order to investigate patterns of depressive symptoms and cognitive functioning over time in relation to the APOE-ε4 allele.
Acknowledgments
Megan Richie was supported by the Summer Training on Aging Research Topics - Mental Health (START-MH) Fellowship Program award from the National Institute of Mental Health (NIMH) with Dr. Bogner as the mentor. Dr. Bogner was supported by a NIMH mentored Patient-Oriented Research Career Development Award (MH67671-01) and an American Heart Association Grant-in-aid. Dr. Morales was supported by a NIMH Mentored Research Scientist Development Award (MH073903). The Spectrum Study was supported by grants MH62210-01, MH62210-01S1, and MH67077 from the NIMH.
References
- 1.Ganguli M, Du Y, Dodge HH, Ratcliff GG, Chang CC. Depressive symptoms and cognitive decline in late life: a prospective epidemiological study. Arch Gen Psychiatry. 2006;63(2):153–60. doi: 10.1001/archpsyc.63.2.153. [DOI] [PubMed] [Google Scholar]
- 2.Blazer D, Swartz M, Woodbury M, Manton KG, Hughes D, George LK. Depressive symptoms and depressive diagnoses in a community population: Use of a new procedure for analysis of psychiatric classification. Archives of General Psychiatry. 1988;45:1078–1084. doi: 10.1001/archpsyc.1988.01800360026004. [DOI] [PubMed] [Google Scholar]
- 3.Wilson RS, Barnes DA, Bennett YL, Bienias CF, de Leon CF, Evans DA. Proneness to psychological distress and risk of Alzheimer disease in a biracial community. Neurology. 2005;64:380–382. doi: 10.1212/01.WNL.0000149525.53525.E7. [DOI] [PubMed] [Google Scholar]
- 4.Woo SL, Prince SE, Petrella JR, Hellegers C, Doraiswamy PM. Modulation of a Human Memory Circuit by Subsyndromal Depression in Late Life: A Functional Magnetic Resonance Imaging Study. Am J Geriatr Psychiatry. 2008 doi: 10.1097/JGP.0b013e318180056a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ, Hulette C, Crain B, Goldgaber D, Roses AD. Association of apolipoprotein E allele e4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 6.Strittmatter WJ, Saunders AM, Schmechel D, et al. Apolipoprotein E: high affinity binding to BA amyloid and increased frequency of type 4 allele in familial Alzheimer's. Proceedings of the National Academy of Sciences. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savitz J, Solms M, Ramesar R. Apolipoprotein E variants and cognition in healthy individuals: a critical opinion. Brain Res Rev. 2006;51(1):125–35. doi: 10.1016/j.brainresrev.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Caselli RJ, Reiman EM, Osborne D, Hentz JG, Baxter LC, Hernandez JL, Alexander GG. Longitudinal changes in cognition and behavior in asymptomatic carriers of the APOE e4 allele. Neurology. 2004;62(11):1990–5. doi: 10.1212/01.wnl.0000129533.26544.bf. [DOI] [PubMed] [Google Scholar]
- 9.Driscoll I, McDaniel MA, Guynn MJ. Apolipoprotein E and prospective memory in normally aging adults. Neuropsychology. 2005;19(1):28–34. doi: 10.1037/0894-4105.19.1.28. [DOI] [PubMed] [Google Scholar]
- 10.Greenwood PM, Lambert C, Sunderland T, Parasuraman R. Effects of apolipoprotein E genotype on spatial attention, working memory, and their interaction in healthy, middle-aged adults: results From the National Institute of Mental Health's BIOCARD study. Neuropsychology. 2005;19(2):199–211. doi: 10.1037/0894-4105.19.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosen VM, Sunderland T, Levy J, Harwell A, McGee L, Hammond C, Bhupali D, Putnam K, Bergeson J, Lefkowitz C. Apolipoprotein E and category fluency: evidence for reduced semantic access in healthy normal controls at risk for developing Alzheimer's disease. Neuropsychologia. 2005;43(4):647–58. doi: 10.1016/j.neuropsychologia.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 12.Negash S, Petersen LE, Geda YE, Knopman DS, Boeve BF, Smith GE, Ivnik RJ, Howard DV, Howard JH, Jr, Petersen RC. Effects of ApoE genotype and mild cognitive impairment on implicit learning. Neurobiol Aging. 2007;28(6):885–93. doi: 10.1016/j.neurobiolaging.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Kim KR, Lee KS, Kim EA, Cheong HK, Oh BH, Hong CH. The effect of the ApoE genotype on the association between head circumference and cognition. Am J Geriatr Psychiatry. 2008;16(10):819–25. doi: 10.1097/JGP.0b013e3181800551. [DOI] [PubMed] [Google Scholar]
- 14.Krishnan KR, Tupler LA, Ritchie JC, Jr, McDonald WM, Knight DL, Nemeroff CB, Carroll BJ. Apolipoprotein E-epsilon 4 frequency in geriatric depression. Biol Psychiatry. 1996;40(1):69–71. doi: 10.1016/0006-3223(95)00424-6. [DOI] [PubMed] [Google Scholar]
- 15.Yen Y, Rebok GW, Gallo J, Yang M, Lung F, Shih C. ApoE4 is associated with late-life depression: a population based study. Am J Geriatr Psychiatry. 2007;15:858. doi: 10.1097/JGP.0b013e3180f63373. [DOI] [PubMed] [Google Scholar]
- 16.Rigaud AS, Latour F, Moulin F, Forette F, Traykov L, Boller F. Apolipoprotein E epsilon4 allele and clinically defined vascular depression. Arch Gen Psychiatry. 2002;59(3):290–1. doi: 10.1001/archpsyc.59.3.290. [DOI] [PubMed] [Google Scholar]
- 17.Butters MA, Sweet RA, Mulsant BH, Ilyas Kamboh M, Pollock BG, Begley AE, Reynolds CF, 3rd, DeKosky ST. APOE is associated with age-of-onset, but not cognitive functioning, in late-life depression. Int J Geriatr Psychiatry. 2003;18(12):1075–81. doi: 10.1002/gps.1006. [DOI] [PubMed] [Google Scholar]
- 18.Lee JS, Potter GG, Wagner HR, Welsh-Bohmer KA, Steffens DC. Persistent mild cognitive impairment in geriatric depression. Int Psychogeriatr. 2007;19(1):125–35. doi: 10.1017/S1041610206003607. [DOI] [PubMed] [Google Scholar]
- 19.Geda YE, Knopman DS, Mrazek DA, Jicha GA, Smith GE, Negash S, Boeve BF, Ivnik RJ, Petersen RC, Pankratz VS, Rocca WA. Depression, apolipoprotein E genotype, and the incidence of mild cognitive impairment: a prospective cohort study. Arch Neurol. 2006;63(3):435–40. doi: 10.1001/archneur.63.3.435. [DOI] [PubMed] [Google Scholar]
- 20.Hwang JP, Yang CH, Hong CJ, Lirng JF, Yang YM, Tsai SJ. Association of APOE genetic polymorphism with cognitive function and suicide history in geriatric depression. Dement Geriatr Cogn Disord. 2006;22(4):334–8. doi: 10.1159/000095599. [DOI] [PubMed] [Google Scholar]
- 21.Bogner HR, Wittink M, Merz JF, Straton JB, Cronholm PF, Rabins PV, Gallo JJ. Personal characteristics of older primary care patients who provide a buccal swab for APOE testing and banking of genetic material: The Spectrum Study. Community Genetics. 2004;7(4):202–10. doi: 10.1159/000082263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stadnyk K, Calder J, Rockwood K. Testing the measurement properties of the Short Form-36 health survey in a frail elderly population. Journal of Clinical Epidemiology. 1998;51:827–835. doi: 10.1016/s0895-4356(98)00061-4. [DOI] [PubMed] [Google Scholar]
- 23.Wittchen HU. Reliability and validity studies of the WHO--Composite International Diagnostic Interview (CIDI): a critical review. J Psychiatr Res. 1994;28(1):57–84. doi: 10.1016/0022-3956(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 24.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 25.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 26.Benton AL, Hamsher K. Multilingual Aphasia Examination. Iowa City: AJA Associates; 1983. [Google Scholar]
- 27.Brandt J. The Hopkins verbal learning test: Development of a new memory test with six equivalent forms. Clincial Neuropsychologist. 1991;5:125–142. [Google Scholar]
- 28.Hogervorst E, Combrinck M, Lapuerta P, Rue J, Swales K, Budge M. The Hopkins Verbal Learning Test and screening for dementia. Dement Geriatr Cogn Disord. 2002;13(1):13–20. doi: 10.1159/000048628. [DOI] [PubMed] [Google Scholar]
- 29.Snow WG, Tierney MC, Zorzitto ML. One year test-retest reliability of selected neuropsychological tests in older adults. Journal of Clinical and Experimental Neuropsychology. 1988;10:60. [Google Scholar]
- 30.desRosiers G, Kavanagh D. Cognitive assessment in closed head injury: Stability, validity, and parallel form for two neuropsychological measures of recovery. International Journal of Clinical Neuropsychology. 1987;9:162–173. [Google Scholar]
- 31.Addya K, Wang YL, Leonard DG. Optimization of Apolipoprotein E Genotyping. Mol Diagn. 1997;2(4):271–276. doi: 10.1054/MODI00200271. [DOI] [PubMed] [Google Scholar]
- 32.Gallo JJ, Cooper-Patrick L, Lesikar S. Depressive symptoms of whites and African Americans aged 60 years and older. J Gerontol B Psychol Sci Soc Sci. 1998;53(5):P277–86. doi: 10.1093/geronb/53b.5.p277. [DOI] [PubMed] [Google Scholar]
- 33.McCutcheon A. Latent Class Analysis. Beverly Hills: Sage University Press; 1987. [Google Scholar]
- 34.Akaike H. Factor analysis and the AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- 35.Schwartz G. Estimating the dimension of a model. The Annals of Statistics. 1978;6:461–464. [Google Scholar]
- 36.Wang CP, Brown CH, Bandeen-Roche K. Residual diagnostics for Growth Mixture Models: Examining the impact of a preventive intervention on multiple trajectories of aggressive behavior. Journal of the American Statistical Association. 2005;100(3):1054–1076. [Google Scholar]
- 37.Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry. 2002;59(10):877–83. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- 38.Ravaglia G, Forti P, Lucicesare A, Rietti E, Pisacane N, Mariani E, Dalmonte E. Prevalent depressive symptoms as a risk factor for conversion to mild cognitive impairment in an elderly Italian cohort. Am J Geriatr Psychiatry. 2008;16(10):834–43. doi: 10.1097/JGP.0b013e318181f9b1. [DOI] [PubMed] [Google Scholar]
- 39.Ayalon L, Mackin S, Arean PA, Chen H, McDonel Herr EC. The role of cognitive functioning and distress in suicidal ideation in older adults. J Am Geriatr Soc. 2007;55(7):1090–4. doi: 10.1111/j.1532-5415.2007.01237.x. [DOI] [PubMed] [Google Scholar]
- 40.Crane MK, Bogner HR, Brown GK, Gallo JJ. The link among depressive symptoms, negative cognitive bias, and memory complaints in older adults. Aging and Mental Health. 2007;11(6):708–715. doi: 10.1080/13607860701368497. [DOI] [PMC free article] [PubMed] [Google Scholar]