Abstract
Limitation of infarct size by ischemic/pharmacological pre- and post-conditioning involves activation of a complex set of cell-signaling pathways. Multiple lines of evidence implicate the mitochondrial permeability transition pore (mPTP) as a key end-effector of ischemic/pharmacological pre- as well as post-conditioning. Increasing the ROS-threshold for mPTP-induction enhances the resistance of cardiomyocytes to oxidant stress and results in infarct size reduction. Here we survey and synthesize the current knowledge about the role of glycogen synthase kinase-3β (GSK-3β) in cardioprotection, including pre- and post-conditioning. Activation of a wide spectrum of cardioprotective signaling pathways is associated with phosphorylation and inhibition of a discrete pool of GSK-3β relevant to mitochondrial signaling. Therefore, GSK-3β has emerged as the integration point of many of these pathways and plays a central role in transferring protective signals downstream to target(s) that act at or in proximity to the mPTP. Bcl-2-family proteins and mPTP-regulatory elements such as ANT and CyP-D (possibly VDAC) may be the functional downstream target(s) of GSK-3β. Gaining a better understanding of these interactions to control and prevent mPTP-induction when appropriate will enable us to decrease the negative impact of the reperfusion-induced ROS-burst on the fate of mitochondria and perhaps allow us to limit propagation of damage throughout and between cells and consequently, to better limit infarct size.
Keywords: Mitochondria, ROS-induced ROS release, permeability transition pore
Introduction
Tissue reperfusion after ischemia, while ultimately necessary for cell survival, is a “double-edged sword” because it is also associated with further damage, known as reperfusion injury.1 Once the artery blockage is removed blood flow-triggered reoxygenation aggravates the injury suffered during the ischemia, resulting in further cell damage. A similar process can be mimicked in experimental settings, e.g., in isolated cardiomyocytes or neurons exposed to prolonged hypoxia followed by reoxygenation. The lack of oxygen results in a situation in which the restoration of circulation rapidly leads to oxidative damage through the induction of excess oxidative stress. Therefore, the improvement in tissue function and survival after reperfusion has often been substantially negatively affected by this damaging phenomenon.
Reperfusion-induced elevation in cytotoxic reactive oxygen species (ROS), mostly in and around mitochondria2 (reviewed in3, 4) can trigger opening of the so-called mitochondrial permeability transition pore (mPTP), that is accompanied by the immediate dissipation of the mitochondrial membrane potential, Δψm, with detrimental consequences for the affected mitochondrion (originally proven by Griffiths and Halestrap5). We have developed a method to quantify the mPTP-susceptibility to ROS-induction in individual mitochondria inside intact cardiomyocytes. Using this method, we have demonstrated a phenomenon we call ROS-induced ROS release (RIRR), in which the mPTP induced by an elevated level of “triggering ROS” is followed by an additional ROS burst that occurs simultaneously with mitochondrial Δψm dissipation.6-8 Therefore, RIRR is responsible for a large fraction of the oxidative damage detected in cells after reperfusion7, and ROS rather than Ca2+ appears to be the more important mPTP-trigger in excitable cells such as cardiomyocytes and neurons.9, 10 Occasionally, synchronous cycles of mPTP-induction and closure, manifested as oscillations of the mitochondrial Δψm, are observed in groups of contiguous mitochondria. The propagation of RIRR-induced mitochondrial depolarizations through a large portion of a cell, and even spreading among neighboring cardiomyocytes may result in pathological disturbances in excitability throughout the whole of the heart and contribute to lethal cardiac dysrhythmias.7, 8, 11, 12
Cardiomyocte survival after hypoxia-reoxygenation has been negatively correlated to the fraction of cellular mitochondria that undergo mPTP-induction.9 Furthermore, that agents such as diazoxide limit mPTP-induction by increasing the mPTP-ROS threshold (resulting in cell protection) provides confirmatory evidence for the causative role of mPTP-induction during ischemia/reperfusion injury. Ischemic/pharmacological preconditioning and postconditioning are probably the two most widely recognized methods of achieving such cell protection. These protective mechanisms are adaptive cellular responses to protocols which involve several brief cycles of ischemia-reperfusion applied either before a prolonged ischemic insult or during the first moments of reperfusion.13, 14 Intrinsic cell defense mechanisms triggered by various pharmacologic interventions (reviewed in 15) or induced acidosis 10, 16-18 result in comparable levels of protection.
Activation at a variety of points along the various signaling pathways results in comparable levels of protection of cardiomyocytes from reperfusion injury. For example, ligand-activation of a variety of cell-surface receptors (such as adenosine, muscarinic, endothelin, angiotensin, bradykinin, etc.) has been shown to be effective in cardioprotection, as has direct activation of their downstream mediator-enzymes, for example, PKA, PKB/AKT, PKC, PKG, and glycogen synthase kinase-3β (GSK-3β). Pharmacologic activation of the putative mitochondrial ATP-dependent K+ channel (mitoK-ATP) as well as treatment with CyP-D ligands (e.g., cyclosporin A (CsA) and sanglifehrin A (SfA)) also triggers similar levels of cell protection (reviewed in15, 19).
Given the diversity of the upstream pathways, we hypothesized that there might be a mechanism that would serve to integrate these inputs and then convey the signal to the end-effector, the mPTP. Recently we proposed that the most likely candidate for this point of convergence is GSK-3β.9, 19 This review brings together current knowledge related to the role of GSK-3β in cardioprotection. First, the molecular characterization of GSK-3, its physiological roles, and the complex regulatory network of this kinase will be discussed. The focus will then shift to the involvement of GSK-3β in cell protection: upstream protective signaling pathways that converge on GSK-3β and trigger cardioprotection will be highlighted, as will the downstream targets of GSK-3β that act at, or in proximity to, the mPTP to increase the mPTP-ROS-threshold, and based on available information, a model of the mPTP regulation by GSK-3β will be proposed.
1. Regulation of GSK-3
1.1 Molecular characterization
GSK-3 is a serine/threonine kinase that was originally identified as an enzyme that phosphorylates and down regulates glycogen synthase, the rate-limiting enzyme of glycogen metabolism.20 Although subsequent work has shown that its role and importance extends far beyond intermediary metabolism, nevertheless, the original nomenclature remains in use. GSK-3 is highly evolutionarily conserved, present in every eukaryotic species examined to-date, and has been implicated in a multiplicity of critical regulatory roles in a variety of cell types. GSK-3 is found in the cytosol, mitochondria and nucleus of cells,21 and ∼50 substrates have been identified. GSK-3 activity has been associated with many cell processes, including the regulation of multiple transcription factors, the Wnt-pathway, NF-κB, ER-stress, embryogenesis, apoptosis and cell survival, cell-cycle progression, cell migration, etc. (reviewed in22). GSK-3 has been linked to a diverse variety of human disorders, including neurodegenerative diseases, sleep disorders, psychiatric disorders, stroke, diabetes, parenchymal renal disease and cancer.
Two mammalian isoforms of GSK-3, α and β, have been identified. They are encoded by distinct genes; expressed GSK-3α is 51 kDa, whereas GSK-3β is 47 kDa. The size difference is due to a glycine-rich extension at the N-terminus of GSK-3α. The two isoforms are highly homologous within their kinase domains (∼98% identity) but share only ∼36% identity in the last 76 C-terminal residues.23 GSK-3 from different species displays a high degree of homology. Unlike most kinases, GSK-3 is highly active in the basal-state (i.e., unstimulated cells) and usually exerts a tonic-negative, inhibitory effect on its downstream pathways, and becomes serine-phosphorylated and inactivated in response to stimulation of its upstream pathways. The ideal phospho-acceptor site for GSK-3 is a serine or threonine 4 residues upstream of an already phosphorylated hydroxyamino acid.24 Phosphorylation within the amino-terminal domain of GSK-3α (Ser21) or GSK-3β (Ser9) results in enzyme inactivation of GSK-3, and consequently, due to relief of its tonic-inhibition, in activation of downstream targets.25 Recently, it was demonstrated that p-38 mitogen-activated protein kinase (MAPK)-dependent phosphorylation of Ser389/Thr390 (mouse/human) may also result in GSK-3 inhibition.26 In resting cells, the active state of GSK-3 is correlated with autophosphorylation at tyrosine 279/216 (GSK-3α/GSK-3β, respectively) within the catalytic domain “T-loop”. This tyrosine-phosphorylation does not seem to be required for kinase activity, but rather seems to increase its overall catalytic efficiency.27
Relatively less is known about the respective functional roles of these two kinases. Despite their similar biochemical properties and substrate recognition, GSK-3α and GSK-3β are almost, but not always, functionally identical and interchangeable. For example, it has been proposed that GSK-3α might have a positive role in the build-up of amyloid-peptide in amyloid plaques while GSK-3β might play a negative role.28 A genetic knock-in approach (GSK-3α S21A knock-in and GSK-3β S9A knock-in mice) revealed a potential functional difference between the two isoforms in the heart during pressure overload. Based on this study, it was proposed that while Ser9 phosphorylation of GSK-3β might mediate pathological hypertrophy, Ser21 phosphorylation of GSK-3α might be involved in compensatory activation of cell proliferation in the heart.29 Also, as will be discussed, GSK-3β but not GSK-3α is involved in cardioprotection.9 Differential spatial/temporal distributions of these two GSK-3-isoforms have also been demonstrated. Due to its exceptional therapeutic potential, this multifunctional kinase has become an attractive target for drug development for more than a decade. Thus, a better understanding of the potentially unique roles of each isoform in response to stimulation of various signaling pathway is necessary.
1.2 Inhibitors
Three classes of GSK-3 inhibitors have been described: 1) cations that compete for the Mg2+ binding site, 2) competitive inhibitors that act on the ATP binding pocket, and, 3) non-competitive inhibitors that interact with the substrate domain. Lithium ion is the best known GSK-3inhibitor and probably functions by competing with the binding of Mg2+, an essential enzyme cofactor. A number of potent and fairly selective small-molecule GSK-3 inhibitors have now been developed that do not discriminate between GSK-3α and GSK-3β; most act as effective competitors at the ATP-binding site of these enzymes. Unfortunately, in many cases the ATP-binding-pocket shares a high degree of homology across a wide range of protein kinases, thus the specificity of these inhibitors is always a concern (reviewed in30). The availability of X-ray crystallography data for GSK-3 has allowed rational drug-design approaches to be utilized in the search for new inhibitors of these proteins. For example, structure-activity studies guided the development of 2,4-disubstituted thiadiazolidinones (TDZDs), the first ATP-noncompetitive GSK-3 inhibitor.31
2. Convergence of distinct cell-protection mechanisms via GSK-3β
2.1 Cell-surface receptors in preconditioning/postconditioning
Both ischemic preconditioning and postconditioning are known to be receptor-mediated (e.g.,15, 32). Downey's group originally reported that ischemic preconditioning involves A1-adenosine receptor activation.33 Activation of several other Gi-coupled receptors prior to a prolonged ischemic insult can also trigger the preconditioned state. Recently, ischemic postconditioning has been linked to activation of several types of cell-surface receptors, including adenosine-A1 and bradykinin-B2 receptors,32, 34 and it has been accepted that postconditioning can also be achieved by treatment with a variety of drugs at the time of reperfusion, for example by adenosine (and its derivatives), bradykinin, opioids, norepinephrine, angiotensin, acetylcholine, erythropoietin, etc. (e.g.,15, 35).
In contrast to preconditioning, the relative timing of postconditioning to the evolving pathologic process makes it feasible to be applied in clinical settings. Shortly after ischemic postconditioning was discovered,14 its practical applicability had been successfully tested in pilot clinical trials on patients with acute myocardial infarction. Repeated short cycles of angioplasty balloon-inflations/deflations at the stenosis site of the infarct-related artery, just before the final reperfusion stage, significantly decreased infarct size.36, 37 Pharmacological alternatives to ischemic postconditioning, with the mPTP being the primary target of intervention, are currently under development. In a small clinical trial, a single bolus of CsA, administered just before primary reperfusion yielded encouraging results with respect to reducing infarct size.38 There may also be a critical role for controlled acidosis at the time of reperfusion (discussed later;16). Therefore, the moment of early reperfusion provides a new opportunity for intervention to reduce infarct size and potentially to improve clinical outcomes. The success of postconditioning with catheter reperfusion, CsA, and acidosis is very promising and a niche for future research to safely and efficiently engage protection against reperfusion injury. Whether or not GSK-3β or other enzyme cascades are involved in these latter protocols (i.e., CsA, acidosis), which probably involve some form of “direct” mPTP-desensitization, remains to be determined.
2.2 Diverse signaling induces the preconditioned/postconditioned state
The various surface-receptor agonist-mediated signal transduction pathways involved in cell protection include at least the following kinases (singly, or in combination, depending on circumstances): PI3K/AKT/PKB, PKC, PKG, p70S6K, ERK1/2, MAPK and GSK-3β (reviewed by15, 19, 39, 40). Early on, the putative mitochondrial mitoKATP emerged as a mediator or a possible end-effector of preconditioning. Pharmacologic activation of the mitoKATP (e.g., using diazoxide at an appropriate dose, as well as many others) was shown to induce a protective state sensitive to inhibition by mitoKATP blockers such as 5-hydroxydecanoate (5-HD) and glybenclamide.41, 42 In isolated cardiomyocytes, utilizing only glucose, treatment with diazoxide, acting presumably via mitoKATP, was associated with an increase in oxygen consumption, regulatory mitochondrial-swelling and the production of a redox-signal; furthermore, these actions of diazoxide were abolished by 5-HD.9 In the control experiments, 5-HD itself, absent diazoxide, had no effect on any of these parameters,9 suggesting the likely specificity of its action under these conditions rather than the nonspecific interference with β-oxidation (which should play little if any role when glucose is the only oxidative substrate present), possibly seen in other experimental conditions in isolated mitochondria.43, 44 Importantly, both ischemic preconditioning/postconditioning and diazoxide-induced protection are blocked by the ROS-scavenger N-acetylcysteine,45, 46 and these processes appear to act via redox-activation of PKC.9, 32, 47, 48
Currently, it is accepted that activation of NOS, or the application of “NO-donors” induce cell protection.7, 49, 50 These studies demonstrated that not only ROS but also reactive nitrogen species can play a “second messenger” role in the signal transduction cascade involved in certain forms of cell protection.
2.3 mPTP is a key end-effector of cell protection
The mPTP was first described as a phenomenon by Haworth and Hunter in a pivotal work published in 1979.51 Soon it became evident that the mPTP is a significant player in cell death during reperfusion injury and that drugs, such as CsA, acting directly on presumptive components of the mPTP could exert a cardioprotective effect.52, 53 Furthermore, transient acidosis, apparently also acting directly on mPTP components/regulatory elements,18, 54 increases mPTP-ROS threshold10 and decreases infarct size (e.g.,16, 17). It should be pointed out that these approaches, likely acting at or near the mPTP, probably do not (necessarily) involve an elaborate (or even any) kinase cascade. On the other hand, ischemic preconditioning/postconditioning as well as various drugs that involve one or more kinase signaling pathways, have also been shown to promote cell survival by limiting mPTP-induction.9, 53, 55, 56 For the past 20 years, the more or less accepted dogma described the model of the mPTP as a proteinaceous complex comprised mainly of the voltage-dependent anion channel, adenine nucleotide translocase and cyclophilin D (VDAC–ANT–CyP-D), and also containing putative regulatory elements such as the peripheral benzodiazepine receptor, Bcl-2-family members, hexokinase II (HKII) and mitochondrial creatine kinase (comprehensively reviewed by Crompton57). Recent evidence strongly casts doubt on the validity of this classical model. Based on the genetic deletion of the presumptive individual “pore” components, ANT, VDAC and CyP-D,58-64 it now appears that the core structure of the pore remains unidentified, and that what were thought to be essential pore components are probably mPTP regulatory elements that are not required for pore-formation (reviewed in10). The role of phosphate and the inorganic phosphate carrier (PiC) in mPTP regulation has also been revisited in two recent studies. In one study, a new model for the mPTP was proposed in which a Ca2+-triggered conformational change of the PiC (possibly providing the mPTP pore-forming component), facilitated by CyP-D and possibly regulated by ANT-(±ligand)-binding, induces pore opening.65 The second study highlighted the mPTP-desensitizing role of Pi, specifically, the role of a Pi regulatory site on the mPTP that is masked by CyP-D. Thus blocking the CyP-D by CsA (or similar compounds) or CyP-D ablation, in the presence of Pi, would result in mPTP desensitization.66 This remains an interesting although controversial area of research.
2.4 Distinct upstream protection signaling mechanisms converge on GSK-3β
In 2002, Murphy's lab showed that ischemic preconditioning results in Ser9 phosphorylation and hence inhibition of GSK-3β, and that direct but isoform non-selective pharmacologic inhibition of GSK-3 protects against ischemia/reperfusion injury and reduces infarct size.67 Since then, extensive evidence implicating GSK-3β as a critical element in preconditioning and postconditioning has emerged. It was convincingly demonstrated that both preconditioning9, 67-69 and postconditioning70 involve inhibition of GSK-3β, and that administration of GSK-3 inhibitors 24 hrs before ischemia (delayed protection),71 shortly before ischemia (preconditioning)67, 69, 72-76 or just before reperfusion (postconditioning)69, 70, 73, 74, 77-79 resulted in reduced infarct size. Additionally, treatment of permeabilized cardiomyocytes with an active (nonphosphorylated) form of recombinant GSK-3β facilitates mPTP-induction, an effect blocked by CsA or by addition of a GSK-3 inhibitor or by inactivating the GSK-3β via its preincubation with catalytically-active PKA (presumably by causing Ser9 phosphorylation).80
Moreover, substantial evidence implicates GSK-3β as the critical mediator of pharmacologic pre- and postconditioning triggered by diverse agents applied before ischemia or at reperfusion. Such examples include cardioprotection induced by opioid agonists,73, 74 erythropoietin,69, 81, 82 phosphodiesterase-5 inhibitor sildenafil,50 PARP inhibition,83 anesthetics propofol and isoflurane,84-86 bradykinin,79 A1/A2 adenosine agonist NECA,77 exogenous Zn2+,87 acute PKCδ inhibition,88 and PKCε-activating peptides.75 Several free radical-scavenging compounds, aside from scavenging ROS, could also activate pro-survival kinases acting through GSK-3β-inhibition by its phosphorylation.89, 90 Delayed protection triggered by lipopolysacharide pretreatment91 as well as local adenovirus-mediated gene delivery of the vasoactive peptides, adrenomedullin,92 or kallikrein/kinin,93 are also dependent on GSK-3β-inhibition. It is noteworthy that cell protection mediated by GSK-3β-inhibition is not limited only to the heart, because the existence of a similar network of pro-survival kinases which can confer cell protection via inhibition of GSK-3β, has also been demonstrated in brain and kidney.10, 94-96
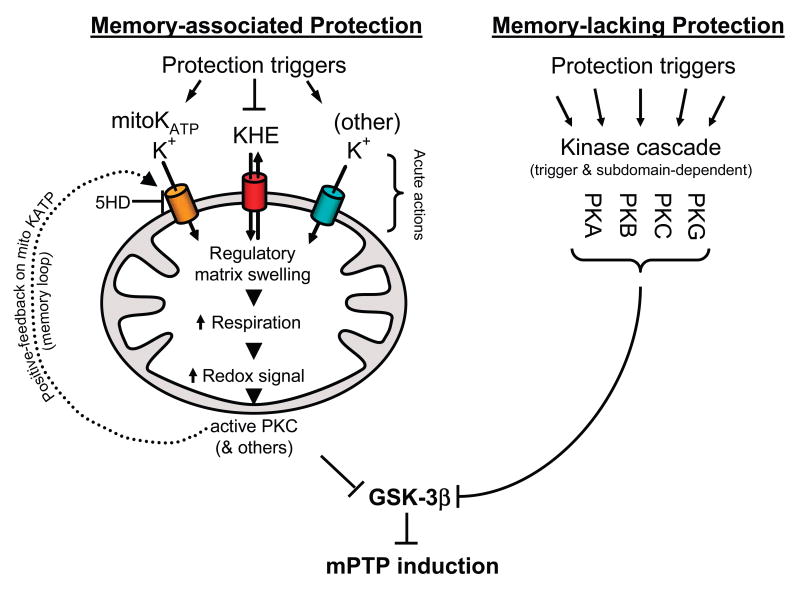
To gain a better understanding of the mechanisms underlying signaling pathways that lead to cell protection, we had undertaken an extensive study of a range of different triggers and pharmacologic agents that are known to induce a cardioprotective state.9 We proposed that protection signaling could be separated into two main mechanistic categories (Figure 1): memory-associated signaling, which results in what is known as preconditioning that persists for hours beyond the exposure to the trigger, and memory-lacking signaling, which results in relatively short-lived cell protection once the triggering agent (and likely the phosphorylation status of one or more key proteins) is cleared from the system. Ischemic and pharmacologic preconditioning (the latter induced by, for example, mitoKATP-openers, Na/H-exchange (NHE) inhibitors, δ-opioids, bradykinin, adenosine, or leptin), via complex kinase cascades (which include AKT, eNOS, NO, PKG and PKC; reviewed in15, 97), both trigger memory-associated signaling that is accompanied by mitochondrial influx and/or retention of K+, subsequent mitochondrial regulatory volume swelling (of merely ∼2-4%), slight-to-moderate activation of respiration, and production of ROS at levels serving as a local redox signal.9, 98 This ROS (i.e., redox) signal in turn activates a local pool of PKC, which in turn transmits the signal downstream to a local GSK-3β pool. In addition to its downstream effect on GSK-3β, we proposed that this PKC signal also serves to maintain the memory of the process in a positive feedback loop by sustaining the activation state of the mitoKATP9 (Figure 1). The experimental evidence to support this latter notion was for the first time demonstrated by Marbán's group.99
Figure 1. Scheme of enzyme-signaling-dependent protection against mPTP-induction.
Memory-associated protection (left), induced by triggers conveying upstream signaling either via kinases (including AKT, eNOS, guanylyl cyclase, PKG and PKC; not shown), or acting directly, engages mitochondrial targets associated with an increased K+-influx into, or retention by, mitochondria (induced by mitoKATP openers, inhibitors of mitochondrial K+/H+ exchange, as well as by agents that may increase K+-influx into mitochondria via other channels, such as BKCa), consequent regulatory mitochondrial matrix swelling, increased respiration producing a redox signal due to moderately increased ROS production which results in local PKC activation. Activated PKC, in turn, signals in a positive-feedback loop to mitoKATP producing the system memory which enables sustained (∼hours) inhibition of GSK-3β and consequent prolonged protection against mPTP-induction. Memory-lacking protection (right) triggered by a multitude of cell-surface receptor ligands or pharmacologic agents causing activation of diverse signaling pathways (e.g., PKA, PKB, PKC, PKG) that can bypass the mitochondrial volume regulatory mechanism and still converge on GSK-3β resulting in relatively short term protection of mPTP, determined, in part, by the duration of exposure to the triggering signal.
Therefore, mitoKATP could act as both activator and mediator of the cell protection. Consequently, phosphorylation-induced inactivation of the mitochondria-associated GSK-3β pool (which is but a small fraction of the entire cellular pool) transmits a downstream signal that results in protection by increasing the mPTP-induction ROS-threshold9 (reviewed in100).
Alternatively, memory-lacking signaling induced by, e.g., insulin, IGF, GLP-1, β2-adrenoreceptor activation, Li+ and small molecule GSK-3β inhibitors, erythropoietin and probably estrogen results in activation of one or more of the multiple signal transduction pathways, including PI3K, PKA, PKB/AKT, PKG, PKC and their downstream targets including GSK-3β.9 Recently, exogenous zinc was added to the list of agents exerting PI3K-GSK-3β-mediated cardioprotection. The effect of Zn2+ was not blocked by 5-HD,87 suggesting independence from mitoKATP mechanisms and that memory-lacking signaling might be involved. Memory-lacking protection signaling is not associated with regulatory mitochondrial swelling; however, it still requires the convergence of multiple signaling pathways that result in increasing the mPTP ROS-induction threshold. Certain drugs (e.g., adenosine) probably trigger elements consistent with both types of protection.9
Experiments using Li+, other small molecule GSK-3 inhibitors, as well as GSK-3β gene manipulation (knock-down and signal-resistant transgene expression) studies demonstrate that GSK-3β specifically is the point of integration for all the types of signaling tested. We concluded that both the memory-associated and the memory-lacking protection require GSK-3β, and that this enzyme, located proximally (in the sense of a signaling cascade) to the mPTP complex, acts as a master switch to convey a multiplicity of protective signals to their final point, the mPTP.9 Whether other kinase-signaling cascade can bypass this intersection under ordinary circumstances (i.e., in non-genetically-altered backgrounds which are unlikely to be confounded by unusual adaptations) remains to be determined.
Considerable and mounting evidence, summarized in this review, supports the key role of GSK-3β in cell protection. However, two recent papers question the significance of the pro-survival kinases, including the importance of GSK-3β inhibition, in cell protection.101, 102 Based on the analysis of the phosphoprotein levels in the mitochondria isolated from preconditioned hearts it was concluded that protein kinase-(including GSK-3β-) mediated changes in mitochondrial phosphoprotein levels, may not be required for the preconditioning-induced inhibition of the mPTP, but rather that preconditioning-induced reduction in oxidative stress is responsible for mPTP inhibition.101 In the second work, while postconditioning conferred significant cardioprotection, detected levels of phosphorylated PKB/AKT, ERK1/2, p70S6K and GSK-3β in whole tissue extracts from pig hearts were not significantly different between the postconditioned and control groups.102 The latter observation is in apparent contrast to a previous study also in pig heart, which reported that ischemic pre- and postconditioning induced significant increases (versus control) of the PKB/AKT, ERK1/2 and P70S6K phosphorylation, (although, only preconditioning and not postconditioning was cardioprotective).103 Furthermore, there may be unique aspects to the post-conditioning response in pigs versus other species.
These seeming contradictions related to the apparent role of kinases in cell protection might reflect some intrinsic methodological issues related to the studied phenomenon: for example, GSK-3β is a multifunctional kinase subserving discrete domain signaling functions across virtually all cell sub-compartments, and the specificity of the stimulus would be achieved, at least in part by selectively targeting/inhibiting the GSK-3β in regional compartments and microdomains. Whole cell or tissue lysate immunoblots would almost certainly but poorly resolve critical changes in mitochondrial sub-compartments or other subdomains.9 Indeed, we showed dramatically different whole-cell patterns of GSK-3β-phosphorylation immunostaining comparing diazoxide, HOE694, and insulin, but with notable overlap in the region of the sarcomeric/mitochondrial microdomain, and each afforded similar levels of protection.9 The antibody choice for detection of the Ser9-phosphorylated-GSK-3β is also very important. We have tested different commercially available antibodies and have found significant differences in their ability to specifically recognize the phosphorylation status of the Ser9-GSK-3β yielding large differences in apparent signal strength on immunoblotting. Furthermore, regional differences in the total and phosphorylated GSK-3β levels have been reported (e.g., persistently stunned region versus nonstunned region104 or ischemic versus normal zone73). While the location is understandably critical, the importance of timing, duration of the signal, level of GSK-3β inhibition and possible species differences also need to be stressed (reviewed in105). Additionally, it has also been found that particularly rigorous tissue extraction procedures are required for unambiguous detection of GSK-3β phosphorylation. Preparation of extracts without such adequate precautions resulted in almost complete loss of protein phosphorylation suggesting that the state of phosphorylation may be labile and/or transient and, hence, might be lost during certain mitochondrial isolation and purification protocols.72
Consequently, in light of the considerable weight of evidence summarized in this review, it is entirely premature to dismiss the role of kinases in most forms of cell protection signaling. We should point out that the foregoing discussion does not necessarily apply to scenarios such as acute acidosis, CsA or SfA exposures, which likely exert more direct effects on the mPTP (and on its proximal regulatory elements), as discussed elsewhere in this review.
2.5 Significance of GSK-3β versus GSK-3α
Because the available GSK-3 inhibitors are isoform unspecific, genetic manipulation approaches have necessarily been applied to determine the respective roles of the GSK-3β and GSK-3α isoforms in cell protection. RNAi-mediated gene silencing has been employed as a tool to independently control individual GSK-3-isoform levels in rat neonatal cardiomyocytes. Silencing of GSK-3β resulted in cell protection, while comparably decreasing the level of GSK-3α had no such effect.9 Similarly, silencing of GSK-3β in H9c2 cardiomyocytes increased cell tolerance against H2O2-induced cell death while silencing of GSK-3α had no effect.106 To confirm the unique role of the GSK-3β isoform in cell protection, transgenic mice (GSK-3β-S9A) with cardiac-specific expression of a constitutively active, signal resistant form of GSK-3β were also employed. Indeed, induction of both hypoxic and pharmacologic preconditioning failed in myocytes isolated from animals with this signal-resistant form of GSK-3β.9 Similarly, ischemic postconditioning failed to reduce infarct size in GSK-3β-S9A transgenic mice, while CsA, acting directly on the mPTP-regulatory element, CyP-D, was still able to induce protection.70 Additionally, in H9c2 myocytes transfected with constitutively active GSK-3β-S9A, erythropoietin106 as well as exogenous zinc87 failed to protect cells against oxidative stress. In contrast, cell transfected with “kinase-dead” GSK-3β-plasmids (K85M and K85R) were constitutively protected against oxidative stress.87, 106
Experiments on transgenic mice overexpressing FrzA in cardiomyocytes under a conditional transgene expression approach further strengthen the importance of GSK-3β in preconditioning. FrzA/sFRP-1 is a secreted antagonist of the Wnt/Frizzled pathway expressed in the heart, and it is able to significantly decrease the phosphorylation of GSK-3β at Ser9 independently of the PI3K/AKT signaling pathway (instead it probably involves activation of the PKC pathway). Overexpression of FrzA blocked the preconditioning-induced phosphorylation of GSK-3β, with a consequent increase in infarct size.107 These examples provide additional strong support for the likely indispensable role played by GSK-3β in integrating a host of upstream protective signaling pathways and conveying their signals to downstream target(s) located proximal to the mPTP, with the outcome of increasing the mPTP ROS-threshold.
Recently, a different mouse model was used in an attempt to verify the role of GSK-3 in cell protection. In these experiments, a genetically modified mouse with a knock-in of a signal-resistant GSK-3α and GSK-3β that cannot be phosphorylated on Ser21, and Ser9, respectively, was used. In contrast to the extensive evidence summarized above, in this instance, both preconditioning and postconditioning protocols were able to protect the heart of this homozygous GSK-3 double-knock-in mouse model. Therefore, in this particular mouse model, phosphorylation of GSK-3β on Ser9 was apparently not required for induction of protection.108
However, some specific and unexpected systemic phenotypic alterations have been noted in this double-knock-in model, possibly because of the pan-tissue (pan-cellular) expression of the knock-in transgenes. This unexpected set of phenotypes could result from the complex interplay of systemic changes initiated by and resulting from separate transgene-related perturbations of multiple organ systems feeding back and sensed throughout the body, causing further secondary and tertiary level changes likely unrelated to, and confounding, the primary transgene effect in a given tissue because it is not acting in isolation. The systemic alterations in the homozygous GSK-3 double-knock-in mouse model include: significantly higher body temperature, blood pressure, food and water intake, fecal excretion, glomerular filtration rate, urinary flow rate, urine osmolarity, as well as urinary excretion of Na+, K+, and urea,109 none of which are observed when the signal-resistant GSK-3β transgene expression is organ-restricted. It has been documented that inhibition of GSK-3β results in decreased expression of nephrin, a protein critically important for the integrity of the glomerular slit membrane,110 thus it was expected that genetic knock-in of a signal resistant GSK-3 would be protective against glomerural injury. Surprisingly, instead, development of the glomerular injury with spontaneous proteinuria was observed.111 Furthermore, in this specific model, additional discrepancies were found in studies focused on hepatic gene transcription. In the liver, insulin-triggered GSK-3-inhibition (i.e., phosphorylation of GSK-3α and β on Ser21, and Ser9, respectively) attenuates expression of the gluconeogenic genes, phosphoenolpyruvate carboxykinase, glucose-6-phosphatase, as well as insulin-like growth factor binding protein-1, while activation or overexpression of GSK-3 antagonizes the insulin effect. Surprisingly, in this insulin-insensitive double-knock-in mouse regulation of gluconeogenic genes was similar to that observed in wild-type.112 Because GSK-3 is involved in many different cell activities, the requisite specificities of these processes require a complex set of regulatory mechanisms, which for example, involves balancing the activities of priming kinases and phosphatases. Indeed, Lipina et al.112 hypothesized that the lack of a clear phenotype in this double knock-in model could be attributed to “functional compensation” by the priming kinases and phosphatases. Interestingly, an alternative pathway for the GSK-3β inactivation has been demonstrated recently. The activity of GSK-3β can be potently suppressed by MAPK-dependent phosphorylation of Ser389/Thr390 (mouse/human).26 Since p-38 MAPK activation can induce cardioprotection,113 this may explain both how p38-mediated protection operates in WT mice as well as how the knock-in mice may have developed alternative ways (e.g., utilizing p38 MAPK) to negatively regulate GSK-3β in the absence of the Ser9 regulatory site.
To resolve these apparent contradictions, found in multiple organ systems, with the vast array of data leading to the opposite conclusions from independent laboratories, further experimentation with this specific knock-in model and comparison with other models is necessary. As with many transgenic studies, unforeseen model-specific functional compensations might occur in a pan-cellular knock-in model that could mask or distort the anticipated phenotype resulting from the chronic absence of an inhibitable enzyme such as GSK-3 that participates in so many functions critical to cell development and homeostasis. Thus, in addition to the expected change(s) of function in each organ system due to its intrinsically altered genes, there are also likely potent extrinsic effects due to the complex interplay of systemic changes manifest by all of the altered organ systems upon each other. Chronic adaptations in a given organ system thus represent the complex and likely unpredictable integration of both intrinsic and extrinsic systemic mechanisms that potentially confound interpretation. It is well recognized from previous biochemical or pharmacological studies that complex functions might be altered in a particular genetically modified mouse.114 Region-specific and inducible knock-out may avoid some of the ontogenetic consequences inherent to conventional systemic knock-in/knockouts.115 Although certain TG mouse adaptations under the circumstances described above may in some functional way substitute for “WT” GSK-3 signaling, at this point, the vast weight of evidence, summarized in this review, still supports that GSK-3β serves as the convergence point of at least a significant proportion of signaling pathways conveying the protection signal to the mPTP under ordinary circumstances.
3. Downstream targets of GSK-3β proximal to mPTP
3.1 Bcl-2 family proteins as the downstream targets of GSK-3β
Recent publications in the field of cell protection reflect an intense search for the downstream molecular target(s) of GSK-3β and how they interact with the mPTP. For example, predicted GSK-3β phosphorylation motifs exist in Bcl-2 and the Bcl-2-binding protein, Bis 9 (although others are likely to exist). Since the function of Bcl-2 is known to be regulated via phosphorylation, a decrease in phosphorylation of serine-70, serine-87, or threonine-69, each of which is a potential target motif of GSK-3β, may represent an activation signal that has been shown to increase the antiapoptotic effect in certain cell types.116, 117 Additionally, in H9c2 cardiomyocytes, both the erythropoietin-induced inhibition of GSK-3β and a knock-down of GSK-3β resulted in decreased mitochondrial translocation of the “pro-apoptotic” protein, BAX.106 It has also been reported that in hematopoietic cell lines, interleukin-3 withdrawal-induced apoptosis is caused by GSK-3β-activation, which results in phosphorylation and degradation of the “anti-apoptotic” Bcl-2 family member, Mcl-1.118 GSK-3β-dependent phosphorylation and mitochondrial translocation of Bax has also been detected during neuronal apoptosis.119 Similarly, overexpression of GSK-3β has been shown to augment staurosporine-induced apoptosis in the neuroblastoma cell line, an effect which could be blocked by Li+.21
Although the works cited above seem to imply that GSK-3β-inhibition is typically pro-survival, it had been demonstrated that under certain conditions and in specific cell types, for example, involving recruitment of alternative members of the Bcl-2 family of proteins, GSK-3β-inhibition could also have a pro-apoptotic effect (reviewed in120). This typically applies for certain cancer cell lines that are characterized by apoptotic cell death (contrary to necrotic cell death, typically occurring, e.g., after an infarction of heart or brain, and causally linked to mPTP-induction58, 63). For example, in melanoma cell lines, sorafenib-induced apoptosis was enhanced by pharmacologic GSK-3β-inhibition. In this work sorafenib-induced GSK-3β-activation resulted in down-modulation of the pro-apoptotic Bcl-2 family member Noxa, which in turn weakened the lethality of the drug. In this scenario, GSK-3β-inhibition enhanced apoptosis by preventing down-regulation of the pro-apoptotic protein Noxa, an effect that was accompanied by reduction in the levels of the anti-apoptotic protein Mcl-1.121
We have to emphasize that for the most part information regarding the relationship between GSK-3β activity and function of Bcl-2 family of proteins is based on studies concerning apoptotic cell death. It appears that GSK-3β-inhibition plays a role in two mechanistically independent pro-survival effects, exerting both protection against stress-induced apoptosis and protection against necrosis. New data, based on experiments with the CyP-D knockout mouse, demonstrated that mPTP-induction, in cells such as cardiomyocytes, is most likely relevant to necrotic cell death and not to apoptosis.58, 60, 63 Although certain questions remain, nevertheless, it seems prudent that future discussions related to the pro-survival role of GSK-3β have to discriminate between necrotic cell death pathways in excitable cells such as cardiomyocytes triggered by mPTP-induction, and apoptotic death pathways typically studied in and relevant to various cancer cell lines.
Recent evidence supports the direct functional role of the Bcl-2-protein family in GSK-3β-related cardiomyocyte protection against mPTP-induction by ROS.9, 10 A direct link between the induction of preconditioning to protect against ischemia/reperfusion injury and GSK-3β phosphorylation-dependent modulation of mitochondrial Bcl-2 protein levels has been demonstrated.72 PKG activation and enhanced phosphorylation of GSK-3β, with a subsequent increase in the Bcl-2/BAX ratio, was implicated in preconditioning induced by inhibition of phosphodiesterase-5.122
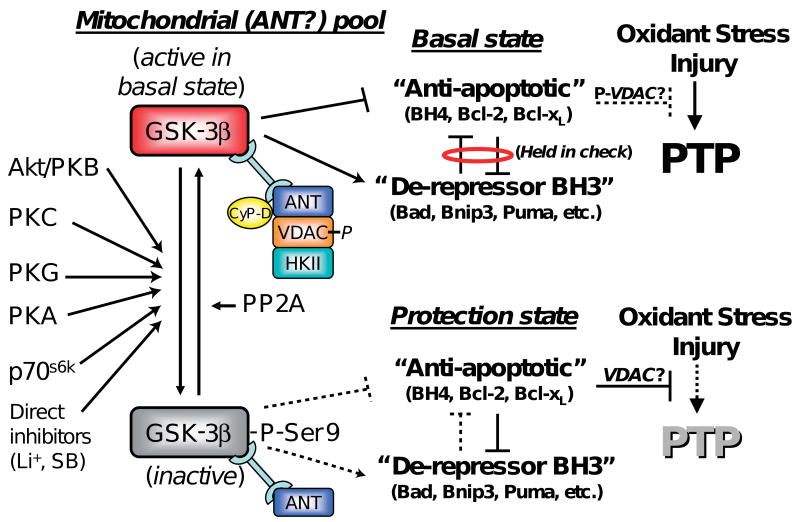
In addition to direct modulation of VDAC activity by phosphorylation, VDAC activity could be regulated by the binding of Bcl-2 family of proteins to VDAC. The conserved N-terminal homology (BH4) domain of BcL-xL has been shown to be necessary and sufficient in preventing apoptotic mitochondrial dysfunction and cell death in HeLa cells by directly binding to VDAC and blocking its activity.123 A similar degree of protection as achieved by different pharmacological agents was obtained by using a peptide comprised of the BH4-domain of BcL-xL fused to the protein-transduction domain of HIV-TAT (TAT-BH4). TAT-BH4 could reduce infarct size and protect the heart against ischemia-reperfusion injury.124 In a model of isolated cardiomyocytes, this peptide was also shown to increase the ROS-threshold for mPTP-induction.9, 10 It has also been demonstrated that preconditioning of cardiomyocytes triggered by a Na+/H+ inhibitor (HOE694) was attenuated by HA14-1, a small, cell-permeable non-peptidic ligand that is thought to bind to the Bcl-2 surface-pocket and block its biological action. Additionally, the enhancement of the mPTP ROS-threshold triggered by either HOE694, insulin, or Li+ (each acting via separate upstream mechanisms but converging via GSK-3β-inhibition) was each blocked by the BH3-peptide inhibitor of Bcl-2, a peptide derived from the BH3-domain of Bad that binds to Bcl-2 with high affinity, while a negative-control peptide with only a single amino acid change (L151A) rendering it more than one order of magnitude less avid for Bcl-2 binding, was completely ineffective to block protection in parallel experiments.10 Moreover, HA14-1 and additional small organic ligands (e.g., BH3Is and Chelerythrine) that share the ability to bind the BH3 domain of Bcl-2, specifically sensitized the mPTP to opening both in isolated mitochondria and in intact cells.125 Correspondingly, hearts treated with GSK-3 inhibitors showed increased levels of VDAC-bound Bcl-2 in mitochondria72 (see below). Thus, it was concluded that drugs acting upstream of GSK-3β (via PKC, PKB, P70S6-kinase, etc.) as well as by direct inhibition of GSK-3, all appear to afford protection by altering the functional balance of BH4- and BH3- domain protein related mechanisms10 (Figure 2).
Figure 2. Proposed model of mPTP modulation by GSK-3β.
The phosphorylation state of the mitochondrial-(ANT)-associated pool of GSK-3β contributes to the balance of Bcl-2-family protein effects, the result of which determines the resistance of the mPTP to oxidant stress. Basal state (top): the local GSK-3β pool (active in the basal state) binds ANT in a complex with phosphorylated VDAC, CyP-D and possibly other mPTP-regulatory elements. The basal state is established by the mutual antagonism holding in check the activities of the “anti-apoptotic” BH4 and the “de-repressor” BH3-only domain Bcl-2 protein family members. Note that certain direct inhibitors of GSK-3β do not necessarily change GSK-3β activity via phosphorylation-related mechanisms. Protection state (bottom): the protection signal induces inactivation/phosphorylation of this GSK-3β subdomain pool resulting in a shift in interaction within the mPTP regulatory complex elements because of subsequent modulation of the balance between opposing Bcl-2 family members in favor of the “anti-apoptotic” elements with consequent protection being manifested by an increase in mPTP-ROS threshold (modified from10).
In conclusion, the examples listed above provide strong functional evidence for the role of the Bcl-2 family of proteins in preconditioning-induced cardioprotection. We propose that this would also likely hold true for post-conditioning related mechanisms (although not necessarily in the case of the acute effects of acidosis or CsA, etc.)
3.2 Regulatory roles of cyclophilin D, ANT and VDAC
The physical identity of the mPTP remains a mystery. In view of new evidence based on genetic knockout studies, ANT and CyP-D (and possibly VDAC) are now being recognized not as essential core components of the pore but rather as pore regulators (see above). Experiments on CyP-D-knockout mice demonstrated that opening of the mPTP likely results in necrotic but not apoptotic cell death.58, 60, 63 The ability of the CyP-D ligand, CsA, to desensitize the mPTP was lost in the CyP-D-knockout mice. CyP-D possesses a peptidyl-prolyl cis-trans isomerase (PPIase) activity, which seems to play an important role in suppression of apoptosis126 but is probably not involved in mPTP-desensitization by CsA.127 Therefore, depending on circumstances, CyP-D binding and its enzymatic activity may appear to play different roles depending on the mechanism and type of cell death. Probably in tissues such are heart and brain, where reperfusion injury-triggered mPTP is a central concern, inhibition of CyP-D by CsA or by related ligands has a protective role while in cancer cells PPIase activity of CyP-D is likely responsible for suppression of apoptosis (for a current discussion see128). Recently, an intriguing new model of mPTP regulation by CyP-D was put forth which highlighted the underlying protective role of Pi. According this model the presence of CyP-D tends to prevent the intrinsic mPTP-desensitizing action of Pi by blocking the Pi-regulatory site on the mPTP (thus, CsA or genetic deletion of CyP-D may act by unmasking the direct mPTP-desensitizing action of Pi).66
A highly conserved region of the VDAC protein, amino acids 51 to 55, represents a GSK-3β phosphorylation consensus-motif. Experiments in HeLa cells showed that chemotherapeutic drug–induced cytotoxicity is increased by inhibition of the PI3-kinase/AKT pathway that leads to activation of mitochondrial GSK-3β, together with significant elevation of the levels of phosphorylated VDAC. VDAC phosphorylation by GSK-3β was associated with dissociation of HKII from VDAC and release of HKII from mitochondria.129 Moreover, forced detachment of HKII from mitochondria by treatment of cells with a peptide that interferes with VDAC binding resulted in activation of cell death pathways,129 an outcome that could be prevented by CyP-D- and ANT-inhibitors.130 In particular, we found that HKII detachment substantially decreased the mPTP-ROS-threshold in cardiomyocytes while a control peptide had no effect.130 Recently, it has been demonstrated that cardioprotection induced by GSK-3 inhibitors results in a significant decrease in VDAC phosphorylation along with increased levels of Bcl-2 in mitochondria and, specifically, increased Bcl-2 affinity for VDAC. Under these circumstances, transmission of the cardioprotective signal from GSK-3β to VDAC might be direct or mediated by phosphatases, as suggested by Das et al.72. For example, the serine/threonine protein phosphatase-2A (PP2A, subunit B) possesses a predicted consensus site for GSK-3β phosphorylation.9 It is plausible that GSK-3β regulation of PP2A adjacent to the mPTP complex site could also participate in transmitting the protection signal to downstream targets. Indeed, it was proposed that in 293T cells the cell death signal triggered by ceramide and staurosporine results in activation of PP2A in concert with inhibition of PKA/PKC and consequent dephosphorylation of BAD. The change in phosphorylation status of BAD modulates the interaction between Bad, BcL-xL and VDAC that results in mPTP-sensitization to Ca2+.131
The search for the downstream targets of GSK-3β in mitochondria lead to the observation that in mitochondrial sucrose gradient fractions GSK-3β co-sedimented with mPTP-regulatory/associated proteins, ANT and VDAC in the controls, and that it also appeared as phosphorylated GSK-3β in the same fraction in insulin treated hearts.9 In accordance with this finding, cardioprotection afforded by ischemic preconditioning in combination with infusion of erythropoietin resulted in increased levels of both total and phosphorylated GSK-3β in mitochondria.82 In this study, co-immunoprecipitation experiments demonstrated that the preconditioned heart shows a significantly enhanced association of phosphorylated GSK-3β with ANT (but not with VDAC), together with decreased levels of CyP-D co-immunoprecipitated with ANT. The authors speculated that in the preconditioned heart, an increase in phospho-GSK-3β binding to ANT results in reduced affinity of ANT to CyP-D, with consequent suppression of mPTP-opening and enhanced myocardial protection,82 a mechanism supported by certain parallels to that observed with CsA. Furthermore, it has been demonstrated that oxidative stress results in increased binding of CyP-D to the mitochondrial membranes.132 This is in agreement with the hypothesis that binding of mitochondrial CyP-D to ANT in the inner mitochondrial membrane tends to promote the mPTP.133 Interestingly, though, apoptotic cell death, induced by forced detachment of mitochondrial HKII in fibroblasts, was associated with a decreased interaction between ANT and CyP-D130 a scenario that might not extrapolate to mPTP-related survival mechanisms in cardiomyocytes.
3.3 Proposed model of mPTP modulation by GSK-3β
To summarize the role of GSK-3β in cardioprotection, we propose the following model of mPTP-protection against oxidant stress in cardiomyocytes (Figure 2): In the basal state, a GSK-3β subdomain pool relevant to mitochondrial signaling is active (i.e., not phosphorylated) and binds ANT, forming a dynamic complex with phosphorylated VDAC and CyP-D. In the basal state, the so-called “anti-apoptotic” members of the Bcl-2-protein family (e.g., BH4-domain containing proteins such as Bcl-2, Mcl-1 and Bcl-xL) would be held in-check in a functional sense by “de-repressor”, BH3-domain-only containing proteins (Bad, Bnip3, PUMA etc.) that act as BH4 de-repressors. We need to emphasize that our use of the obsolete Bcl-2-descriptive term, “anti-apoptotic”, is applied here in the context of protection against, primarily, necrotic cell death due to mPTP-inhibition, and the term “de-repressor” is being used in the sense of “opposing BH4 but not activating cell death per se” (i.e., to distinguish it from the BH3-containing death-effector proteins, e.g., BAX, BAK, etc.). According to this scheme, GSK-3β in the active (basal) state may participate by modulating the interaction between mPTP-regulatory components i.e., ANT and CyP-D (maybe VDAC), as well as the binding of various Bcl-2-family proteins. These interactions can contribute to maintaining a balance between opposing influences of these members of the Bcl-2-family proteins, the “anti-apoptotic” BH4-related proteins and the de-repressors BH3-only proteins thus producing the basal state of mPTP resistance to pore-induction. Kinase-signaling-dependent forms of ischemic/pharmacologic pre- or postconditioning results in the protection state which is associated with the phosphorylation and therefore inactivation of GSK-3β, which leads directly (or indirectly, by involving dephosphorylation of VDAC and the release of both VDAC and CyP-D from the GSK-3β-bound pool of ANT) to a shift in the balance within the Bcl-2 family of proteins in favor of “anti-apoptotic” proteins. Released, dephosphorylated VDAC exhibits increased affinity to HKII, as well as increased binding to BH4-domain-containing species, i.e., Bcl-2 and BcL-xL. These interactions also result in inhibition of VDAC activity which in turn may play some role in reducing cell death.72 Diminishing the interaction between VDAC-ANT-CyP-D may also participate in inducing this shift in the balance within the Bcl-2 family of proteins in favor of “anti-apoptotic” proteins, resulting in an increase in the ROS-threshold for mPTP-induction. It should be mentioned that VDACs are dispensable for mPTP formation, and currently there is no direct evidence about their mPTP-regulatory role either,59, 130 so the actual mechanism here remains uncertain. We submit that the model, as it is presented here, may require revision as new data become available.
Conclusion
Numerous lines of evidence suggest that the mPTP is a key end-effector of ischemic/pharmacologic pre- and post-conditioning. Reduction of infarct size could be achieved by increasing the resistance of cardiomyocytes against oxidant stress by increasing the mPTP-ROS-threshold. Since mPTP-induction is also associated with a ROS-burst which can be propagated to neighboring mitochondria and cells causing further mPTP-induction via ROS-induced ROS release, this protection will simultaneously reduce the magnitude of the reperfusion ROS-burst, which would simulate an “antioxidant-like” effect.
This review reflects recent research findings regarding signaling pathways activated during pre- and post-conditioning. It highlights the pro-survival role of acutely inhibiting a mitochondrial-associated microdomain pool of GSK-3β, and it portrays this kinase as an integration point where a wide spectrum of upstream protective signaling pathways converges and then conveys a protection signal to the mPTP against ROS stress. While in most scenarios the integration point seems to belong to GSK-3β, for the sake of logic and completeness we can not rule out the possibility that in certain circumstances GSK-3β may not be indispensable (e.g., chronic adaptations to a genetic GSK-3β functional deficiency) and another kinase could take its functional place or could be unmasked as an alternative integration point. Downstream of this GSK-3β mechanism, possibly acting through effects on mPTP-regulatory proteins ANT and CyP-D (and possible VDAC), engagement of the Bcl-2 family of proteins (and the regulation of the balance of BH4 versus BH3 actions) appears to transmit the protection signal to control the resistance of the mPTP to oxidant stress. Direct inhibition of GSK-3β has been shown to increase the mPTP-ROS threshold and to reduce infarct size, which has sparked interest in GSK-3β inhibitors as potential tools in eliciting cardioprotection in clinical settings. Because GSK-3β is a multifunctional kinase, widely distributed in many cellular compartments and serving a multitude of other cellular functions, some undesired effects may result from “unspecific” application of GSK-3β inhibitors, and in particular, over a chronic time frame.134 Therefore, caution should be exercised when considering GSK-3β as a therapeutic target. The intensive search for downstream targets of the mitochondrial pool of GSK-3β could expedite the identification of the mPTP regulatory elements, provide new insight into their interactions, and ultimately could assist in resolving the identity of the mPTP.
Acknowledgments
Sources of Funding: This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Aging.
Footnotes
Diclosures: None
References
- 1.Braunwald E, Kloner RA. Myocardial reperfusion: a double-edged sword? J Clin Invest. 1985;76:1713–1719. doi: 10.1172/JCI112160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jennings RB, Sommers HM, Smyth GA, Flack HA, Linn H. Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. Arch Pathol. 1960;70:68–78. [PubMed] [Google Scholar]
- 3.Zweier JL, Talukder MA. The role of oxidants and free radicals in reperfusion injury. Cardiovasc Res. 2006;70:181–190. doi: 10.1016/j.cardiores.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 4.Di Lisa F, Bernardi P. Mitochondria and ischemia-reperfusion injury of the heart: fixing a hole. Cardiovasc Res. 2006;70:191–199. doi: 10.1016/j.cardiores.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 5.Griffiths EJ, Halestrap AP. Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem J. 1995;307:93–98. doi: 10.1042/bj3070093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brady NR, Elmore SP, van Beek JJ, Krab K, Courtoy PJ, Hue L, Westerhoff HV. Coordinated behavior of mitochondria in both space and time: a reactive oxygen species-activated wave of mitochondrial depolarization. Biophys J. 2004;87:2022–2034. doi: 10.1529/biophysj.103.035097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med. 2000;192:1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta. 2006;1757:509–517. doi: 10.1016/j.bbabio.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 9.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113:1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Juhaszova M, Wang S, Zorov DB, Nuss HB, Gleichmann M, Mattson MP, Sollott SJ. The identity and regulation of the mitochondrial permeability transition pore: where the known meets the unknown. Ann N Y Acad Sci. 2008;1123:197–212. doi: 10.1196/annals.1420.023. [DOI] [PubMed] [Google Scholar]
- 11.Akar FG, Aon MA, Tomaselli GF, O'Rourke B. The mitochondrial origin of postischemic arrhythmias. J Clin Invest. 2005;115:3527–3535. doi: 10.1172/JCI25371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aon MA, Cortassa S, Akar FG, O'Rourke B. Mitochondrial criticality: a new concept at the turning point of life or death. Biochim Biophys Acta. 2006;1762:232–240. doi: 10.1016/j.bbadis.2005.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 14.Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 15.Yellon DM, Downey JM. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev. 2003;83:1113–1151. doi: 10.1152/physrev.00009.2003. [DOI] [PubMed] [Google Scholar]
- 16.Cohen MV, Yang XM, Downey JM. The pH hypothesis of postconditioning: staccato reperfusion reintroduces oxygen and perpetuates myocardial acidosis. Circulation. 2007;115:1895–1903. doi: 10.1161/CIRCULATIONAHA.106.675710. [DOI] [PubMed] [Google Scholar]
- 17.Lundmark JA, Trueblood N, Wang LF, Ramasamy R, Schaefer S. Repetitive acidosis protects the ischemic heart: implications for mechanisms in preconditioned hearts. J Mol Cell Cardiol. 1999;31:907–917. doi: 10.1006/jmcc.1998.0931. [DOI] [PubMed] [Google Scholar]
- 18.Nicolli A, Petronilli V, Bernardi P. Modulation of the mitochondrial cyclosporin A-sensitive permeability transition pore by matrix pH. Evidence that the pore open-closed probability is regulated by reversible histidine protonation. Biochemistry. 1993;32:4461–4465. doi: 10.1021/bi00067a039. [DOI] [PubMed] [Google Scholar]
- 19.Juhaszova M, Rabuel C, Zorov DB, Lakatta EG, Sollott SJ. Protection in the aged heart: preventing the heart-break of old age? Cardiovasc Res. 2005;66:233–244. doi: 10.1016/j.cardiores.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 20.Rylatt DB, Aitken A, Bilham T, Condon GD, Embi N, Cohen P. Glycogen synthase from rabbit skeletal muscle. Amino acid sequence at the sites phosphorylated by glycogen synthase kinase-3, and extension of the N-terminal sequence containing the site phosphorylated by phosphorylase kinase. Eur J Biochem. 1980;107:529–537. [PubMed] [Google Scholar]
- 21.Bijur GN, Jope RS. Glycogen synthase kinase-3 beta is highly activated in nuclei and mitochondria. Neuroreport. 2003;14:2415–2419. doi: 10.1097/00001756-200312190-00025. [DOI] [PubMed] [Google Scholar]
- 22.Kockeritz L, Doble B, Patel S, Woodgett JR. Glycogen synthase kinase-3--an overview of an over-achieving protein kinase. Curr Drug Targets. 2006;7:1377–1388. doi: 10.2174/1389450110607011377. [DOI] [PubMed] [Google Scholar]
- 23.Woodgett JR. Molecular cloning and expression of glycogen synthase kinase-3/factor A. Embo J. 1990;9:2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woodgett JR, Cohen P. Multisite phosphorylation of glycogen synthase. Molecular basis for the substrate specificity of glycogen synthase kinase-3 and casein kinase-II (glycogen synthase kinase-5) Biochim Biophys Acta. 1984;788:339–347. doi: 10.1016/0167-4838(84)90047-5. [DOI] [PubMed] [Google Scholar]
- 25.Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2:769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- 26.Thornton TM, Pedraza-Alva G, Deng B, Wood CD, Aronshtam A, Clements JL, Sabio G, Davis RJ, Matthews DE, Doble B, Rincon M. Phosphorylation by p38 MAPK as an alternative pathway for GSK3beta inactivation. Science. 2008;320:667–670. doi: 10.1126/science.1156037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughes K, Nikolakaki E, Plyte SE, Totty NF, Woodgett JR. Modulation of the glycogen synthase kinase-3 family by tyrosine phosphorylation. Embo J. 1993;12:803–808. doi: 10.1002/j.1460-2075.1993.tb05715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phiel CJ, Wilson CA, Lee VM, Klein PS. GSK-3alpha regulates production of Alzheimer's disease amyloid-beta peptides. Nature. 2003;423:435–439. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- 29.Matsuda T, Zhai P, Maejima Y, Hong C, Gao S, Tian B, Goto K, Takagi H, Tamamori-Adachi M, Kitajima S, Sadoshima J. Distinct roles of GSK-3alpha and GSK-3beta phosphorylation in the heart under pressure overload. Proc Natl Acad Sci U S A. 2008;105:20900–20905. doi: 10.1073/pnas.0808315106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medina M, Castro A. Glycogen synthase kinase-3 (GSK-3) inhibitors reach the clinic. Curr Opin Drug Discov Devel. 2008;11:533–543. [PubMed] [Google Scholar]
- 31.Martinez A, Alonso M, Castro A, Dorronsoro I, Gelpi JL, Luque FJ, Perez C, Moreno FJ. SAR and 3D-QSAR studies on thiadiazolidinone derivatives: exploration of structural requirements for glycogen synthase kinase 3 inhibitors. J Med Chem. 2005;48:7103–7112. doi: 10.1021/jm040895g. [DOI] [PubMed] [Google Scholar]
- 32.Philipp S, Yang XM, Cui L, Davis AM, Downey JM, Cohen MV. Postconditioning protects rabbit hearts through a protein kinase C-adenosine A2b receptor cascade. Cardiovasc Res. 2006;70:308–314. doi: 10.1016/j.cardiores.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 33.Liu GS, Thornton J, Van Winkle DM, Stanley AW, Olsson RA, Downey JM. Protection against infarction afforded by preconditioning is mediated by A1 adenosine receptors in rabbit heart. Circulation. 1991;84:350–356. doi: 10.1161/01.cir.84.1.350. [DOI] [PubMed] [Google Scholar]
- 34.Xi L, Das A, Zhao ZQ, Merino VF, Bader M, Kukreja RC. Loss of myocardial ischemic postconditioning in adenosine A1 and bradykinin B2 receptors gene knockout mice. Circulation. 2008;118:S32–37. doi: 10.1161/CIRCULATIONAHA.107.752865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tissier R, Berdeaux A, Ghaleh B, Couvreur N, Krieg T, Cohen MV, Downey JM. Making the heart resistant to infarction: how can we further decrease infarct size? Front Biosci. 2008;13:284–301. doi: 10.2741/2679. [DOI] [PubMed] [Google Scholar]
- 36.Staat P, Rioufol G, Piot C, Cottin Y, Cung TT, L'Huillier I, Aupetit JF, Bonnefoy E, Finet G, Andre-Fouet X, Ovize M. Postconditioning the human heart. Circulation. 2005;112:2143–2148. doi: 10.1161/CIRCULATIONAHA.105.558122. [DOI] [PubMed] [Google Scholar]
- 37.Thibault H, Piot C, Staat P, Bontemps L, Sportouch C, Rioufol G, Cung TT, Bonnefoy E, Angoulvant D, Aupetit JF, Finet G, Andre-Fouet X, Macia JC, Raczka F, Rossi R, Itti R, Kirkorian G, Derumeaux G, Ovize M. Long-term benefit of postconditioning. Circulation. 2008;117:1037–1044. doi: 10.1161/CIRCULATIONAHA.107.729780. [DOI] [PubMed] [Google Scholar]
- 38.Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, Elbelghiti R, Cung TT, Bonnefoy E, Angoulvant D, Macia C, Raczka F, Sportouch C, Gahide G, Finet G, Andre-Fouet X, Revel D, Kirkorian G, Monassier JP, Derumeaux G, Ovize M. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359:473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 39.Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: united at reperfusion. Pharmacol Ther. 2007;116:173–191. doi: 10.1016/j.pharmthera.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Murphy E, Steenbergen C. Does inhibition of glycogen synthase kinase protect in mice? Circ Res. 2008;103:226–228. doi: 10.1161/CIRCRESAHA.108.181602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garlid KD, Paucek P, Yarov-Yarovoy V, Murray HN, Darbenzio RB, D'Alonzo AJ, Lodge NJ, Smith MA, Grover GJ. Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels. Possible mechanism of cardioprotection. Circ Res. 1997;81:1072–1082. doi: 10.1161/01.res.81.6.1072. [DOI] [PubMed] [Google Scholar]
- 42.Gross GJ, Fryer RM. Sarcolemmal versus mitochondrial ATP-sensitive K+ channels and myocardial preconditioning. Circ Res. 1999;84:973–979. doi: 10.1161/01.res.84.9.973. [DOI] [PubMed] [Google Scholar]
- 43.Hanley PJ, Gopalan KV, Lareau RA, Srivastava DK, von Meltzer M, Daut J. Beta-oxidation of 5-hydroxydecanoate, a putative blocker of mitochondrial ATP-sensitive potassium channels. J Physiol. 2003;547:387–393. doi: 10.1113/jphysiol.2002.037044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lim KH, Javadov SA, Das M, Clarke SJ, Suleiman MS, Halestrap AP. The effects of ischaemic preconditioning, diazoxide and 5-hydroxydecanoate on rat heart mitochondrial volume and respiration. J Physiol. 2002;545:961–974. doi: 10.1113/jphysiol.2002.031484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen W, Gabel S, Steenbergen C, Murphy E. A redox-based mechanism for cardioprotection induced by ischemic preconditioning in perfused rat heart. Circ Res. 1995;77:424–429. doi: 10.1161/01.res.77.2.424. [DOI] [PubMed] [Google Scholar]
- 46.Forbes RA, Steenbergen C, Murphy E. Diazoxide-induced cardioprotection requires signaling through a redox-sensitive mechanism. Circ Res. 2001;88:802–809. doi: 10.1161/hh0801.089342. [DOI] [PubMed] [Google Scholar]
- 47.Liu GS, Cohen MV, Mochly-Rosen D, Downey JM. Protein kinase C-epsilon is responsible for the protection of preconditioning in rabbit cardiomyocytes. J Mol Cell Cardiol. 1999;31:1937–1948. doi: 10.1006/jmcc.1999.1026. [DOI] [PubMed] [Google Scholar]
- 48.Liu Y, Gao WD, O'Rourke B, Marban E. Synergistic modulation of ATP-sensitive K+ currents by protein kinase C and adenosine. Implications for ischemic preconditioning. Circ Res. 1996;78:443–454. doi: 10.1161/01.res.78.3.443. [DOI] [PubMed] [Google Scholar]
- 49.Cohen MV, Yang XM, Downey JM. Nitric oxide is a preconditioning mimetic and cardioprotectant and is the basis of many available infarct-sparing strategies. Cardiovasc Res. 2006;70:231–239. doi: 10.1016/j.cardiores.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 50.Das A, Xi L, Kukreja RC. Protein kinase G-dependent cardioprotective mechanism of phosphodiesterase-5 inhibition involves phosphorylation of ERK and GSK3beta. J Biol Chem. 2008;283:29572–29585. doi: 10.1074/jbc.M801547200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hunter DR, Haworth RA. The Ca2+-induced membrane transition in mitochondria. I. The protective mechanisms. Arch Biochem Biophys. 1979;195:453–459. doi: 10.1016/0003-9861(79)90371-0. [DOI] [PubMed] [Google Scholar]
- 52.Crompton M, Costi A. A heart mitochondrial Ca2(+)-dependent pore of possible relevance to re-perfusion-induced injury. Evidence that ADP facilitates pore interconversion between the closed and open states. Biochem J. 1990;266:33–39. doi: 10.1042/bj2660033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Javadov SA, Clarke S, Das M, Griffiths EJ, Lim KH, Halestrap AP. Ischaemic preconditioning inhibits opening of mitochondrial permeability transition pores in the reperfused rat heart. J Physiol. 2003;549:513–524. doi: 10.1113/jphysiol.2003.034231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nicolli A, Basso E, Petronilli V, Wenger RM, Bernardi P. Interactions of cyclophilin with the mitochondrial inner membrane and regulation of the permeability transition pore, and cyclosporin A-sensitive channel. J Biol Chem. 1996;271:2185–2192. doi: 10.1074/jbc.271.4.2185. [DOI] [PubMed] [Google Scholar]
- 55.Argaud L, Gateau-Roesch O, Raisky O, Loufouat J, Robert D, Ovize M. Postconditioning inhibits mitochondrial permeability transition. Circulation. 2005;111:194–197. doi: 10.1161/01.CIR.0000151290.04952.3B. [DOI] [PubMed] [Google Scholar]
- 56.Hausenloy DJ, Maddock HL, Baxter GF, Yellon DM. Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning? Cardiovasc Res. 2002;55:534–543. doi: 10.1016/s0008-6363(02)00455-8. [DOI] [PubMed] [Google Scholar]
- 57.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341:233–249. [PMC free article] [PubMed] [Google Scholar]
- 58.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 59.Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D. J Biol Chem. 2005;280:18558–18561. doi: 10.1074/jbc.C500089200. [DOI] [PubMed] [Google Scholar]
- 61.Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR, Wallace DC. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krauskopf A, Eriksson O, Craigen WJ, Forte MA, Bernardi P. Properties of the permeability transition in VDAC1(-/-) mitochondria. Biochim Biophys Acta. 2006;1757:590–595. doi: 10.1016/j.bbabio.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 63.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 64.Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci U S A. 2005;102:12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leung AW, Varanyuwatana P, Halestrap AP. The mitochondrial phosphate carrier interacts with cyclophilin D and may play a key role in the permeability transition. J Biol Chem. 2008;283:26312–26323. doi: 10.1074/jbc.M805235200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Basso E, Petronilli V, Forte MA, Bernardi P. Phosphate is essential for inhibition of the mitochondrial permeability transition pore by cyclosporin A and by cyclophilin D ablation. J Biol Chem. 2008;283:26307–26311. doi: 10.1074/jbc.C800132200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tong H, Imahashi K, Steenbergen C, Murphy E. Phosphorylation of glycogen synthase kinase-3beta during preconditioning through a phosphatidylinositol-3-kinase--dependent pathway is cardioprotective. Circ Res. 2002;90:377–379. doi: 10.1161/01.res.0000012567.95445.55. [DOI] [PubMed] [Google Scholar]
- 68.Kaga S, Zhan L, Altaf E, Maulik N. Glycogen synthase kinase-3beta/beta-catenin promotes angiogenic and anti-apoptotic signaling through the induction of VEGF, Bcl-2 and survivin expression in rat ischemic preconditioned myocardium. J Mol Cell Cardiol. 2006;40:138–147. doi: 10.1016/j.yjmcc.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 69.Nishihara M, Miura T, Miki T, Sakamoto J, Tanno M, Kobayashi H, Ikeda Y, Ohori K, Takahashi A, Shimamoto K. Erythropoietin affords additional cardioprotection to preconditioned hearts by enhanced phosphorylation of glycogen synthase kinase-3 beta. Am J Physiol Heart Circ Physiol. 2006;291:H748–755. doi: 10.1152/ajpheart.00837.2005. [DOI] [PubMed] [Google Scholar]
- 70.Gomez L, Paillard M, Thibault H, Derumeaux G, Ovize M. Inhibition of GSK3beta by postconditioning is required to prevent opening of the mitochondrial permeability transition pore during reperfusion. Circulation. 2008;117:2761–2768. doi: 10.1161/CIRCULATIONAHA.107.755066. [DOI] [PubMed] [Google Scholar]
- 71.Gross ER, Hsu AK, Gross GJ. Delayed cardioprotection afforded by the glycogen synthase kinase 3 inhibitor SB-216763 occurs via a KATP- and MPTP-dependent mechanism at reperfusion. Am J Physiol Heart Circ Physiol. 2008;294:H1497–1500. doi: 10.1152/ajpheart.01381.2007. [DOI] [PubMed] [Google Scholar]
- 72.Das S, Wong R, Rajapakse N, Murphy E, Steenbergen C. Glycogen synthase kinase 3 inhibition slows mitochondrial adenine nucleotide transport and regulates voltage-dependent anion channel phosphorylation. Circ Res. 2008;103:983–991. doi: 10.1161/CIRCRESAHA.108.178970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gross ER, Hsu AK, Gross GJ. Opioid-induced cardioprotection occurs via glycogen synthase kinase beta inhibition during reperfusion in intact rat hearts. Circ Res. 2004;94:960–966. doi: 10.1161/01.RES.0000122392.33172.09. [DOI] [PubMed] [Google Scholar]
- 74.Gross ER, Hsu AK, Gross GJ. The JAK/STAT pathway is essential for opioid-induced cardioprotection: JAK2 as a mediator of STAT3, Akt, and GSK-3 beta. Am J Physiol Heart Circ Physiol. 2006;291:H827–834. doi: 10.1152/ajpheart.00003.2006. [DOI] [PubMed] [Google Scholar]
- 75.Korzick DH, Kostyak JC, Hunter JC, Saupe KW. Local delivery of PKCepsilon-activating peptide mimics ischemic preconditioning in aged hearts through GSK-3beta but not F1-ATPase inactivation. Am J Physiol Heart Circ Physiol. 2007;293:H2056–2063. doi: 10.1152/ajpheart.00403.2007. [DOI] [PubMed] [Google Scholar]
- 76.Obame FN, Plin-Mercier C, Assaly R, Zini R, Dubois-Rande JL, Berdeaux A, Morin D. Cardioprotective effect of morphine and a blocker of glycogen synthase kinase 3 beta, SB216763 [3-(2,4-dichlorophenyl)-4(1-methyl-1H-indol-3-yl)-1H-pyrrole-2,5-dione], via inhibition of the mitochondrial permeability transition pore. J Pharmacol Exp Ther. 2008;326:252–258. doi: 10.1124/jpet.108.138008. [DOI] [PubMed] [Google Scholar]
- 77.Forster K, Paul I, Solenkova N, Staudt A, Cohen MV, Downey JM, Felix SB, Krieg T. NECA at reperfusion limits infarction and inhibits formation of the mitochondrial permeability transition pore by activating p70S6 kinase. Basic Res Cardiol. 2006;101:319–326. doi: 10.1007/s00395-006-0593-4. [DOI] [PubMed] [Google Scholar]
- 78.Park SS, Zhao H, Jang Y, Mueller RA, Xu Z. N6-(3-iodobenzyl)-adenosine-5′-N-methylcarboxamide confers cardioprotection at reperfusion by inhibiting mitochondrial permeability transition pore opening via glycogen synthase kinase 3 beta. J Pharmacol Exp Ther. 2006;318:124–131. doi: 10.1124/jpet.106.101477. [DOI] [PubMed] [Google Scholar]
- 79.Park SS, Zhao H, Mueller RA, Xu Z. Bradykinin prevents reperfusion injury by targeting mitochondrial permeability transition pore through glycogen synthase kinase 3beta. J Mol Cell Cardiol. 2006;40:708–716. doi: 10.1016/j.yjmcc.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 80.Katoh H, Tanaka T, Saotome M, Urushida T, Satoh H, Hayashi H. PKA Inhibited The Opening Of Mitochondrial Permeability Tansiton Pore Induced By Cytosolic GSK3β. Biophys J. 2009;96:531a. [Google Scholar]
- 81.Kim KH, Oudit GY, Backx PH. Erythropoietin protects against doxorubicin-induced cardiomyopathy via a phosphatidylinositol 3-kinase-dependent pathway. J Pharmacol Exp Ther. 2008;324:160–169. doi: 10.1124/jpet.107.125773. [DOI] [PubMed] [Google Scholar]
- 82.Nishihara M, Miura T, Miki T, Tanno M, Yano T, Naitoh K, Ohori K, Hotta H, Terashima Y, Shimamoto K. Modulation of the mitochondrial permeability transition pore complex in GSK-3beta-mediated myocardial protection. J Mol Cell Cardiol. 2007;43:564–570. doi: 10.1016/j.yjmcc.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 83.Kovacs K, Toth A, Deres P, Kalai T, Hideg K, Gallyas F, Jr, Sumegi B. Critical role of PI3-kinase/Akt activation in the PARP inhibitor induced heart function recovery during ischemia-reperfusion. Biochem Pharmacol. 2006;71:441–452. doi: 10.1016/j.bcp.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 84.Feng J, Lucchinetti E, Ahuja P, Pasch T, Perriard JC, Zaugg M. Isoflurane postconditioning prevents opening of the mitochondrial permeability transition pore through inhibition of glycogen synthase kinase 3beta. Anesthesiology. 2005;103:987–995. doi: 10.1097/00000542-200511000-00013. [DOI] [PubMed] [Google Scholar]
- 85.Kamada N, Kanaya N, Hirata N, Kimura S, Namiki A. Cardioprotective effects of propofol in isolated ischemia-reperfused guinea pig hearts: role of KATP channels and GSK-3beta. Can J Anaesth. 2008;55:595–605. doi: 10.1007/BF03021433. [DOI] [PubMed] [Google Scholar]
- 86.Pagel PS, Krolikowski JG, Neff DA, Weihrauch D, Bienengraeber M, Kersten JR, Warltier DC. Inhibition of glycogen synthase kinase enhances isoflurane-induced protection against myocardial infarction during early reperfusion in vivo. Anesth Analg. 2006;102:1348–1354. doi: 10.1213/01.ane.0000202379.61338.37. [DOI] [PubMed] [Google Scholar]
- 87.Chanoit G, Lee S, Xi J, Zhu M, McIntosh RA, Mueller RA, Norfleet EA, Xu Z. Exogenous zinc protects cardiac cells from reperfusion injury by targeting mitochondrial permeability transition pore through inactivation of glycogen synthase kinase-3beta. Am J Physiol Heart Circ Physiol. 2008;295:H1227–H1233. doi: 10.1152/ajpheart.00610.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kostyak JC, Hunter JC, Korzick DH. Acute PKCdelta inhibition limits ischaemia-reperfusion injury in the aged rat heart: role of GSK-3beta. Cardiovasc Res. 2006;70:325–334. doi: 10.1016/j.cardiores.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 89.Toth A, Kovacs K, Deres P, Halmosi R, Czopf L, Hanto K, Kalai T, Hideg K, Sumegi B, Toth K. Impact of a novel cardioprotective agent on the ischaemia-reperfusion-induced Akt kinase activation. Biochem Pharmacol. 2003;66:2263–2272. doi: 10.1016/j.bcp.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 90.Toth A, Halmosi R, Kovacs K, Deres P, Kalai T, Hideg K, Toth K, Sumegi B. Akt activation induced by an antioxidant compound during ischemia-reperfusion. Free Radic Biol Med. 2003;35:1051–1063. doi: 10.1016/s0891-5849(03)00467-2. [DOI] [PubMed] [Google Scholar]
- 91.Ha T, Hua F, Liu X, Ma J, McMullen JR, Shioi T, Izumo S, Kelley J, Gao X, Browder W, Williams DL, Kao RL, Li C. Lipopolysaccharide-induced myocardial protection against ischaemia/reperfusion injury is mediated through a PI3K/Akt-dependent mechanism. Cardiovasc Res. 2008;78:546–553. doi: 10.1093/cvr/cvn037. [DOI] [PubMed] [Google Scholar]
- 92.Yin H, Chao L, Chao J. Adrenomedullin protects against myocardial apoptosis after ischemia/reperfusion through activation of Akt-GSK signaling. Hypertension. 2004;43:109–116. doi: 10.1161/01.HYP.0000103696.60047.55. [DOI] [PubMed] [Google Scholar]
- 93.Yin H, Chao L, Chao J. Kallikrein/kinin protects against myocardial apoptosis after ischemia/reperfusion via Akt-glycogen synthase kinase-3 and Akt-Bad.14-3-3 signaling pathways. J Biol Chem. 2005;280:8022–8030. doi: 10.1074/jbc.M407179200. [DOI] [PubMed] [Google Scholar]
- 94.Gao X, Zhang H, Takahashi T, Hsieh J, Liao J, Steinberg GK, Zhao H. The Akt signaling pathway contributes to postconditioning's protection against stroke; the protection is associated with the MAPK and PKC pathways. J Neurochem. 2008;105:943–955. doi: 10.1111/j.1471-4159.2008.05218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Koh SH, Park Y, Song CW, Kim JG, Kim K, Kim J, Kim MH, Lee SR, Kim DW, Yu HJ, Chang DI, Hwang SJ, Kim SH. The effect of PARP inhibitor on ischaemic cell death, its related inflammation and survival signals. Eur J Neurosci. 2004;20:1461–1472. doi: 10.1111/j.1460-9568.2004.03632.x. [DOI] [PubMed] [Google Scholar]
- 96.Plotnikov EY, Kazachenko AV, Vyssokikh MY, Vasileva AK, Tcvirkun DV, Isaev NK, Kirpatovsky VI, Zorov DB. The role of mitochondria in oxidative and nitrosative stress during ischemia/reperfusion in the rat kidney. Kidney Int. 2007;72:1493–1502. doi: 10.1038/sj.ki.5002568. [DOI] [PubMed] [Google Scholar]
- 97.Downey JM, Davis AM, Cohen MV. Signaling pathways in ischemic preconditioning. Heart Fail Rev. 2007;12:181–188. doi: 10.1007/s10741-007-9025-2. [DOI] [PubMed] [Google Scholar]
- 98.Garlid KD, Dos Santos P, Xie ZJ, Costa AD, Paucek P. Mitochondrial potassium transport: the role of the mitochondrial ATP-sensitive K(+) channel in cardiac function and cardioprotection. Biochim Biophys Acta. 2003;1606:1–21. doi: 10.1016/s0005-2728(03)00109-9. [DOI] [PubMed] [Google Scholar]
- 99.Sato T, Sasaki N, O'Rourke B, Marban E. Adenosine primes the opening of mitochondrial ATP-sensitive potassium channels: a key step in ischemic preconditioning? Circulation. 2000;102:800–805. doi: 10.1161/01.cir.102.7.800. [DOI] [PubMed] [Google Scholar]
- 100.Hanley PJ, Daut J. K(ATP) channels and preconditioning: a re-examination of the role of mitochondrial K(ATP) channels and an overview of alternative mechanisms. J Mol Cell Cardiol. 2005;39:17–50. doi: 10.1016/j.yjmcc.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 101.Clarke SJ, Khaliulin I, Das M, Parker JE, Heesom KJ, Halestrap AP. Inhibition of mitochondrial permeability transition pore opening by ischemic preconditioning is probably mediated by reduction of oxidative stress rather than mitochondrial protein phosphorylation. Circ Res. 2008;102:1082–1090. doi: 10.1161/CIRCRESAHA.107.167072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Skyschally A, van Caster P, Boengler K, Gres P, Musiolik J, Schilawa D, Schulz R, Heusch G. Ischemic postconditioning in pigs: no causal role for RISK activation. Circ Res. 2009;104:15–18. doi: 10.1161/CIRCRESAHA.108.186429. [DOI] [PubMed] [Google Scholar]
- 103.Schwartz LM, Lagranha CJ. Ischemic postconditioning during reperfusion activates Akt and ERK without protecting against lethal myocardial ischemia-reperfusion injury in pigs. Am J Physiol Heart Circ Physiol. 2006;290:H1011–1018. doi: 10.1152/ajpheart.00864.2005. [DOI] [PubMed] [Google Scholar]
- 104.Kim SJ, Peppas A, Hong SK, Yang G, Huang Y, Diaz G, Sadoshima J, Vatner DE, Vatner SF. Persistent stunning induces myocardial hibernation and protection: flow/function and metabolic mechanisms. Circ Res. 2003;92:1233–1239. doi: 10.1161/01.RES.0000076892.18394.B6. [DOI] [PubMed] [Google Scholar]
- 105.Murphy E, Steenbergen C. Inhibition of GSK-3beta as a target for cardioprotection: the importance of timing, location, duration and degree of inhibition. Expert Opin Ther Targets. 2005;9:447–456. doi: 10.1517/14728222.9.3.447. [DOI] [PubMed] [Google Scholar]
- 106.Ohori K, Miura T, Tanno M, Miki T, Sato T, Ishikawa S, Horio Y, Shimamoto K. Ser9 phosphorylation of mitochondrial GSK-3beta is a primary mechanism of cardiomyocyte protection by erythropoietin against oxidant-induced apoptosis. Am J Physiol Heart Circ Physiol. 2008;295:H2079–2086. doi: 10.1152/ajpheart.00092.2008. [DOI] [PubMed] [Google Scholar]
- 107.Barandon L, Dufourcq P, Costet P, Moreau C, Allieres C, Daret D, Dos Santos P, Daniel Lamaziere JM, Couffinhal T, Duplaa C. Involvement of FrzA/sFRP-1 and the Wnt/frizzled pathway in ischemic preconditioning. Circ Res. 2005;96:1299–1306. doi: 10.1161/01.RES.0000171895.06914.2c. [DOI] [PubMed] [Google Scholar]
- 108.Nishino Y, Webb IG, Davidson SM, Ahmed AI, Clark JE, Jacquet S, Shah AM, Miura T, Yellon DM, Avkiran M, Marber MS. Glycogen synthase kinase-3 inactivation is not required for ischemic preconditioning or postconditioning in the mouse. Circ Res. 2008;103:307–314. doi: 10.1161/CIRCRESAHA.107.169953. [DOI] [PubMed] [Google Scholar]
- 109.Boini KM, Bhandaru M, Mack A, Lang F. Steroid hormone release as well as renal water and electrolyte excretion of mice expressing PKB/SGK-resistant GSK3. Pflugers Arch. 2008;456:1207–1216. doi: 10.1007/s00424-008-0483-8. [DOI] [PubMed] [Google Scholar]
- 110.Doble BW, Woodgett JR. Role of glycogen synthase kinase-3 in cell fate and epithelial-mesenchymal transitions. Cells Tissues Organs. 2007;185:73–84. doi: 10.1159/000101306. [DOI] [PubMed] [Google Scholar]
- 111.Boini KM, Amann K, Kempe D, Alessi DR, Lang F. Proteinuria in mice expressing PKB/SGK-resistant GSK3. Am J Physiol Renal Physiol. 2009;296:F153–159. doi: 10.1152/ajprenal.90398.2008. [DOI] [PubMed] [Google Scholar]
- 112.Lipina C, Huang X, Finlay D, McManus EJ, Alessi DR, Sutherland C. Analysis of hepatic gene transcription in mice expressing insulin-insensitive GSK3. Biochem J. 2005;392:633–639. doi: 10.1042/BJ20051046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kabir AM, Cao X, Gorog DA, Tanno M, Bassi R, Bellahcene M, Quinlan RA, Davis RJ, Flavell RA, Shattock MJ, Marber MS. Antimycin A induced cardioprotection is dependent on pre-ischemic p38-MAPK activation but independent of MKK3. J Mol Cell Cardiol. 2005;39:709–717. doi: 10.1016/j.yjmcc.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 114.Picciotto MR, Wickman K. Using knockout and transgenic mice to study neurophysiology and behavior. Physiol Rev. 1998;78:1131–1163. doi: 10.1152/physrev.1998.78.4.1131. [DOI] [PubMed] [Google Scholar]
- 115.Holschneider DP, Shih JC. Genotype to phenotype: challenges and opportunities. Int J Dev Neurosci. 2000;18:615–618. doi: 10.1016/s0736-5748(00)00026-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Haldar S, Jena N, Croce CM. Inactivation of Bcl-2 by phosphorylation. Proc Natl Acad Sci U S A. 1995;92:4507–4511. doi: 10.1073/pnas.92.10.4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ojala PM, Yamamoto K, Castanos-Velez E, Biberfeld P, Korsmeyer SJ, Makela TP. The apoptotic v-cyclin-CDK6 complex phosphorylates and inactivates Bcl-2. Nat Cell Biol. 2000;2:819–825. doi: 10.1038/35041064. [DOI] [PubMed] [Google Scholar]
- 118.Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–760. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 119.Linseman DA, Butts BD, Precht TA, Phelps RA, Le SS, Laessig TA, Bouchard RJ, Florez-McClure ML, Heidenreich KA. Glycogen synthase kinase-3beta phosphorylates Bax and promotes its mitochondrial localization during neuronal apoptosis. J Neurosci. 2004;24:9993–10002. doi: 10.1523/JNEUROSCI.2057-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Beurel E, Jope RS. The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog Neurobiol. 2006;79:173–189. doi: 10.1016/j.pneurobio.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Panka DJ, Cho DC, Atkins MB, Mier JW. GSK-3beta inhibition enhances sorafenib-induced apoptosis in melanoma cell lines. J Biol Chem. 2008;283:726–732. doi: 10.1074/jbc.M705343200. [DOI] [PubMed] [Google Scholar]
- 122.Das A, Xi L, Kukreja RC. Phosphodiesterase-5 inhibitor sildenafil preconditions adult cardiac myocytes against necrosis and apoptosis. Essential role of nitric oxide signaling. J Biol Chem. 2005;280:12944–12955. doi: 10.1074/jbc.M404706200. [DOI] [PubMed] [Google Scholar]
- 123.Shimizu S, Konishi A, Kodama T, Shimizu S, Konishi A, Kodama T, Tsujimoto BH4 domain of antiapoptotic Bcl-2 family members closes voltage-dependent anion channel and inhibits apoptotic mitochondrial changes and cell death. Proc Natl Acad Sci U S A. 2000;97:3100–3105. doi: 10.1073/pnas.97.7.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen M, Won DJ, Krajewski S, Gottlieb RA. Calpain and mitochondria in ischemia/reperfusion injury. J Biol Chem. 2002;277:29181–29186. doi: 10.1074/jbc.M204951200. [DOI] [PubMed] [Google Scholar]
- 125.Milanesi E, Costantini P, Gambalunga A, Colonna R, Petronilli V, Cabrelle A, Semenzato G, Cesura AM, Pinard E, Bernardi P. The mitochondrial effects of small organic ligands of BCL-2: sensitization of BCL-2-overexpressing cells to apoptosis by a pyrimidine-2,4,6-trione derivative. J Biol Chem. 2006;281:10066–10072. doi: 10.1074/jbc.M513708200. [DOI] [PubMed] [Google Scholar]
- 126.Lin DT, Lechleiter JD. Mitochondrial targeted cyclophilin D protects cells from cell death by peptidyl prolyl isomerization. J Biol Chem. 2002;277:31134–31141. doi: 10.1074/jbc.M112035200. [DOI] [PubMed] [Google Scholar]
- 127.Scorrano L, Nicolli A, Basso E, Petronilli V, Bernardi P. Two modes of activation of the permeability transition pore: the role of mitochondrial cyclophilin. Mol Cell Biochem. 1997;174:181–184. [PubMed] [Google Scholar]
- 128.Pastorino JG, Hoek JB. Regulation of hexokinase binding to VDAC. J Bioenerg Biomembr. 2008;40:171–182. doi: 10.1007/s10863-008-9148-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pastorino JG, Hoek JB, Shulga N. Activation of glycogen synthase kinase 3beta disrupts the binding of hexokinase II to mitochondria by phosphorylating voltage-dependent anion channel and potentiates chemotherapy-induced cytotoxicity. Cancer Res. 2005;65:10545–10554. doi: 10.1158/0008-5472.CAN-05-1925. [DOI] [PubMed] [Google Scholar]
- 130.Chiara F, Castellaro D, Marin O, Petronilli V, Brusilow WS, Juhaszova M, Sollott SJ, Forte M, Bernardi P, Rasola A. Hexokinase II detachment from mitochondria triggers apoptosis through the permeability transition pore independent of voltage-dependent anion channels. PLoS ONE. 2008;3:e1852. doi: 10.1371/journal.pone.0001852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Roy SS, Madesh M, Davies E, Antonsson B, Danial N, Hajnoczky G. Bad targets the permeability transition pore independent of Bax or Bak to switch between Ca2+-dependent cell survival and death. Mol Cell. 2009;33:377–388. doi: 10.1016/j.molcel.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Connern CP, Halestrap AP. Recruitment of mitochondrial cyclophilin to the mitochondrial inner membrane under conditions of oxidative stress that enhance the opening of a calcium-sensitive non-specific channel. Biochem J. 1994;302:321–324. doi: 10.1042/bj3020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Halestrap AP, Kerr PM, Javadov S, Woodfield KY. Elucidating the molecular mechanism of the permeability transition pore and its role in reperfusion injury of the heart. Biochim Biophys Acta. 1998;1366:79–94. doi: 10.1016/s0005-2728(98)00122-4. [DOI] [PubMed] [Google Scholar]
- 134.Hirotani S, Zhai P, Tomita H, Galeotti J, Marquez JP, Gao S, Hong C, Yatani A, Avila J, Sadoshima J. Inhibition of glycogen synthase kinase 3beta during heart failure is protective. Circ Res. 2007;101:1164–1174. doi: 10.1161/CIRCRESAHA.107.160614. [DOI] [PubMed] [Google Scholar]