Abstract
A digital microfluidic platform for performing heterogeneous sandwich immunoassays based on efficient handling of magnetic beads is presented in this paper. This approach is based on manipulation of discrete droplets of samples and reagents using electrowetting without the need for channels where the droplets are free to move laterally. Droplet-based manipulation of magnetic beads therefore does not suffer from clogging of channels. Immunoassays on a digital microfluidic platform require the following basic operations: bead attraction, bead washing, bead retention, and bead resuspension. Several parameters such as magnetic field strength, pull force, position, and buffer composition were studied for effective bead operations. Dilution-based washing of magnetic beads was demonstrated by immobilizing the magnetic beads using a permanent magnet and splitting the excess supernatant using electrowetting. Almost 100% bead retention was achieved after 7776 fold dilution-based washing of the supernatant. Efficient resuspension of magnetic beads was achieved by transporting a droplet with magnetic beads across five electrodes on the platform and exploiting the flow patterns within the droplet to resuspend the beads. All the magnetic-bead droplet operations were integrated together to generate standard curves for sandwich heterogeneous immunoassays on Human Insulin and Interleukin-6 (IL-6) with a total time to result of seven minutes for each assay.
Introduction
Immunoassays are among the most sensitive and specific analytical methods [1] that are routinely used in a clinical laboratory and other research applications. Immunoassays make use of the high-affinity and specificity in binding between an antigen and its homologous antibody to detect and quantify the antigen in a sample matrix. In a clinical lab, immunoassays are currently used to test for cardiac markers, hormones, drugs, infectious agents, immune response and tumor markers with new tests being added continuously. Among the various immunoassay formats, heterogeneous immunoassays are the most common due to their higher sensitivity and correspondingly lower detection limits. In heterogeneous immunoassays the antibody-antigen complex is immobilized on a solid phase (well plate or microbead) and unbound molecules from the sample matrix are washed away. Detection is performed using a direct fluorescent label on a secondary antibody (FIA) or an enzyme labeled secondary antibody (ELISA).
Heterogeneous immunoassays are inherently rate-limited by mass transport of antigen or antibodies towards the solid surface and therefore benefit from reduction of dimensions in microfluidics. Diffusion time is proportional to the square of diffusion length and therefore reducing the length scale from millimeters (on a micro titer plate) to tens of microns (in a microfluidic device) reduces the incubation time of the analyte with the antibodies from hours to a few minutes. Stand-alone microfluidic platforms, if there were any, also would offer a high degree of integration and automation at a fraction of the cost of robotic systems, thus significantly reducing labor cost and minimizing human error. In heterogeneous immunoassays implemented in microfluidic systems, the antibodies are usually immobilized either onto the surface of the channel walls or onto micro beads. Immobilization onto surfaces requires additional micro-fabrication processing steps and suffers from poor reproducibility and reliability. In contrast, immobilization onto microbeads is performed as a separate process decoupled from microfabrication of the microfluidic devices while offering significantly larger surface area for binding and therefore better sensitivity [2]. However, bead-based systems still require some mechanism to hold them in place during the separation or washing step in the assays. A common approach to immobilize the beads has been to use micro-fabricated physical barriers that retain the beads while allowing the solution to pass through. Sato et al. used a micro-fabricated dam structure to localize beads in an immunoassay system to detect IgG [3], carcinoembryonic antigen [4] and interferon-gamma [5] where the assay times were reduced by a factor of 70 from 24 hours to 20 minutes. Moorthy et al. [6] described the design and fabrication of a microfluidic system for detection of botulinum toxoid by sandwich ELISA directly from whole blood. This device incorporated a porous filter to separate serum from the blood cells and avidin-agarose beads held by a filter membrane as the solid phase. Christodoulides et al. [7] fabricated micro-machined pits to entrap beads in an immunoassay chip developed for cardiac markers.
An alternative approach for bead-based separation using barriers and filters is to use paramagnetic beads [8] and an external magnetic field for localization of the beads [9]. This configuration permits flexible microfluidic architectures since the fluidics would be isolated from the separation mechanism. There have been several occasions where magnetic beads have been used in microfluidic systems [10,11,12]. However most of them were performed in a channel based continuous flow format, which suffers from the lack of reconfigurability. Clogging of channels is another problem specially observed in bead-based microfluidic assays. Due to the limitations in versatility and functionality in continuous flow-based microfluidic devices, we have developed an alternative paradigm based on discrete droplets manipulated by electrowetting.
There has been considerable interest in droplet-based microfluidic systems [13] in recent years as an alternative paradigm to continuous-flow channel-based systems. Among several approaches that have been reported in the literature for manipulating droplets, microfluidic devices based on electrowetting [14, 15, 16], dielectrophoresis [17] and multiphase flows [18] are the most common. The advantages of manipulating droplets using electrowetting over other techniques has been discussed in detail elsewhere [19]. In this article, we focus on manipulating droplets through electrowetting. Droplets with a wide range of fluid properties, including physiological fluids, have been demonstrated to be compatible for manipulation using electrowetting [20]. Several chemical/biological applications have been demonstrated on electrowetting-based lab-on-a-chip devices including enzymatic assays [21, 22], clinical diagnostics on human physiological fluids [23], multiplexed proteomic sample preparation and analysis by MALDI-MS [24, 25], explosives detection [26], nucleic acid amplification (PCR) [27, 28] and cell based assays [29].
More recently, separation of magnetic beads was demonstrated in an electrowetting-based droplet manipulation system [30]. In this report, magnetic beads were separated by splitting off a droplet using electrowetting with a bead capture efficiency of 98.7% for each step of separation. However, efficient washing could require several separation steps and the bead loss would be compounded after each wash cycle. In a case where nominally 10 such separation cycles are required, the number of beads lost will be 12% of the starting number of beads and this amount of loss would seriously affect precision, functional sensitivity, and clinical sensitivity of the assays leading to erroneous results. Fouillet et al. [31] reported that handling of magnetic beads is compatible with electrowetting and mentioned that magnetic and electrowetting forces can be coupled to implement sample preparation protocols. The magnetic beads (6 mg/mL) were extracted by immobilizing the magnetic bead pellet and transporting the supernatant away using electrowetting. Shikida et al. [32] demonstrated a bead droplet handling mechanism utilizing the differences in wettability and interfacial tension between different liquids. They developed mechanisms for extracting and fusing bead droplets by moving a permanent magnet to extract the beads from one droplet and fuse into another fresh droplet. Apart from this, Lehmann et al. [33] demonstrated a two dimensional magnetic manipulation of microdroplets on a chip suspended in silicone oil wherein the superparamagnetic particles inside the droplets provided the means for magnetic actuation. They proposed the potential of the system in performing an immunoassay by diluting and detecting the secondary antibody of an enzyme-linked immunosorbent assay on chip. To the best of our knowledge, there are no publications on implementation of immunoassays in digital microfluidic systems. In this paper, we demonstrate the ability to manipulate magnetic beads using electrowetting without moving magnets and particularly focus on efficient washing of magnetic beads while maintaining ~100% bead retention. Also, we have integrated all the basic droplet operations such as dispensing, transporting, mixing/incubation and splitting to demonstrate heterogeneous sandwich immunoassays on human insulin and IL-6 using magnetic beads on our digital microfluidic platform with no moving parts.
Droplet- based Magnetic Bead Immunoassay
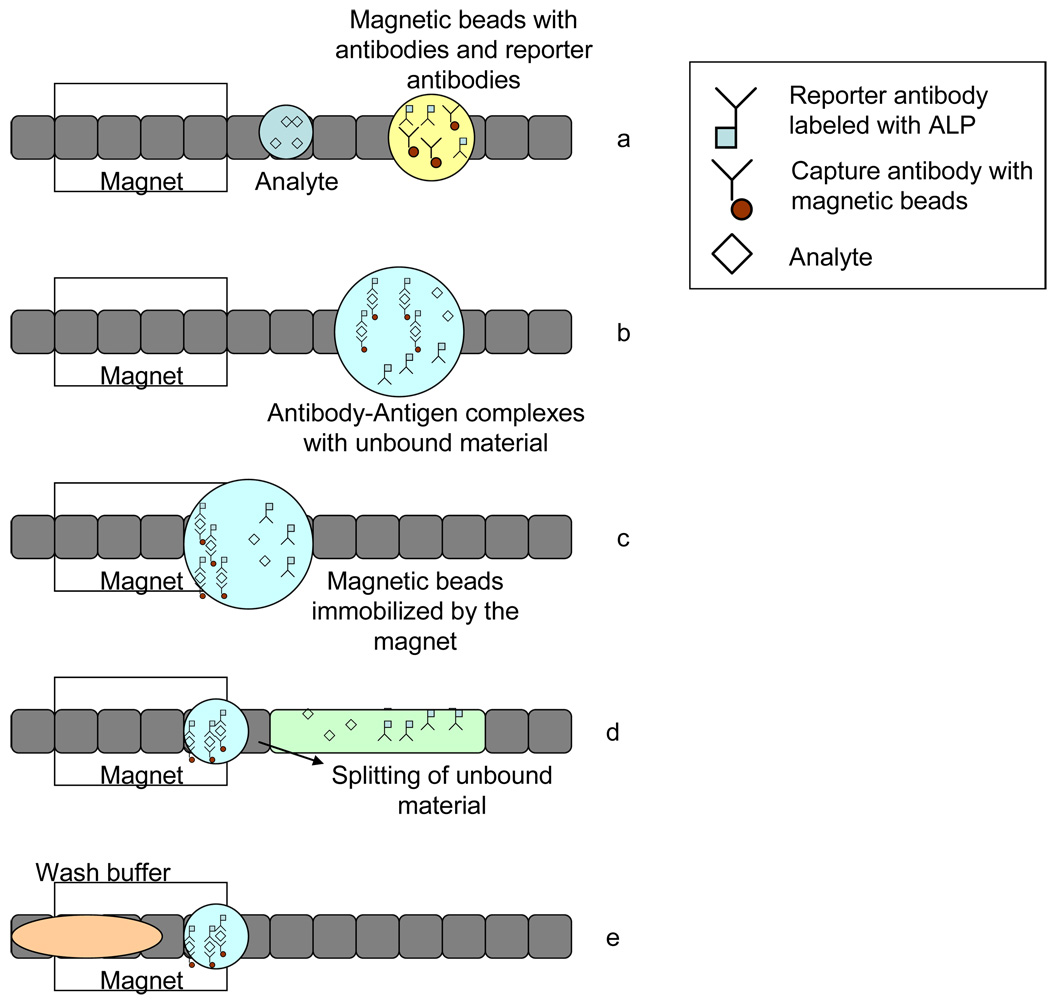
A droplet-based magnetic bead immunoassay protocol consists of the following steps. First dispense a sample droplet and a reagent droplet containing magnetic beads with primary capture antibodies, blocking proteins and reporter secondary antibodies (step 1 in Figure 1). Merge these two droplets, mix and incubate (step 2 in Figure1). After the formation of the capture antibody-antigen-reporter antibody complex, immobilize the magnetic beads (step 3 in Figure 1) and wash away unbound material. Finally, a reagent droplet is added for detection. A schematic of all the key steps is shown in Figure 1.
Figure 1.
Protocol for heterogeneous immunoassay on a digital microfluidics platform, (a) Dispensing of reagents, (b) Incubation, (c) Immobilization of magnetic beads (d) Removal of supernatant and washing (e) Adding fresh wash buffer
Washing of magnetic beads, where unbound molecules are separated and removed, is the most important step towards implementing an immunoassay in a digital microfluidic system. During washing, the magnetic bead-primary antibody-antigen-secondary antibody complex is immobilized using a permanent magnet and then unbound molecules are removed by serial dilution, which involves repeated addition of a wash buffer droplet and splitting away the excess supernatant as shown in steps 4 and 5 in Figure 1. This method of serial dilution is repeated until the supernatant is free of unbound molecules.
For washing, we have explored four key operations on droplets containing magnetic beads viz., magnetic bead attraction, wash efficiency, magnetic bead retention and magnetic bead resuspension. We have integrated the basic droplet operations performed for manipulation of droplets in digital microfluidics, viz., dispensing, transporting, mixing and splitting with the new set of basic operations required for manipulation of magnetic bead-containing droplets to demonstrate sandwich immunoassays.
EXPERIMENTAL METHODS AND MATERIALS
Digital Microfluidic Setup
The electrowetting setup consists of two parallel glass plates where the first one comprises of a photolithographically patterned electrode array for manipulation of droplets and the second one has an indium tin oxide (ITO) coating to serve as a reference electrode. The electrode array is patterned in 200nm thick chrome using standard photolithographic mask making methods. The electrode array is insulated from the droplet by a ~5µm thick Parylene C layer. All surfaces are hydrophobized with a ~200nm Teflon AF coating. Droplets are sandwiched between these two plates and are surrounded by an immiscible filler fluid (1.5cSt silicone oil, DMS-T01, Gelest, Morrisville, Pennsylvania, USA) and the top plate is held in place using spring loaded clips and the filler fluid is immobilized by capillarity. The two planes are separated by a spacer, which defines the height of the droplet. For the experiments reported in this paper, we have used chips with an electrode pitch of L=1.5mm, spacer thickness of H=300 µm and an inter-electrode spacing of 50 µm.
Detection Setup
We have selected chemiluminescence as a detection mechanism for measuring the bound antibody due to the high sensitivity afforded by the technique. Chemiluminescence measurements were obtained in a plane perpendicular to the digital microfluidic chip using a photo multiplier tube (PMT) obtained from Hamamatsu (H9858). The PMT has an 8mm diameter window for light collection, which is much larger than the footprint of a single droplet which is 1.5mm. The PMT was placed at a distance of h=5mm from the upper surface of the ground plane as shown in Figure 1b to maximize the light collected from the droplet.
Reagents
Dynal® MyOne™ Streptavidin magnetic beads (1.05 µm diameter) and Amplex® Ultra red reagent (A36006) were obtained from Invitrogen™ (Carlsbad, California, USA). Biotinylated horseradish peroxidase (HRP) was from EY laboratories (San Mateo, California, USA). Lumigen PS-Atto was obtained from Lumigen Inc. (Southfield, Michigan, USA). Chemiluminescence substrate for HRP (Lumigen PS-Atto) was prepared by mixing equal volumes of PS-Atto A and B solutions. The colorimetric substrate for HRP (Amplex® Ultra red) was prepared by mixing equal volumes of 0.1 mM Amplex Ultra red reagent in DMSO solution and 2 mM hydrogen peroxide. Wash buffer was made with 0.05M Tris-HCl, 0.1M NaCl and 0.01% (w/w) Tween 20.
The Dynal MyOne Streptavidin beads were labeled with HRP by incubating 50 µL of 2 mg/mL magnetic beads with 10µL of 10µg/mL biotinylated HRP for 30 minutes in a microcentrifuge tube. Unbound biotin-HRP was removed by immobilizing the magnetic beads at the bottom of the tube using a permanent magnet and removing the excess supernatant. The beads were then resuspended in excess wash buffer and the process was repeated five times to remove all the excess biotinylated HRP. Immunoassay kits were obtained from Beckman Coulter for insulin and interleukin-6 containing antibodies, magnetic beads and standards.
Magnetic Bead Attraction
Neodymium magnets (ND 42) with different pull forces (1.25lb, 5lb and 10lb) were obtained from KJ Magnetics. A droplet containing magnetic beads was sandwiched between two hydrophobic glass plates with a known gap height (300µm) and the effect of the following parameters on the attraction of magnetic beads was observed qualitatively on a microscope: buffers (phosphate buffered saline (PBS), Tris buffered saline (TBS), PBS and TBS with 0.005% Tween 20, PBS and TBS with 0.01% Tween 20); magnetic pull force with field strength of ~5000 Gauss (1.25 lbs, 5 lbs, and 10 lbs) and position of magnet (such as magnets underneath the droplets, magnets over and underneath the droplet). Images were taken at regular intervals to qualitatively observe the effect of the aforementioned parameters on the efficiency of attraction.
Magnetic Bead Wash Efficiency
Dynal® streptavidin coated magnetic beads (1.05 µm diameter) were resuspended in 10 µg/ml of unmodified HRP in TBS with 0.01 % Tween® 20. One microliter of the magnetic bead solution containing free HRP was pipetted onto the chip and the sandwich filled with 1.5 cSt silicone oil. The magnetic beads were immobilized using a neodymium permanent magnet (ND 42) and the supernatant was removed by activating several contiguous adjacent electrodes to create a slug. The slug was split by switching off the intermediate electrode to remove the supernatant resulting in two asymmetric daughter droplets, a small droplet concentrated with magnetic beads and a large slug with no beads. The bead droplet was washed with 5 µl slugs of TBS with 0.01 % Tween® 20 which were hand dispensed into a hole in the top plate using a pipette and then transported using electrowetting towards the magnetic bead droplet. Using electrowetting, the supernatant was transported away from the magnetic bead droplet towards a hole in the top plate which was then collected after every wash cycle with a pipette inserted through the hole, and transferred to individual wells in a 96-well transparent microtiter plate. The amount of HRP present in the wash slug was measured by adding Amplex® Ultra red substrate to each slug and by reading the absorbance after eight minutes at a wavelength of 570 nm using a BioTEK Synergy™ plate reader. The same experiment was performed manually using conventional bench-scale equipment in tubes wherein the magnetic beads are immobilized by placing a permanent magnet on the bottom of the tube and the supernatant was removed using a pipette. Experimental results obtained from the bench and chips were compared.
Magnetic Bead Retention
Dynal® streptavidin coated magnetic beads (1.05 µm diameter) were labeled with biotinylated HRP as described above and resuspended in 500 µL of TBS with 0.01% Tween 20 after washing. One microliter of biotinylated-HRP labeled magnetic beads was pipetted onto the chip, sandwiched with an ITO coated hydrophobic top plate separated by a 300 µm spacer and the space filled with 1.5 cSt silicone oil. Several droplets of TBS with 0.01% Tween 20 were hand dispensed using a pipette and combined on-chip by electrowetting to form 5 µL slugs. The HRP labeled beads were washed repetitively (5x) through serial dilution of the 1 µL HRP labeled magnetic bead droplet with 5 µL slugs of TBS containing 0.01% Tween 20 by immobilizing the beads with a 0.5 Tesla Neodymium permanent magnet (ND 42) and removing the supernatant using electrowetting as described earlier. This leads to a total dilution of 7,776x of the starting 1 µL bead droplet. The supernatant from each wash was transported using electrowetting to a hole in the top plate through which it was collected, transferred into a single well of a 96-well Costar opaque plate and tested for the presence of HRP. Fifty microliters of chemiluminescence substrate (equal volumes of Lumigen Ultra PS-Atto solution A and solution B) for HRP were added into each well. The chemiluminescence signal was measured on a plate reader (BioTEK Synergy™) to quantify the bead loss that may have occurred during each wash cycle. Measurements were taken for a total of 8 minutes and a reading was taken every 20 seconds. Any loss of magnetic beads would give rise to a chemiluminescence signal in the wash slugs on reaction of the substrate with HRP enzyme on the beads.
Magnetic Bead Resuspension
A 1 µL droplet of Dynal® streptavidin coated magnetic beads labeled with biotinylated HRP was injected onto the electrowetting system as described previously. The droplet of HRP-magnetic beads was shuttled across a set of six electrodes for 30 seconds at a switching rate of 4 Hz and an actuation voltage of 100V. The resuspension of magnetic beads within the droplet and the cycle number at which it occurred was determined by visual inspection in the presence and absence of an external permanent magnet.
Reagents for Human Insulin and Interleukin-6 Immunoassays
The immunoassay kits obtained from Beckman Coulter for insulin and IL-6 contain primary capture antibodies immobilized on paramagnetic beads, secondary reporter antibodies labeled with bovine alkaline phosphatase (ALP) and blocking proteins. For the insulin immunoassay, mouse monoclonal anti-insulin coupled to paramagnetic particles in Tris buffer and mouse monoclonal anti-insulin conjugated to alkaline phosphatase were used as the primary and secondary antibodies. Mouse IgG in HEPES buffer with a BSA matrix was used as blocking solution to prevent non-specific binding. For the IL-6 immunoassay, paramagnetic beads coated with goat anti-mouse IgG: mouse anti-human IL-6 monoclonal antibody and goat anti-human IL-6 alkaline phosphatase (bovine) conjugate in BSA matrix were used as the primary and secondary antibodies. A blocking solution of porcine, goat, bovine and mouse proteins suspended in a surfactant matrix was used to prevent non specific binding. Standards of six different concentrations (S0 through S5) for both IL-6 and insulin were also provided along with the kits. The clinically relevant ranges for IL-6 and Insulin are (1–100 pg/mL) and (50–500 pmol/mL) respectively for humans. The concentrations of the standards included in the kits cover a large dynamic range. The digital microfluidic system described in the previous section was used to perform the immunoassays.
Protocol for Droplet Based Sandwich Immunoassays on Insulin and Interleukin-6
Magnetic beads coated with the primary antibody, secondary antibody labeled with alkaline phosphatase and the blocking proteins are mixed off-chip in an Eppendorf tube and 3 µL of the reagent mixture was pipetted onto an electrode on the digital microfluidic lab-on-a-chip. One microliter of the sample (insulin or IL-6 standard) was pipetted onto an adjacent electrode on the lab-on-a-chip (Step a in Figure1). The two droplets were sandwiched using an ITO coated glass plate, the height of the droplet being 300 µm. The space between the chip and the top plate was filled with 1.5 cSt silicone oil. The reagent droplet and the sample droplet were transported and merged using electrowetting at an actuation voltage of 100 VDC and a switching frequency of 1 Hz. The reagent-sample mixture was incubated by shuttling the merged droplet on a set of six electrodes for 2 minutes (Step b in Figure 1). The shuttling of the droplet ensured efficient mixing of the magnetic beads even in the presence of a permanent magnet placed underneath the chip. From our previous work [34], it was observed that shuttling the droplet over four electrodes at a droplet switching speed of 1 Hz resulted in complete mixing within 40 seconds. By extrapolating the data, if a droplet is shuttled over 6 electrodes it would take approximately 28 seconds for complete mixing. To ensure complete mixing in the current droplet configuration where the aspect ratios are different, we chose to incubate (shuttle) the droplet for 2 minutes over six electrodes. After incubation, the magnetic beads with the antigen-antibody complex were immobilized with the help of the magnet and the unbound secondary antibody was removed using the protocol described in the “Washing” section where the magnetic beads were washed for 5 times using 5 µL of wash buffer introduced with a pipette through a hole in the top plate. One microliter droplet of Lumigen APS-5 was loaded into the digital microfluidic chip from a hole in the top plate and merged with the washed magnetic beads using electrowetting and the chemiluminescence kinetics were read for 4 minutes with the photon multiplier tube placed over the chemiluminescent droplet. Immunoassays were performed for insulin and IL-6 standards (S0 through S4). Each experiment was performed in duplicate using a new chip each time. Standard curve data for IL-6 and Insulin were fit using Sigma Plot . No weighting parameters were included in the fit.
RESULTS and DISCUSSION
Magnetic Bead Attraction
Magnetic beads must be attracted and immobilized on chip in order to perform washing. We have evaluated several parameters that influence the attraction of magnetic beads to find the conditions for efficient and quick attraction of beads towards the magnet, to avoid aggregation between beads and to avoid sticking to the surfaces of the chip. These parameters include the buffer in which the beads are suspended, pull force of the magnet to immobilize the beads and the position of the magnet relative to the bead droplet. A matrix of experiments was designed to explore each of these parameters and to identify the conditions for efficient attraction of the beads in a droplet by taking periodic images using a microscope. Qualitative analysis was performed on all the images to choose the most suitable conditions for efficient attraction of magnetic beads. Attraction of magnetic beads using a permanent magnet was observed to depend on the composition of the buffer. While the beads were attracted towards the magnet in just PBS and TBS, we have noted that the streptavidin coated beads aggregated irreversibly between one another or to the surface when in buffer alone. Images of droplets with a surfactant (0.01% Tween 20) in the buffer showed no aggregates and the beads readily resuspended upon removal of the magnet. Magnetic beads were attracted in the same amount of time for magnets with different pull forces (20 seconds) and it was observed that a magnet with a lower pull force (1.25lb) prevented aggregation of the beads. However, when a magnet with 5 lb and 10 lb pull force was used for attraction; the images showed irreversible aggregation of the beads, wherein the clumps of beads would not resuspend upon removal of the magnet. We chose to use a single magnet with a pull force of 1.25lbs underneath the droplet for ease of implementation.
Wash Efficiency
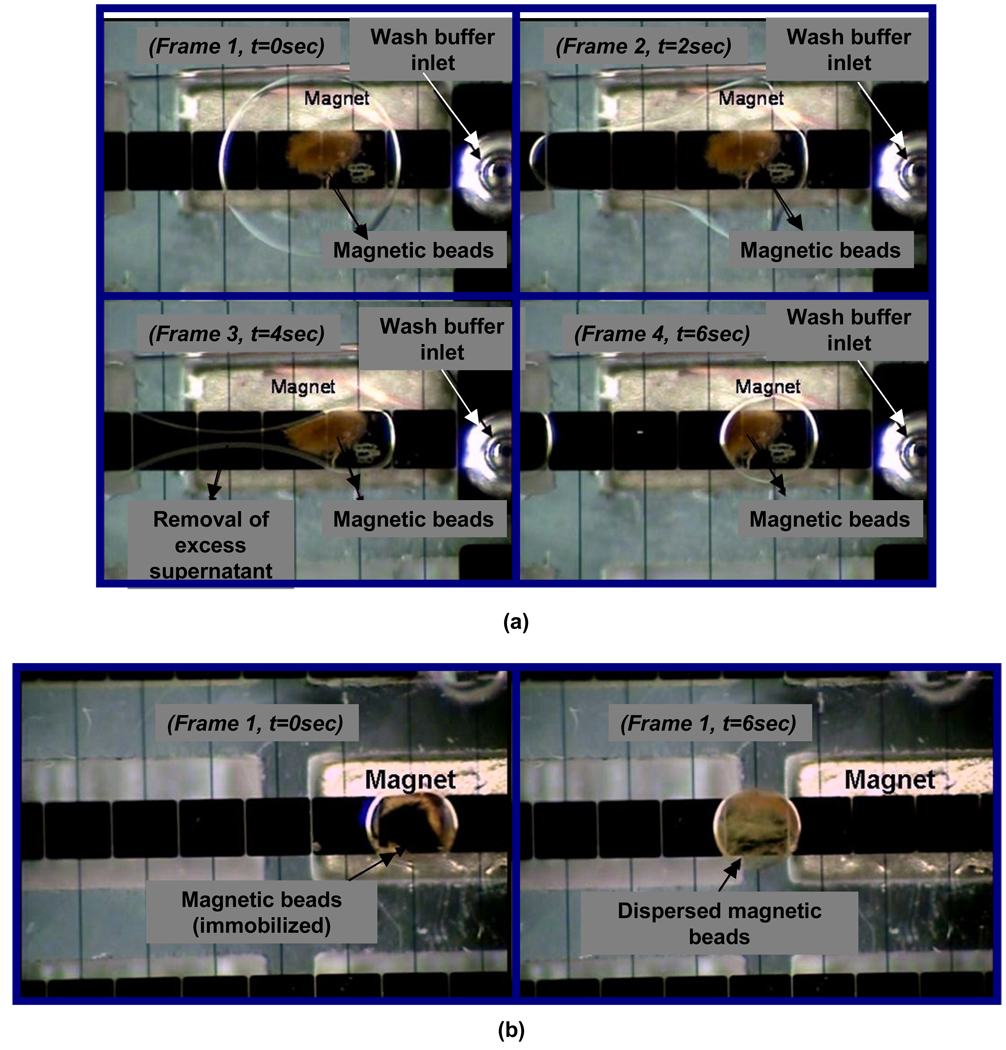
As mentioned above, heterogeneous immunoassays require an additional separation step to remove the excess unreacted reagents after binding the antibodies to the antigen. Separation of the excess unreacted reagents from the magnetic beads in a tube is performed by placing a permanent magnet at the edge of the tube and removing the supernatant using a pipette whereas on the chip, magnetic beads are attracted by the magnet underneath while a wash droplet is merged and the supernatant droplet split off. The attracted magnetic beads are then dispersed by adding excess wash buffer, vortexing and the process of separation is repeated. Washing essentially comprises of this process of separation and dispersion of magnetic beads. We evaluated the efficiency of washing of streptavidin coated magnetic beads for an immunoassay on the digital microfluidic platform by suspending magnetic beads and free HRP in a droplet and then performed washing operations as described above. The supernatant wash droplets were analyzed for the amount of HRP after each wash cycle to determine washing efficiency. The magnetic beads were immobilized using a neodymium permanent magnet (ND 42) (frame 1 in Figure 2a) and the supernatant was removed by activating several contiguous adjacent electrodes to create a slug (frame 2 in Figure 2a). The slug was then split by switching off the intermediate electrode to remove the supernatant as shown in frame 3 of Figure 2a resulting in two asymmetric daughter droplets, a small droplet concentrated with magnetic beads and a large slug with no beads (frame 4, Figure 2a). Even though the image shows splitting on the magnet, the splitting point is usually placed a few electrodes away from the magnet so that the effect of the magnetic field is minimized on the supernatant droplet which minimizes the loss of magnetic beads into the supernatant droplet.
Figure 2.
Figure 2a- Washing of magnetic beads by removing the excess supernatant on chip, Figure 2b- Resuspension of magnetic beads
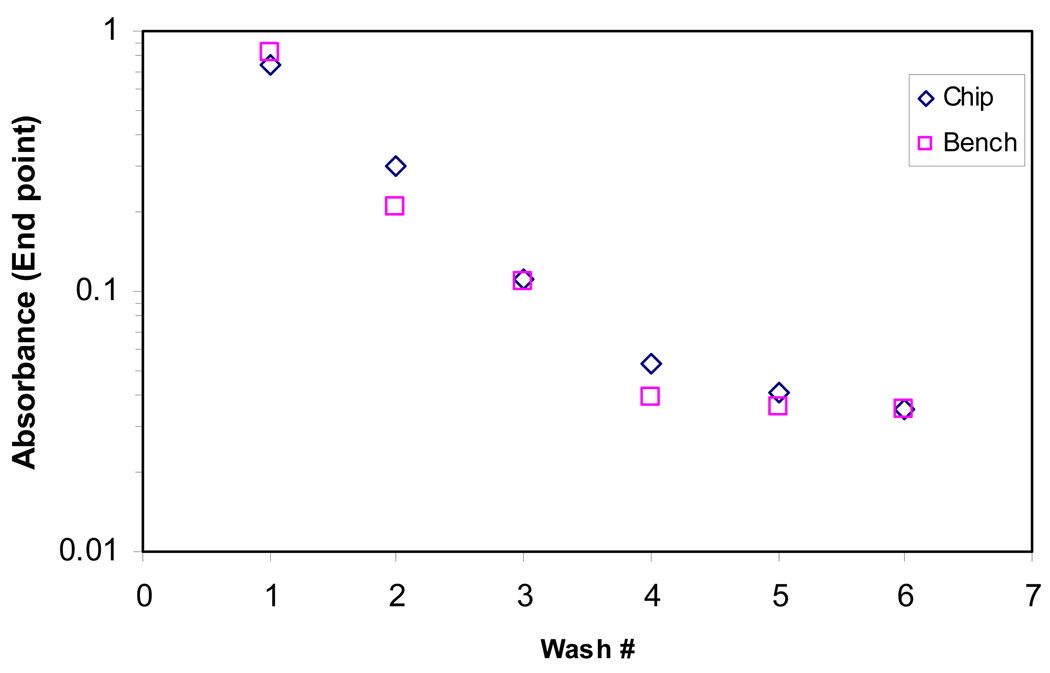
In the wash experiment, the bead droplet was washed with 5 µl slugs of TBS containing 0.01 % Tween® 20 and the supernatant was collected after every wash using a pipette through a hole in the top plate and transferred to individual wells in a 96-well transparent microtiter plate. The amount of HRP present in each wash slug was measured to determine the efficiency of the wash protocol by adding Amplex® Ultra red substrate to each slug and reading the change in absorbance after eight minutes at a wavelength of 570 nm using a BioTEK Synergy™ plate reader. End point absorbance measurements were taken after 8 minutes of the enzymatic reaction of the substrate in the supernatant. For comparison, the same experiment was performed manually using conventional bench-scale equipment in tubes with the same reagents and wash volumes as described. Figure 3 shows the end point absorbance of the supernatant at 8 minutes after each wash cycle obtained from the experiments performed on bench and on chip. A negative control of the wash buffer and the substrate was also measured on the same plate reader. On the bench scale process, the supernatant is almost completely removed and replaced with fresh wash buffer for each step of washing, whereas it is performed through serial dilution on chip. Hence the end point value was higher for the bench experiment for the first wash cycle since a higher amount of HRP was removed on bench in the first wash cycle. However, since most of the free HRP was removed in the first wash cycle on bench, supernatant from the subsequent wash cycles had lesser signal than that of the chip. Although there is a significant difference in the washing protocol between the chip and bench, the absorbance reached the values of the negative control in the same number of wash cycles in both the cases (Figure 3).
Figure 3.
Comparison of washing performed on bench and on chip
Magnetic Bead Retention
If the magnetic field and interfacial tension of the liquids are not adjusted accordingly, considerable number of beads can be lost during the removal of supernatant in our dilution-based washing strategy where the process of immobilization and removal of supernatant is repeated several times until desirable washing levels are achieved. Any loss of beads would incur a loss in the signal because of removal of the bound antigen along with the lost beads. Since the beads would be in vast excess for ligand binding assays, it is possible that magnetic beads without bound antigens could also be lost however the loss of beads could be unevenly spread between beads with and without antigens and this could add to significant variation between assays. We have performed experiments to quantify the bead loss that could have occurred, while washing the magnetic beads on the digital microfluidic chip, by performing chemiluminescence assays to identify losses of even single beads.
The presence of HRP labeled magnetic beads in the supernatant droplet (slugs) was measured to determine the extent of bead loss during washing performed on chip. As described in the methods, the HRP labeled magnetic beads were washed by serial dilution with 5 µl slugs of TBS with Tween 20 until a dilution of 7,776x was obtained. The supernatant droplets from each wash cycle were collected through a hole in the top plate and the amount of HRP in each wash droplet was measured using an enzymatic chemiluminescence reaction in a microtiter plate. Measurements were taken for a total of 8 minutes and a reading was taken every 20 seconds. The presence of HRP in the wash slug is an indication of bead loss during the wash protocol - the greater the signal, the more substantial the loss of beads. A standard curve of HRP-labeled magnetic beads was prepared ranging from 2 mg/ml to 2×10−9 mg/ml by serial dilution of beads with TBS containing 0.01% Tween 20. Five microliters of each standard were transferred into a well of a 96-well Costar opaque plate and the velocity of the HRP reaction determined as described above. The mean velocity of the chemiluminescence reaction was calculated by taking the slope of the first five (100 seconds) chemiluminescence measurements for each well. The starting solution had 7×109−12×109 beads per milligram of beads and the starting bead concentration was at 2 mg/mL. The mean velocity obtained for the negative control (Tris buffered saline with 0.01% Tween 20) was 900 milli Lum/min whereas that obtained for the supernatant from the 7776-fold dilution based washing protocol was 1350 milli Lum/min. Interpolating from the data in Table 1, the number of beads that were lost during the washing steps can be estimated to be in the range of 2×10−7 to 2×10−6 mg/mL. In other words, 1.4–24 beads were lost from 14–24×106 beads during the 7,776x washing step performed with 5 µL of wash buffer solution. Hence, the bead retention efficiency is ~100%.
Table 1.
Concentration of HRP labeled magnetic beads versus Chemiluminescent signal produced
| Concentration of HRP labeled magnetic beads (mg/mL) |
Mean Velocity of the Chemiluminescent signal (milli Lum/min) |
|---|---|
| 2 × 10−8 | 1000 |
| 2 × 10−7 | 1200 |
| 2 × 10−6 | 1500 |
| 2 × 10−5 | 2700 |
| 2 × 10−4 | 6000 |
Magnetic Bead Resuspension
Magnetic beads have a tendency to settle down due to gravity and form aggregates even in the absence of magnetic field. Apart from this, the beads also tend to aggregate if they are exposed to strong magnetic fields for a long time. It is necessary to keep the beads dispersed well enough to avoid formation of aggregates because aggregation of beads effectively reduces the surface area available for binding thereby slowing down reaction kinetics and eventually affecting the time to result and sensitivity of the assay. Also, interstices in magnetic bead aggregates can hold unbound species leading to ineffective washing, yielding less sensitive assays and sometimes inaccuracies between assays due to differing amounts of unbound species held in the interstices. As noted earlier, the attraction of magnetic beads depends on presence or absence of surfactants in the buffer. Since resuspension of beads is an inverse process of attraction of beads, both function well if the beads do not aggregate.
Resuspension is required during incubation and for steps immediately following separation in washing for further processing of the droplets away from the magnets. Moving the magnets away is one option but since our system is designed to have no moving parts, the droplets can be moved away from the influence of the magnet. During bead attraction, the magnetic force applied on the magnetic beads, surface properties of the beads, and the interfacial tension of the droplet are chosen such that the beads stay in the buffer droplet and do not partition into oil. However, the beads need to be moved away from the magnet for uniform resuspension within the droplet and this can be achieved by utilizing the interfacial tension of the receding edge of the droplet to drag the beads along the direction of droplet transport. If the pull force of the magnet is very high (>= 5lb) and the concentration of the magnetic beads is 10 mg/mL or greater, then the beads would partition out of the droplet and it will not be possible to resuspend the beads. It is not sufficient to just move the droplet away from the magnet to achieve resuspension. Based on our earlier work [34], we shuttle the droplet to influence bead resuspension utilizing the recirculation patterns within the droplet that are developed during transport, which essentially mimics the vortexing action produced on bench scale equipment. Also, due to the optimum balance achieved between the magnetic force and interfacial tension of the liquid suspending the magnetic beads, the beads can be immobilized allowing the supernatant to be split off during the washing operation while the droplet with the beads can be transported away from the magnet resuspending the beads completely even in the presence of a magnet. A 1 µL droplet of HRP labeled magnetic beads was injected onto the electrowetting system as described previously. Extrapolating from the results obtained from our previous work [34], complete mixing can be obtained in 6 seconds when a droplet is shuttled over 6 electrodes at a switching speed 4 Hz. In this paper, the droplet of magnetic beads was shuttled across a set of six electrodes for 30 seconds at a switching rate of 4 Hz and an actuation voltage of 100V to ensure complete dispersion. Resuspension of magnetic beads within the droplet was obtained after 1 cycle even in the presence of an external permanent magnet as shown frame 2 in Figure 2b.
Immunoassays on Human Insulin and Interleukin-6
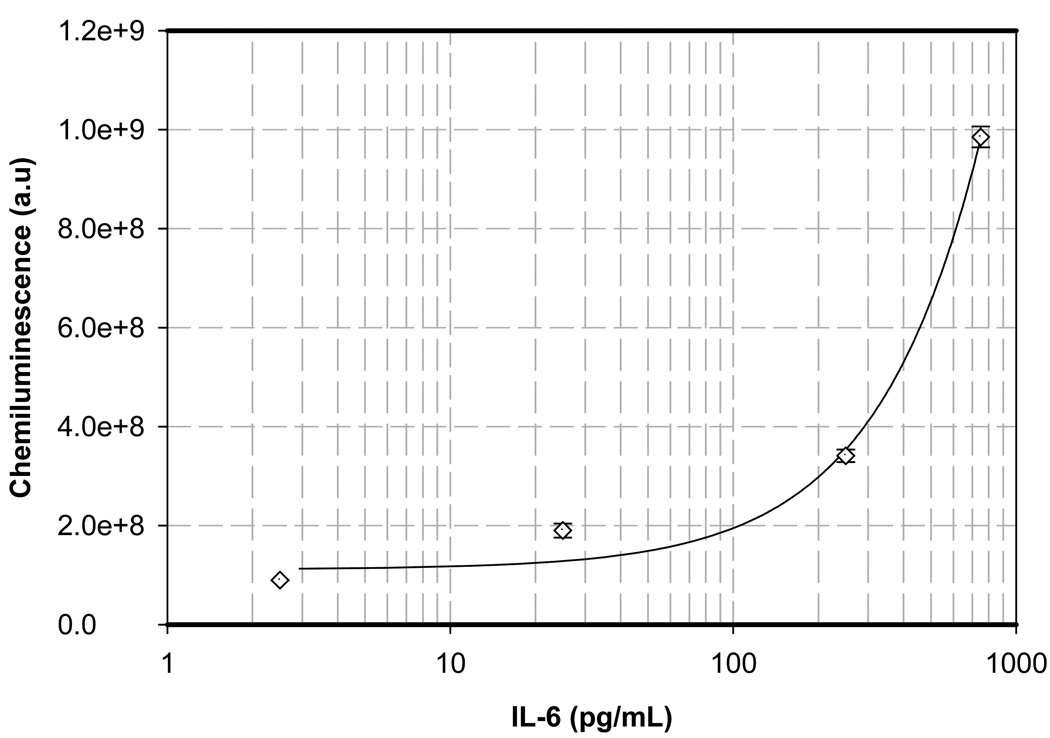
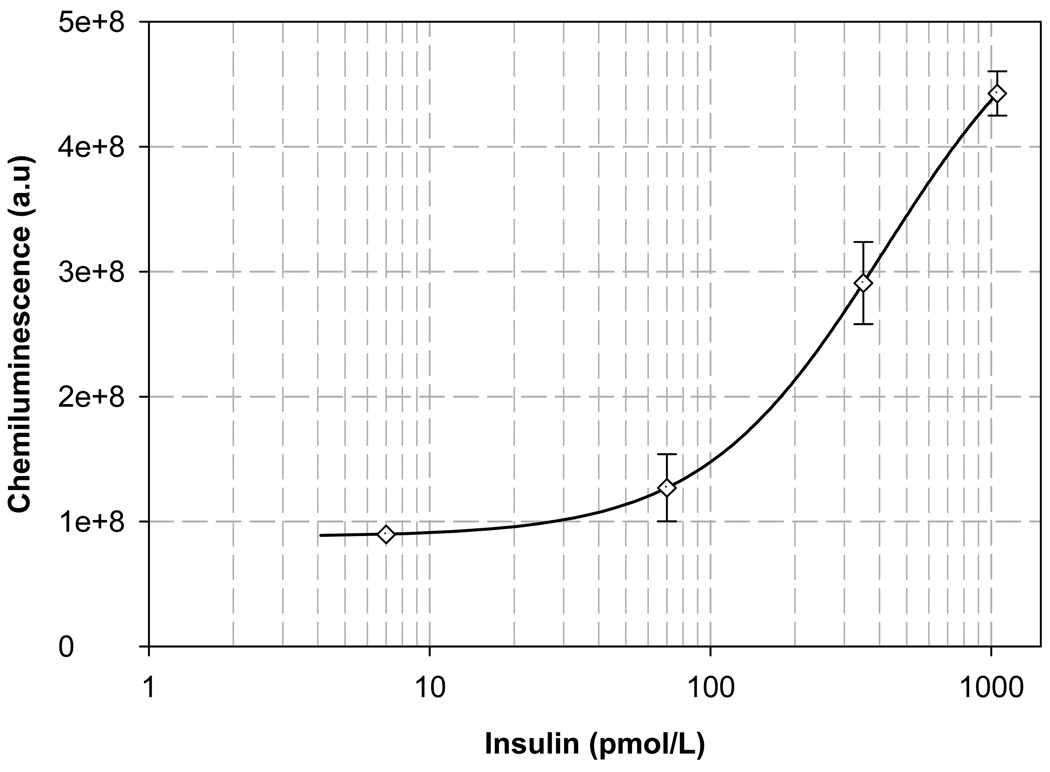
All the basic steps described above have been integrated to demonstrate performance of heterogeneous sandwich immunoassays on chip on both insulin and IL-6 using samples of known concentration as described in the methods section. After addition of APS-5 substrate, kinetic curves were obtained for chemiluminescence for each individual standard for insulin and IL-6. Area under the curves were calculated and plotted against the concentration of the analytes to obtain standard curves for both the analytes (Figure 4 and Figure 5). Each experiment on each analyte at each concentration was performed in duplicate. Experiments were repeated on the bench in tubes with the same volumes. Incubation and washing was performed in tubes and the washed magnetic beads with the antibody-antigen sandwich were pipetted onto the chip and the chemiluminescence readings were obtained in the same way using the same PMT module, to compare the results obtained on chip for both insulin and IL-6. The error bars in Figure 4 and Figure 5 represent the standard deviation between the two measurements performed on chip. The chemiluminescence readings obtained from the bench were compared to that obtained from the chip by plotting the bench readings on the x-axis and the chip readings on the y-axis. A straight line with a slope of 1.0572 and 0.9781 with an r2 (correlation coefficient) value of 0.9916 and 0.9812 was obtained for IL-6 and Insulin respectively. Chemiluminescence data were measured as electrical current on an analog PMT and then digitized and hence are represented as arbitrary units.
Figure 4.
Standard curve for IL-6 generated on a digital microfluidic chip
Figure 5.
Standard curve for Insulin generated on a digital microfluidic chip
Conclusions
In this paper we have successfully demonstrated the manipulation of magnetic beads on a digital microfluidic chip to perform heterogeneous immunoassays. We have demonstrated magnetic bead transport, resuspension, immobilization and efficient washing of magnetic beads using the basis set of instructions (dispense, transport, mix, and split) in digital microfluidics paradigm. The droplet of magnetic beads was shuttled across a set of six electrodes for 30 seconds at a switching rate of 4 Hz and an actuation voltage of 100V for incubation, mixing, and resuspension. Complete dispersion of magnetic beads within the droplet was obtained after 1 cycle even in the presence of an external permanent magnet.
Different parameters affecting the magnetic bead immobilization were identified and optimized. It was found that the buffer in which the beads were resuspended should have a surfactant (0.01% Tween 20) to avoid irreversible aggregation in the presence of strong magnetic field. Permanent magnets with different pull forces were used to immobilize the beads and a neodymium permanent magnet with a pull force of 1.25 lbs was chosen to avoid irreversible aggregation of the beads. The interfacial tension and magnetic forces were optimized to allow for attraction of the beads during washing without any significant bead loss while also allowing for transport of the droplet away from the magnet after washing is completed. Almost 100% bead retention was achieved during washing (7,776-fold) of the magnetic beads. An efficient serial dilution-based washing protocol to remove the unbound material from the supernatant was developed and tested on the lab-on-a-chip. Heterogeneous sandwich immunoassay using magnetic beads were demonstrated for the first time on a droplet-based digital microfluidic platform. Standard curves were obtained for insulin and IL-6, which compared well with the results obtained on bench. Our future work is to integrate all the operations to perform a completely automated magnetic bead based heterogeneous immunoassay on physiological samples such as blood, serum and plasma on a digital microfluidic system.
Acknowledgments
This work was partially supported by a grant (R43 CA114993) from the National Cancer Institute, Bethesda, Maryland, USA.
References
- 1.Gosling JP. Immunoassays. UK: Oxford University Press; 2000. [Google Scholar]
- 2.Verpoorte E. Beads and Chips: New recipes for analysis. Lab on a chip. 2003;3 doi: 10.1039/b313217j. 60N-68N. [DOI] [PubMed] [Google Scholar]
- 3.Sato K, Tokeshi M, Odake T, Kimura H, Ooi T, Nakao M, Kitamori T. Integration of an immunosorbent assay system: Analysis of secretory human immunoglobulin A on polystyrene beads in a microchip. Analytical Chemistry. 2000;72(6):1144–1147. doi: 10.1021/ac991151r. [DOI] [PubMed] [Google Scholar]
- 4.Sato K, Tokeshi M, Kimura H, Kitamori T. Determination of carcinoembryonic antigen in human sera by integrated bead bed immunoassay in a microchip for cancer diagnosis. Analytical Chemistry. 2001;73(6):1213–1218. doi: 10.1021/ac000991z. [DOI] [PubMed] [Google Scholar]
- 5.Sato K, Yamanaka M, Takahashi H, Tokeshi M, Kimura H, Kitamori T. Microchip-based immunoassay system with branching multichannels for simultaneous determination of interferon-gamma. Electrophoresis. 2002;23(5):734–739. doi: 10.1002/1522-2683(200203)23:5<734::AID-ELPS734>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 6.Moorthy J, Mensing GA, Kim D, Mohanty S, Eddington DT, Tepp WH, Johnson EA, Beebe DJ. A colorimetric, disposable botulinum toxin enzyme-linked immunosorbent system. Electrophoresis. 2004;25:1705–1713. doi: 10.1002/elps.200405888. [DOI] [PubMed] [Google Scholar]
- 7.Christodoulides N, Tran M, Floriano PN, Rodriquez M, Goodey A, Ali M, Neikirk D, McDevitt JT. A microchip-based multi analyte assay system for the assessment of cardiac risk. Analytical Chemistry. 2002;74:3030–3036. doi: 10.1021/ac011150a. [DOI] [PubMed] [Google Scholar]
- 8.Gijs M. Magnetic bead handling on chip: new opportunities. Microfluidics Nanofluidics. 2004;1:22–40. [Google Scholar]
- 9.Pamme N. Magnetism and microfluidics. Lab on a chip. 2006;6:24–38. doi: 10.1039/b513005k. [DOI] [PubMed] [Google Scholar]
- 10.Choi JW, Oh KW, Thomas JH, Heineman WR, Halsall HB, Nevin JH, Helmicki AJ, Henderson HT, Ahn CH. An integrated microfluidic biochemical detection system for protein analysis with magnetic bead sampling capabilities. Lab on a chip. 2002;2:27–30. doi: 10.1039/b107540n. [DOI] [PubMed] [Google Scholar]
- 11.Hayes MA, Polson NA, Phayre AN, Garcia AA. Flow-based microimmunoassay. Analytical Chemistry. 2001;73:5896–5902. doi: 10.1021/ac0104680. [DOI] [PubMed] [Google Scholar]
- 12.Bronzeau S, Pamme N. Simultaneous bioassays in a microfluidic channel on plugs of different magnetic particles. Analytica Chimica Acta. 2008;609:105–112. doi: 10.1016/j.aca.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 13.Teh S, Lin R, Hung L, Lee A. Droplet microfluidics. Lab Chip. 2008;8:198–220. doi: 10.1039/b715524g. [DOI] [PubMed] [Google Scholar]
- 14.Pollack MG, Shenderov AD, Fair RB. Electrowetting-based actuation of droplets for integrated microfluidics. Lab Chip. 2002;2(1):96–101. doi: 10.1039/b110474h. [DOI] [PubMed] [Google Scholar]
- 15.Cho SK, Moon H, Kim CJ. Creating, transporting, cutting and merging liquid droplets by electrowetting-based actuation for digital microfluidic circuits. Journal of microelectromechanical systems. 2003;12(1):70–80. [Google Scholar]
- 16.Mugele F, Baret JC. Electrowetting: From basics to applications. Journal of Physics: Condensed matter. 2005;17:R705–R774. [Google Scholar]
- 17.Gascoyne PRC. Dielectrophoresis-based programmable fluidic processors. Lab on a chip. 2004;4:299–309. doi: 10.1039/b404130e. [DOI] [PubMed] [Google Scholar]
- 18.Song H, Tice J, Ismagilov R. A microfluidic system for controlling reaction networks in time. Angew. Chem. Int. Ed. 2003;42:767–772. doi: 10.1002/anie.200390203. [DOI] [PubMed] [Google Scholar]
- 19.Fair RB. Digital Microfluidics: is a true lab-on-a-chip possible? Microfluidics Nanofluidics. 2007;3:245–281. [Google Scholar]
- 20.Srinivasan V, Pamula VK, Pollack MG, Fair RB. Clinical diagnostics on human whole blood, plasma, serum, urine, saliva, sweat, and tears on a digital microfluidic platform. Proc. μTAS. 2003:1287–1290. [Google Scholar]
- 21.Srinivasan V, Pamula VK, Fair RB. A droplet-based microfluidic lab-on-a-chip for glucose detection. Anal. Chim. Acta. 2004;507(1):145–150. [Google Scholar]
- 22.Miller EM, Wheeler AR. A digital microfluidics approach to homogeneous enzyme assays. Analytical Chemistry. 2008;80(5):1614–1619. doi: 10.1021/ac702269d. [DOI] [PubMed] [Google Scholar]
- 23.Srinivasan V, Pamula VK, Fair RB. An integrated digital microfluidic lab-on-a-chip for clinical diagnostics on human physiological fluids. Lab Chip. 2004;4:310–315. doi: 10.1039/b403341h. [DOI] [PubMed] [Google Scholar]
- 24.Srinivasan V, Pamula VK, Paik P, Fair RB. Protein stamping for MALDI mass spectrometry using an electrowetting-based microfluidic platform. In SPIE Optics East, Lab-on-a-Chip: Platforms, Devices and Applications. 2004 [Google Scholar]
- 25.Moon H, Wheeler AR, Garrell RL, Loo JA, Kim CJ. An integrated digital microfluidic chip for multiplexed proteomic sample preparation and analysis by MALDI-MS. Lab Chip. 2006;6:1213–1219. doi: 10.1039/b601954d. [DOI] [PubMed] [Google Scholar]
- 26.Pamula VK, Srinivasan V, Chakrapani H, Fair RB, Toone EJ. A droplet based lab-on-a-chip for colorimetric detection of nitroaromatic explosives. In Proceedings of IEEE MEMS 2005 Conference; 2005. [Google Scholar]
- 27.Pollack MG, Paik PY, Shenderov AD, Pamula VK, Dietrich FS, Fair RB. Investigation of electrowetting-based microfluidics for real-time PCR applications. In Proceedings of MicroTAS 2003 Conference; 2003. [Google Scholar]
- 28.Chang Y, Lee G, Huang F, Chen Y, Lin J. Integrated polymerase chain reaction chips utilizing digital microfluidics. Biomedical microdevices. 2006;8:215–225. doi: 10.1007/s10544-006-8171-y. [DOI] [PubMed] [Google Scholar]
- 29.Nad IB, Yang H, Park PS, Wheeler AR. Digital microfluidics for cell-based assays. Lab chip. 2008;8:519–526. doi: 10.1039/b717759c. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Zhao Y, Cho SK. Efficient in-droplet separation of magnetic particles for digital microfluidics. Journal of Micromech. and Microeng. 2007;17:2148–2156. [Google Scholar]
- 31.Fouillet Y, Jary D, Chabrol C, Claustre P, Peponnet C. Digital microfluidic design and optimization of classic and new fluidic functions for lab on a chip systems. Microfluidics and Nanofluidics. 2008;4:159–165. [Google Scholar]
- 32.Shikida M, Takayanagi K, Inouchi K, Honda H, Sato K. Using wettability and interfacial tension to handle droplets of magnetic beads in a micro-chemical-analysis system. Sensors and Actuators B. 2006;113:563–569. [Google Scholar]
- 33.Lehmann U, Hadjidj S, Parashar VK, Rida A, Gijs MAM. Two dimensional magnetic manipulation of microdroplets on a chip as a platform for bioanalytical applications. Sensors and Actuators B. 2006;117:457–463. [Google Scholar]
- 34.Paik P, Pamula VK, Fair RB. Electrowetting-based droplet mixers for microfluidic systems. Lab chip. 2003;3:28–33. doi: 10.1039/b210825a. [DOI] [PubMed] [Google Scholar]