Abstract
Psoriasis is a chronic inflammatory disorder characterized by T cell dysregulation and a chronic inflammatory infiltrate within the epidermis. Several cytokines play an important role in the pathogenesis of psoriasis, including interleukin-12 (IL-12) and IL-23. These cytokines act via induction of pro-inflammatory cytokines which promote chronic inflammation and auto-reactivity. Ustekinumab is a fully human monoclonal antibody against the common p40 subunit of IL-12 and IL-23. Two randomized, double-blind, placebo-controlled trials of ustekinumab have demonstrated significant and prolonged efficacy in the treatment of plaque psoriasis. Adverse events were generally similar across treatment and control groups. Studies are ongoing to assess the long term safety and efficacy profiles of ustekinumab.
Keywords: ustekinumab, psoriasis, plaque
Introduction
Psoriasis is a chronic systemic inflammatory disease which primarily affects the skin and joints. The pathogenesis of psoriasis is multifactorial, with genetic, environmental, and immunologic factors contributing to the phenotype. Although the inheritance pattern for psoriasis remains unclear, a definite genetic predisposition has been noted through sibling and twin studies.1,2 Evidence for the role of environmental or external factors in psoriasis has been observed clinically by the appearance of psoriatic lesions at sites of physical trauma, known as Koebner’s phenomenon. Infections, especially streptococcal infections of the upper respiratory tract, have also been recognized as triggers of psoriasis.3
Immunologic mechanisms in psoriasis are complex, and are mediated by an aberrant T cell response to an unknown pathogen in the skin. The importance of T cells in the pathogenesis of psoriasis is supported by the response to treatment with compounds that act on lymphocytes, such as cyclosporine.4 In addition, psoriasis may develop for the first time after bone marrow transplantation from a donor with psoriasis.5 Animal models demonstrate that T cell dysregulation occurs without prior epithelial abnormalities, and appears to be dependent upon a susceptible environment.6 The cell mediated immune response in the skin of psoriasis patients is thought to be tilted toward a T helper-1 (Th1) response with over-expression of pro-inflammatory cytokines such as interferon-gamma (IFNγ).7 Several cytokines have been implicated in the pathogenesis of psoriasis, including tumor necrosis factor-alpha (TNFα), IFNγ, interleukin-12 (IL-12), IL-17 and, more recently, IL-23.
The use of biologic therapies in psoriasis is based on targeting one or more of the steps in the cellular immune response. Three fundamental modes of action are being explored: decreasing the number of pathogenic T cells, blocking T cell migration and adhesion, and inhibiting effector cytokines.8 Current therapies have demonstrated clinical efficacy; however, several problems exist. First, non-specific suppression of T cell function leads to global immune suppression, which underlies many of the toxicities associated with conventional systemic psoriasis treatment. Additionally, long term safety and efficacy data for recently developed therapies are just becoming available.9,10 There remains a need for therapies with long term clinical efficacy and safety profiles, which would improve patient compliance and satisfaction.
Ustekinumab is a fully human monoclonal antibody to the shared p40 subunit of IL-12 and IL-23. Studies in animal models demonstrated the drug’s ability to block IL-12 and IL-23 binding to their receptors, with inhibition of downstream signaling. Clinical trials in humans have shown ustekinumab to have favorable short term clinical efficacy and safety profiles. Long term studies are ongoing.
Immune mechanisms in psoriasis
Current research suggests that the T cells in psoriasis become reactive to an unknown antigen in the skin, which results in persistent T cell activation and low level expression of cytokines and their receptors.11 The cell mediated immune response has classically been characterized by the nature of infiltrating CD4+ T helper (Th) cells, with polarization toward a Th1 or Th2 response being dependent upon the cytokine milieu and other local factors. The Th1 tilted immune response is characterized by a strong cytotoxic response directed against intracellular pathogens, whereas the Th2 response involves antibody production by B cells.12 Psoriasis has classically been thought of as a Th1 tilted, cell mediated disorder, with infiltration of leukocytes into the dermis observed before any obvious epidermal changes occur.13 T cells play a key role in the pathogenesis of psoriasis via induction of an inflammatory cytokine cascade, with subsequent acceleration of the growth of epidermal and vascular cells.14
The first step in the cellular immune response involves the interaction of a naïve T cell with an antigen-presenting cell (APC), which leads to T cell activation and differentiation. This initial interaction involves the T-cell receptor (TCR) complex on the surface of the T cell, which binds specifically to an antigen-presenting MHC receptor on the APC. Co-stimulatory signals are required for T cell activation. One co-stimulatory signal is the interaction of CD40 on APCs with CD40 ligand (CD40L) on T cells, which results in the synthesis of high levels of the pro-inflammatory cytokine IL-12 by mature dendritic cells.15,16 IL-12 is the main cytokine in regulating the mitotic activation and differentiation of naïve T cells to type 1 cells – CD4+ Th1 cells, which can subsequently induce clonal expansion of type 1 cytotoxic (Tc1) CD8+ T cells, the main effector cell in psoriatic lesions.17 The increased expression of IL-12 in psoriatic skin, along with increased IL-12 receptor expressed on lesional T cells could drive the differentiation to a Type 1 mediated immune response seen in both skin lesions and peripheral circulating lymphocytes in patients with psoriasis.18,19
IL-12 is also an important regulator of the cell-mediated immune response, by inducing the expression of pro-inflammatory cytokines such as IFNγ and TNFα.20 These cytokines play a role in the innate immune response via stimulation of phagocytic activity, facilitating T cell infiltration into the epidermis, and inducing keratinocyte proliferation.21 IFNγ also may play an important role in enhancing survival of keratinocytes, since keratinocytes derived from psoriatic plaques were found to be resistant to apoptosis compared to normal skin samples.22 Recent studies have implicated the importance of IL-23, a newly discovered cytokine which shares a common p40 subunit with IL-12, in psoriasis. IL-23 acts on different effector cell populations, including memory T cells and a novel T helper cell population, known as Th17 cells.23 Through their elaboration of IL-17 and other cytokines important in the inflammatory response, Th17 cells are thought to have an increasingly important role in autoimmune and chronic inflammatory disorders such as psoriasis.24
IL-12 is a heterodimeric cytokine composed of two disulfide linked subunits, known as p40 and p35.25 While p35 is expressed ubiquitously at low levels, p40 expression is limited to cells that elaborate IL-12: monocytes, macrophages, dendritic cells, neutrophils, and B cells.26,27 IL-12 plays an essential role in mediating the innate immune response via enhancing the production of pro-inflammatory cytokines, particularly IFNγ, by natural killer (NK) cells and T cells.28,29 Other roles of IL-12 include promotion of Langerhans cell maturation in the skin, and increasing cytotoxicity of CD8+ T cells and NK cells.30 The gene encoding the p40 subunit of IL-12 is highly inducible by microbial products such as lipopolysaccharide (LPS).31 Therefore IL-12 production can be induced in a T cell-independent fashion by cells of the innate immune system, as well as by T cell interaction with APCs. IFNγ also promotes further IL-12 production, potentiating a positive feedback loop.32 Dysregulation of any of the steps in this pro-inflammatory cascade can lead to chronic inflammatory changes, as seen in psoriasis and other Th1-mediated disorders.
As mentioned above, IL-12 is the key effector cytokine in commitment to a Th1 response, which is thought to be important in inflammatory and autoimmune conditions. However, experiments in mouse models of inflammation have demonstrated that deficiency of IL-12 or IFNγ does not protect from the development of autoimmune diseases, and that often these mice have a more severe clinical picture than their wild type counterparts.33 This led to the discovery of IL-23, a novel biologically active cytokine which consisted of a p19 subunit linked to the p40 subunit of IL-12.34 It is produced primarily by activated macrophages and dendritic cells, and is thought to play an important role in linking the innate and adaptive arms of the immune response.35 Similarly to IL-12, IL-23 induces the production of IFNγ by Th1 cells, however at a much less efficient rate.36 Instead, IL-23 has unique cellular targets – memory T cells and a recently discovered subset of Th cells, termed Th17 cells because they produce IL-17.21 Th17 cells play an important role in the pathogenesis of autoimmune and inflammatory conditions, and actually perform several of the functions originally attributed to IL-12 and Th1 cells. IL-23 also acts on memory T cells, as evidenced by the expression of functional IL-23 receptor on the surface of human peripheral blood memory T cells.37 Contrary to the role of IL-12 in inducing a Th1 tilted immune response, IL-23 may play a more dominant role in maintaining chronic inflammation in psoriasis and regulating end-stage inflammatory processes. Further evidence for the role of IL-23 in the pathogenesis of psoriasis is supported by repeated intradermal injection of IL-23 in mice, which leads to psoriasis-like changes including epidermal hyperplasia and inflammation.38 In human psoriasis lesions, increased expression of IL-23 subunits compared to healthy controls was noted.39
Cytokine function is dependent upon binding to functional receptor on cell surfaces. The IL-12 receptor is composed of two subunits, β1 and β2, which form a heterodimer to allow high-affinity IL-12 binding. The p40 subunit of IL-12 interacts with IL-12Rβ1, whereas p35 binds to IL-12Rβ2, which is the subunit involved in signal transduction.40 Expression of the β2 subunit of IL-12R on activated T cells and NK cells is maintained by IFNγ.41,42 IL-12 receptor is also expressed on dendritic cells, forming a positive feedback loop for IL-12 expression.43 The IL-23 receptor is also composed of two subunits: IL-12Rβ1, which is also a subunit of the IL-12 receptor, and a second subunit which is unique to IL-23, known as IL-23R.44 The p40 subunit of IL-23 interacts with IL-12Rβ1, whereas the p19 subunit binds to IL-23R. Polymorphisms of the human IL-23 receptor complex are associated with several autoimmune and inflammatory diseases, including inflammatory bowel disease, psoriasis, and myocardial infarction.45 The IL-23 receptor has been detected on the surface of activated and memory T cells, NK cells, macrophages, monocytes, and dendritic cells.42,46
Animal models have helped to delineate the unique and overlapping functions of IL-12 and IL-23. Mice deficient in IL-12p40, p35, or IL-12Rβ1 have similar phenotypes of decreased IFNγ secretion and NK cell activity, and impaired Th1 differentiation.47,48 However, in a mouse model of colitis it was noted that p40−/− mice were more immunocompromised than p35−/− mice, owing to the lack of both IL-12 and IL-23 in the p40−/− mice.49 IL-12p40−/− mice showed compromised Toxoplasma gondii clearance, which was associated with decreased IFNγ production. Subsequent administration of IL-23 only prolongs, but does not maintain, viability in the IL-12p40−/− mice, suggesting that the presence of IL-23 cannot fully compensate for the absence of IL-12.50 In terms of autoimmune diseases, mice deficient in IL-12p40 or IL-12Rβ1 were found to be resistant to experimental auto-immune encephalitis (EAE), a murine model of multiple sclerosis, whereas p35 deficient mice still develop EAE. In addition, IL-12p35 deficient mice were noted to have more severe phenotypes of autoimmune diseases than their wild type counterparts.51 In humans, increased expression of IL-12 p40 and IL-23p19 was noted in psoriatic skin lesions, whereas p35 expression was not increased.52 IL-12p40 appears to be critical for the development of autoimmune diseases, since knockout mice are resistant, whereas IL-12 itself is dispensable – this finding emphasizes the unique role of IL-23 in autoimmune inflammation.
Th17 cells probably play a role in modulating the normal host immune response, as receptors for Th17 cytokines are located on epithelial and stromal cell types.53 Evidence for the unique role of Th17 cells is supported by the fact that T cells producing IL-17 do not produce Th1 or Th2 cytokines.54,55 Instead, the cytokine profile of Th17 cells in humans includes IL-17A, IL-17F, IL-22, and IL-6.56 Th17 cytokines were found to be more abundant in lesional and non-lesional skin of psoriasis patients than in healthy controls.35 Cytokines produced by Th17 cells regulate the expression of antimicrobial peptides, supporting the role of Th17 in providing a link between the adaptive immune response and non-specific immunity.57 The differentiation of naïve CD4+ T cells to Th17 cells is stimulated in humans by IL-23.35 In humans, higher levels of IL-17 producing cells were associated with more severe disease in autoimmune conditions such as multiple sclerosis and rheumatoid arthritis.58 The function of Th17 cells in the skin has been studied in models of psoriasis, and this cell lineage was found to play a role in initiating hyperkeratosis and acanthosis, activating downstream inflammation, chemotaxis of monocytes and neutrophils, T cell migration and activation, and neovascularization.59 Th17 cells also produce several cytokines which have been implicated in the keratinocyte hyperproliferation response in psoriasis, including IL-20 and IL-22.60
IL-23 is an essential cytokine in promoting the development of Th17 cells. Mice with EAE and CIA demonstrated a correlation between level of IL-23 and development of Th17 cells.61 Additionally, mice lacking IL-23 have no Th17 cells, and were found to be resistant to EAE and CIA.34 This link has also been demonstrated in human autoimmune diseases. Patients with Crohn’s disease had a 20-fold increase in Th17 cells and IL17+ macrophages compared to healthy controls, which the authors concluded may have been due to increased IL-23.43,56 Since one of the primary functions of Th17 cells is the elaboration of cytokines including IL-17, the function of this cytokine has been studied in autoimmune and inflammatory conditions, including psoriasis. In the skin, IL-17 plays a role in regulation of innate immunity in keratinocytes by increasing the expression of genes for pro-inflammatory cytokines and anti-microbial molecules.51 IL-17 also activates many cell types including dendritic cells, macrophages, neutrophils, T cells, and epithelial cells.57
The above findings support the importance of interleukins-12 and -23 in the pathogenesis of psoriasis. Interfering with either or both of these cytokines may prove to be useful in psoriasis treatment; however, the implications of blocking the actions of these cytokines long term are unknown.
Ustekinumab: a monoclonal antibody to the shared p40 subunit of IL-12 and IL-23
Clinical trials
Ustekinumab is a novel human monoclonal antibody that binds with high affinity to the p40 subunit of IL-12 and IL-23. The antibody acts to neutralize the bioactivity of these cytokines, by blocking interactions with IL-12Rβ1 and preventing subsequent downstream signaling. Inhibition of IFNγ production by mitogen-stimulated CD3+ T lymphocytes was demonstrated after administration of anti-IL-12p40.62 Previously known as CNTO-1275, it inhibits the T cell-mediated immune response that drives several autoimmune diseases, including psoriasis. Ustekinumab is absorbed and eliminated slowly; the average half-life was 20 to 24 days, and the drug demonstrated linear pharmacokinetics with a dose-dependent increase in serum drug concentration.63
Mouse models have demonstrated the potential for treatment of autoimmune diseases with antibody directed against IL-12p40.64,65 In a marmoset model of EAE, weekly injection of CNTO-1275 resulted in delayed and diminished symptom development compared to control animals, as well as decreased white matter changes by MRI. Additionally, disease progression in the setting of pre-existent EAE was delayed by treatment with CNTO-1275.66 Neutralizing antibody to IL-12p40 also abolished psoriatic lesions in mice.67 Ustekinumab has also been tested in human autoimmune diseases, and has demonstrated efficacy in early studies in both multiple sclerosis and Crohn’s disease.68,69
The first phase 1 trial of ustekinumab in psoriasis involved 18 patients with moderate to severe psoriasis, on an open label dose escalation trial with 4 intravenous (IV) doses: 0.1, 0.3, 1, and 5 mg/kg.70 Study participants had to have at least 3% body surface area involvement for inclusion in the study. Psoriasis Area and Severity Index (PASI) scores were assessed at baseline and during the 16-week follow-up period. A sustained and dose-dependent improvement in PASI was observed, with 83% of all active treatment group patients obtaining an improvement in PASI greater than 50%, and 67% of study subjects obtaining a 75% improvement in PASI from baseline (PASI 75). Clearing of psoriatic plaques was noted as early as two weeks after infusion, and maximal benefit appeared at 12 weeks for the majority of subjects.
A second phase 1 dose escalation trial of 21 patients with plaque psoriasis was a randomized controlled trial of a single subcutaneous (SC) injection of ustekinumab versus placebo.71 Patients were randomized to receive a SC injection dose of 0.27, 0.675, 1.35, or 2.7 mg/kg, or placebo. The primary endpoint was PASI 75, which was followed during the 24-week study. Subjects treated with ustekinumab experienced a significant, dose-dependent improvement in symptoms, with 77% of all active treatment subjects obtaining PASI 75 between weeks 4 and 24. PASI 75 was achieved in 3 out of 5 subjects in the 0.27 mg/kg group, 4 of 4 in the 0.675 mg/kg group, 2 of 4 in the 1.35 mg/kg group, and 4 of 4 in the 2.7 mg/kg group. No subjects in the placebo group achieved PASI75. Skin lesions from study participants were biopsied at baseline and after 1 week to assess the expression of pro-inflammatory cytokines in response to treatment with ustekinumab. In subjects with sustained PASI improvement (defined as at least 70% PASI improvement through week 16), the expression of IFNγ, IL-8, TNFα, and IL-12p40 and IL-23p19 subunits were decreased compared to baseline; these changes preceded clinical response and histological improvement of lesions.72 Patients who did not achieve sustained PASI improvement greater than 70% did not demonstrate a decrease in pro-inflammatory cytokines at one week post-treatment. Interestingly, baseline levels of TNFα in skin biopsy specimens correlated with clinical response, which suggests that this cytokine may be used in the future to predict therapeutic responsiveness to ustekinumab.73
A phase II double-blind, placebo-controlled trial of 320 patients with moderate-to-severe plaque psoriasis was also performed by Krueger et al with study subjects randomized to receive 1 of 4 subcutaneous dosing regimens of ustekinumab (one 45 mg dose, one 90 mg dose, 4-weekly 45 mg doses, or 4-weekly 90 mg doses) or placebo.74 Subjects with a sub-optimal response received an additional injection of the originally assigned dose at week 16, and subjects in the placebo group crossed over to receive a single 90 mg injection of ustekinumab at week 20. The primary endpoint of the study was achievement of PASI 75 at week 12, which was achieved in 52%, 59%, 67%, and 81%, respectively, of the aforementioned groups. In contrast, 2% of subjects who received placebo achieved PASI 75, with a p value of <0.001. In addition to evaluation of PASI, the physician’s global assessment (PGA) was also used in efficacy evaluations throughout the 32-week study. PGA scores, which were determined by treating physicians and ranged from 1 (clear) to 6 (worse), were significantly better in all active treatment groups versus placebo.
PGA scores were also used to determine which study subjects were eligible to receive an additional dose of ustekinumab; 37% of patients received one 90 mg dose based on PGA scores of 2 or greater at week 16. Study authors noted that PGA scores remained relatively stable throughout week 24, suggesting that this extra dose did not improve clinical outcomes in those patients who did not originally respond to ustekinumab. This observation further supports the notion that there may be a unique population of responders to anti-IL-12/23 antibody, possibly defined by baseline cytokine profiles.
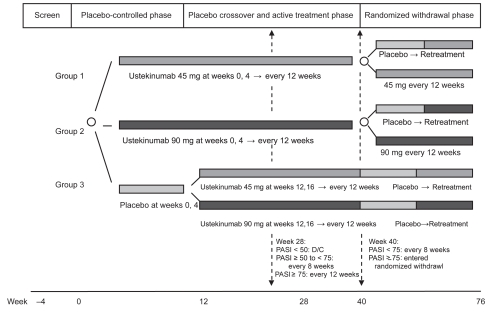
Two phase 3 trials were performed to assess long term safety and efficacy of ustekinumab in large patient cohorts.75,76 The randomized, double-blind, placebo-controlled trials, known as PHOENIX 1 and PHOENIX 2, consisted of nearly 2000 patients combined, and had similar study designs (Figure 1). The PHOENIX 1 study design was SC administration of 45 mg or 90 mg of ustekinumab at weeks 0 and 4, then every 12 weeks thereafter. The study was conducted in three phases: a placebo-controlled phase (weeks 0–12), placebo-crossover and active treatment phase (weeks 12–40), and a randomized withdrawal phase (weeks 40–76). During the placebo crossover phase, half of study subjects originally assigned to placebo received 45 mg injections every 12 weeks, and the other half received 90 mg injections. As in previous studies, the primary endpoint was PASI 75, which was achieved in approximately 67% of patients in both active treatment groups, versus 3% of placebo group patients (Table 1). Similarly to previous studies, onset of efficacy was noted by week 2, and maximal efficacy was observed between weeks 20 and 24. Patients originally randomized to placebo achieved similar response rates after crossover at week 12. After re-randomization at week 40, time to loss of PASI 75 response was better in patients receiving maintenance therapy than in the withdrawal of treatment group. The median time to loss of PASI75 in patients withdrawn from treatment was reported as 15 weeks. Rebound psoriasis was not reported in patients withdrawn from treatment. Among all active treatment groups, PASI 75 response was sustained through week 76.
Figure 1.
Study design of PHOENIX 1 and PHOENIX 2 through week 76, which included a placebo-controlled phase (week 0–12), placebo crossover and active treatment phase (week 12–40), and randomized withdrawal phase (week 40–76). In PHOENIX1, subjects who were initially randomized to receive ustekinumab and achieved long term response (defined as at least 75% improvement in PASI from baseline) were re-randomized at week 40 to maintenance ustekinumab or withdrawal from treatment until loss of response. In PHOENIX2, partial responders (subjects achieving ≥ 50% but < 75% improvement in PASI from baseline) were re-randomized at week 28 to continue dosing every 12 weeks or escalate to dosing every 8 weeks.
Abbreviations: D/C, discontinued; PASI, psoriasis area and severity index.
Table 1.
Improvement in psoriasis area and severity index (PASI) score
| Week 12
|
Week 28
|
||||||
|---|---|---|---|---|---|---|---|
| Ustekinumab 45 mg | Ustekinumab 90 mg | Placebo | Ustekinumab 45 mg | Ustekinumab 90 mg | Placebo to ustekinumab 45 mg | Placebo to ustekinumab 90 mg | |
| PHOENIX 1 | n = 255 | n = 256 | n = 255 | n = 250 | n = 243 | n = 123 | n = 119 |
| PASI 50 | 83.5% | 85.9% | 10.2% | 91.2% | 96.3% | 95.9% | 98.3% |
| PASI 75 | 67.1% | 66.4% | 3.1% | 71.2% | 78.6% | 65.9% | 84.9% |
| PASI 90 | 41.6% | 36.7% | 2.0% | 49.2% | 55.6% | 44.7% | 62.2% |
| PASI 100 | 12.5% | 10.9% | 0.0% | 20.8% | 29.2% | 19.5% | 33.6% |
| PHOENIX 2 | n = 409 | n = 411 | n = 410 | n = 397 | n = 400 | n = 193 | n = 194 |
| PASI 50 | 83.6% | 89.3% | 10.0% | 92.9% | 95.0% | 93.3% | 95.4% |
| PASI 75 | 66.7% | 75.7% | 3.7% | 69.5% | 78.5% | 69.9% | 78.9% |
| PASI 90 | 42.3% | 50.9% | 0.7% | 44.8% | 54.3% | 42.5% | 51.5% |
| PASI 100 | 18.1% | 18.2% | 0.0% | 18.6% | 29.5% | 15.5% | 21.6% |
Notes: PASI 50 indicates at least 50% improvement in PASI from baseline. The primary endpoint, PASI 75 at week 12, was statistically significant in the placebo-controlled phase with a p value < 0.0001 vs placebo. Placebo crossover occurred at week 12, with study subjects randomized to receive either ustekinumab 45 mg or 90 mg at weeks 12, 16, and every 12 weeks thereafter, or placebo.
PHOENIX 2 was similar in design to PHOENIX 1, but with dosing intensification for those subjects who did not respond fully to ustekinumab. Partial responders were defined as patients who achieved greater than 50% but less than 75% improvement in PASI scores from baseline. At week 28, partial responders were re-randomized to continue with their current dosing regimen (45 mg or 90 mg ustekinumab every 12 weeks), or to increase dosing frequency to every 8 weeks at their originally assigned dose. Primary endpoint results for PHOENIX 2 paralleled PHOENIX 1 results, with PASI 75 achieved in 75.7% and 66.7%, respectively, of patients in the 90 mg and 45 mg groups. At week 52, median improvement in PASI from baseline was around 95% in all active treatment subjects. There were 93 (22.7%) partial responders in the 45 mg group, and 65 (15.8%) in the 90 mg group. In the 90 mg group, 22 of 33 partial responders achieved PASI 75 after increasing dosing frequency to every 8 weeks. This observation may have been mediated by increased serum drug concentration with shortened dosing intervals, but does not explain the improvement fully since dosing intensification did not improve clinical outcomes in partial responders in the 45 mg group. Subgroup analysis of partial responders revealed a tendency toward higher bodyweight, more severe disease by PGA, longer duration of skin disease, previous failure of biologic agent(s), and a higher incidence of psoriatic arthritis. Only higher body weight was a statistically significant predictor of partial response to treatment with ustekinumab every 12 weeks. In addition, 12.7% of partial responders were found to have antibodies to ustekinumab, and serum trough levels of ustekinumab at week 28 were 2 to 3 times lower than levels in responders.
Adverse events
SC and IV administrations of ustekinumab were generally well tolerated, with the majority of adverse events being mild in intensity. The most commonly reported adverse events included upper respiratory symptoms, headache, arthralgias, transient increase in creatine phosphokinase (CPK), and lymphopenia. Adverse events were relatively evenly distributed across placebo and study groups. Importantly, changes in levels of T lymphocyte and NK cell groups were noted in early studies but these changes did not appear to be consistent or dose-dependent, and did not correlate with any clinical symptoms. Injection site reactions were generally mild and not considered to be clinically relevant.
In the phase 2 study, adverse events were high in both active treatment and placebo groups (79% and 72%, respectively). Serious adverse events, defined as requiring hospitalization, occurred in 4% of patients treated with ustekinumab and 1% of patients in the placebo group. Two patients receiving ustekinumab were hospitalized for infection, and three for cardiovascular events. Contrary to findings of the phase I studies, there were no significant differences observed in changes in absolute lymphocyte counts or lymphocyte subsets between treatment and placebo groups. Antibodies to ustekinumab developed in 5.1% of phase 3 study participants through week 76; these were predominantly low titer and not associated with injection site reactions. No anaphylactic reactions were observed in either large scale study.
Patients with psoriasis have an increased risk of cardiovascular events.77 In the phase 2 trial of ustekinumab two patients experienced myocardial infarction and one suffered a stroke during the placebo-controlled phase. Subsequent larger phase 3 studies revealed no increased risk of cardiovascular side effects. One patient in the placebo and group and one patient in the active treatment group experienced an adverse cardiac event, including a sudden cardiac death in the active treatment group.
Since the Th1 response is important for clearance of intracellular pathogens and defense against malignancies, the incidence of these adverse effects was considered to be especially important in the evaluation of ustekinumab.78,79 During PHOENIX 1 and PHOENIX 2, two serious infections occurred during the placebo-controlled phase in patients receiving ustekinumab 90 mg, one case each of cellulitis and herpes zoster. Patients receiving maintenance ustekinumab therapy did not experience increased rates of adverse events compared to patients in the randomized withdrawal group.
Patients were screened for tuberculosis (TB) before enrollment. Exclusion criteria included symptoms of active TB. However patients with a newly identified positive skin test (“latent TB”) could be included if appropriate treatment was initiated before or along with first administration of the study agent. No cases of TB, latent reactivation of TB, other mycobacterial infections or salmonella infections were observed.
Cutaneous malignancies were reported in two patients during the placebo-controlled phase of PHOENIX 2, a squamous cell cancer in the placebo group and a basal cell cancer in the 90 mg treatment group. No differences were observed in common laboratory tests between active treatment and placebo groups, including liver function tests, fasting glucose, or hemoglobin A1c levels.
Although the incidence of serious infections was low in studies to date, concern remains for the risk of generalized impaired immunity after treatment with ustekinumab, since IFNγ and IL-12 are essential for cell mediated immunity. Additionally, blocking both IL-12 and IL-23 through their common p40 subunit may also impair the Th17 response, which is thought to be important in normal keratinocyte host immunity.
IL-23 knockout mice were demonstrated to have impaired clearance of bacterial and parasitic pathogens, decreased numbers of NK cells, and deficient Th cell development.80 They also have a defect in re-activation of memory T cells, which leads to impairment in the humoral immune response and delayed type hypersensitivity reactions.81 In human subjects suffering from severe mycobacterial and salmonella infections, defects in the genes encoding IL-12p40 and IL-12Rβ1 were detected.82,83 These observations suggest continued vigilant observation of patients treated with this agent.
Studies to investigate potential limiting factors in the use of ustekinumab were performed in monkeys.68 Embryo-fetal toxicity studies performed during the period of organogenesis did not reveal maternal or fetal abnormalities. Nevertheless, study participants to date have been predominantly males, since women of childbearing age were excluded from participation. Additionally, studies were conducted in a monkey model of asthma, which showed that ustekinumab did not exacerbate the pulmonary function of study monkeys following two IV doses at 50 mg/kg, spaced 4 weeks apart. Further research is needed to determine whether these results will translate to human subjects.
Conclusion
The above results support the strategy of development of monoclonal antibodies directed against Th1 cytokines involved in the pathogenesis of autoimmune and chronic inflammatory responses. Although there are limitations in comparison across studies, current data indicate that clinical efficacy of ustekinumab compares favorably with the highest efficacy reported for currently available biologic therapies.84,85,86 Additionally, the long half-life of ustekinumab allows for prolonged efficacy with infrequent dosing schedules, which may improve treatment adherence. Treatment with subcutaneous injections of ustekinumab every 12 weeks appeared to be efficacious for most psoriasis patients, but intensification to every 8 week therapy at 90 mg may be necessary to elicit a full response in a subset of patients. Partial responders, which could represent approximately 20% of the total population, were found to have lower serum drug levels than PASI 75 responders, indicating an association between drug levels and clinical response. Identification of partial responders may be facilitated by demographic characteristics, such as increased body weight or lack of response to other biologic agents, or possibly by cytokine profiles. Residual skin disease may be mediated by alternate immune pathways in these individuals. In patients withdrawn from ustekinumab therapy in phase 3 trials, psoriasis gradually recurred, showing that temporary blockade of IL-12 and IL-23 did not reverse the underlying causal mechanism of psoriasis. Therefore, sustaining clinical response and quality of life improvements could require indefinite maintenance dosing. Current studies did not reveal any major safety concerns in blocking IL-12 and IL-23 for up to 18 months; however, uncommon events or events that occur after longer exposure cannot be excluded based on current study data. For this reason, the PHOENIX 1 and PHOENIX 2 studies have been extended for a total of 5 years.
It is important to note that serious adverse events may not be noted in newly approved medications until post-marketing surveillance studies. This may be in part because some serious adverse events are very uncommon, and are not detected until a drug has been used in a large number of patients. Additionally, patients participating in clinical trials have specific inclusion and exclusion criteria which may not be applicable to the general population. A recent study examined the nature and frequency of safety-related regulatory actions for biologic drugs following approval, including dear health care professional letters and black box warnings.87 The probability of a safety-related regulatory action for a newly approved biologic agent was 14% 3 years after approval, and 29% 10 years after approval. Additionally, biologics first in class to obtain approval had a higher risk for a safety-related regulatory action, with a hazard ratio of 3.7. Therefore, providers and patients should be vigilant in monitoring patients being treated with ustekinumab, as serious adverse events may only become apparent during post-marking surveillance.
Footnotes
Disclosures
Robert E Kalb has served as a consultant and/or investigator for Abbott, Astellas, Amgen, Centocor (the manufacturer of ustekinumab), and Genentech.
Jenna O’Neill has no conflicts of interest.
References
- 1.Watson W, Cann HM, Farber EM, Nall ML. The genetics of psoriasis. Arch Dermatol. 1972;105:197–207. [PubMed] [Google Scholar]
- 2.Farber EM, Nall ML, Watson W. Natural history of psoriasis in 61 twin pairs. Arch Dermatol. 1974;109:207–211. [PubMed] [Google Scholar]
- 3.Owen CM, Chalmers RJ, O’Sullivan T, Griffiths CE. Antistreptococcal interventions for guttate and chronic plaque psoriasis. Cochrane Database Syst Rev. 2000;2:CD001976. doi: 10.1002/14651858.CD001976. [DOI] [PubMed] [Google Scholar]
- 4.Mueller W, Herrmann B. Cyclosporine A for psoriasis. N Engl J Med. 1979;301:555. doi: 10.1056/NEJM197909063011016. [DOI] [PubMed] [Google Scholar]
- 5.Gardembas-Pain M, Ifrah N, Foussard C, Boasson M, Saint Andre JP, Verret JL. Psoriasis after allogenic bone marrow transplantation. Arch Dermatol. 1990;126:1523. doi: 10.1001/archderm.1990.01670350139033. [DOI] [PubMed] [Google Scholar]
- 6.Boyman O, Hefti HP, Conrad C, Nickoloff BJ, Suter M, Nestle FO. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-alpha. J Exp Med. 2004;199:731–736. doi: 10.1084/jem.20031482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Austin LM, Ozawa M, Kikuchi T, et al. The majority of epidermal T cells in psoriasis vulgaris lesions can produce type I cytokines, IFN-γ, IL-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a Type I differentiation bias is also measured in circulating blood T cells in psoriatic patients. J Invest Dermatol. 1999;113:752–759. doi: 10.1046/j.1523-1747.1999.00749.x. [DOI] [PubMed] [Google Scholar]
- 8.Schon MP, Boehncke WH. Psoriasis. N Engl J Med. 2005;352:1899–1912. doi: 10.1056/NEJMra041320. [DOI] [PubMed] [Google Scholar]
- 9.Leonardi C, Menter A, Hamilton T, Caro I, Xing B, Gottlieb AB. Efalizumab: results of a 3-year continuous dosing study for the long-term control of psoriasis. Br J Dermatol. 2008;158:1107–1116. doi: 10.1111/j.1365-2133.2008.08548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tyring S, Gordon KB, Poulin Y, et al. Long-term safety and efficacy of 50 mg of etanercept twice weekly in patients with psoriasis. Arch Dermatol. 2007;143:719–26. doi: 10.1001/archderm.143.6.719. [DOI] [PubMed] [Google Scholar]
- 11.Bos JD, Hulsebosch HJ, Krieg SR, Bakker PM, Cormane RH. Immunocompetent cells in psoriasis. In situ immunophenotyping by monoclonal antibodies. Arch Dermatol Res. 1983;275:181–189. doi: 10.1007/BF00510050. [DOI] [PubMed] [Google Scholar]
- 12.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 13.Braun-Falco O, Schmoeckel C. The dermal inflammatory reaction in initial psoriatic lesions. Arch Dermatol Res. 1977;258:9–16. doi: 10.1007/BF00582862. [DOI] [PubMed] [Google Scholar]
- 14.Krueger JG. Pathogenic interactions of keratinocytes and T lymphocytes in psoriasis. In: Roenigk HH, Maibach HI, editors. Psoriasis. New York: Marcel Dekker; 1998. pp. 315–327. [Google Scholar]
- 15.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184(2):747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng X, Kasran A, Warmerdam PA, de Boer M, Ceuppens JL. Accessory signaling by CD40 for T cell activation: induction of Th1 and Th2 cytokines and synergy with interleukin-12 for interferon-gamma production. Eur J Immunol. 1996;26:1621–1627. doi: 10.1002/eji.1830260732. [DOI] [PubMed] [Google Scholar]
- 17.Austin LM, Ozawa M, Kikuchi T, Walters IB, Krueger JG. The majority of epidermal T cells in psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J Invest Dermatol. 1999;113:752–759. doi: 10.1046/j.1523-1747.1999.00749.x. [DOI] [PubMed] [Google Scholar]
- 18.Yawalkar N, Karlen S, Hunger R, Brand CU, Braathen LR. Expression of interleukin-12 is increased in psoriatic skin. J Invest Dermatol. 1998;111:1053–1057. doi: 10.1046/j.1523-1747.1998.00446.x. [DOI] [PubMed] [Google Scholar]
- 19.Trepicchio WL, Ozawa M, Walters IB, et al. Interleukin-11 therapy selectively downregulates type I cytokine proinflammatory pathways in psoriasis lesions. J Clin Invest. 1999;104:1527–1537. doi: 10.1172/JCI6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Germann T, Gately MK, Schoenhaut DS, et al. Interleukin-12/T cell stimulating factor, a cytokine with multiple effects of T helper (Th1) but not on Th2 cells. Eur J Immunol. 1993;23:1762–1770. doi: 10.1002/eji.1830230805. [DOI] [PubMed] [Google Scholar]
- 21.Torti DC, Feldman SR. Interleukin-12, interleukin-23, and psoriasis: current prospects. JAAD. 2007;57(6):1059–1068. doi: 10.1016/j.jaad.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Nickoloff BJ. The immunologic and genetic basis of psoriasis. Arch Dermatol. 1999;135:1104–1110. doi: 10.1001/archderm.135.9.1104. [DOI] [PubMed] [Google Scholar]
- 23.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 24.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nature Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gately MK, Desai BB, Wolitzky AB, et al. Regulation of human lymphocyte proliferation by a heterodimeric cytokine, IL-12 (cytotoxic lymphocyte maturation factor) J Immunol. 1991;147(3):874–882. [PubMed] [Google Scholar]
- 26.Babik JM, Adams E, Tone Y, Fairchild PJ, Tone M, Waldmann H. Expression of murine IL-12 is regulated by translational control of the p35 subunit. J Immunol. 1999;162(7):4069–4078. [PubMed] [Google Scholar]
- 27.Watford WT, Moriguchi M, Morinobu A, O’Shea JJ. The biology of IL-12: coordinating innate and adaptive immune responses. Cytokin Growth Factor Rev. 2003;14:361–368. doi: 10.1016/s1359-6101(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 28.Micallef MJ, Ohtsuki T, Kohno K, et al. Interferon-γ-inducing factor enhances T helper I cytokine production by stimulated human T cells: synergism with interleukin-12 for interferon-γ production. Eur J Immunol. 1996;26(7):1647–1651. doi: 10.1002/eji.1830260736. [DOI] [PubMed] [Google Scholar]
- 29.Lauwerys BR, Renauld JC, Houssiau FA. Synergistic proliferation and activation of natural killer cells by interleukin-12 and interleukin-18. Cytokine. 1999;11(11):822–830. doi: 10.1006/cyto.1999.0501. [DOI] [PubMed] [Google Scholar]
- 30.Gran B, Zhang GX, Rostami A. Role of the IL-12/IL-23 system in the regulation of T-cell responses in central nervous system inflammatory demyelination. Crit Rev Immunol. 2004;24:111–128. doi: 10.1615/critrevimmunol.v24.i2.20. [DOI] [PubMed] [Google Scholar]
- 31.Ma X, Trinchieri G. Regulation of interleukin-12 production in antigen-producing cells. Adv Immunol. 2001;79:55–92. doi: 10.1016/s0065-2776(01)79002-5. [DOI] [PubMed] [Google Scholar]
- 32.Ma X, et al. The interleukin-12 p40 gene promoter is primed by interferon-γ in monocytic cells. J Exp Med. 1996;183(1):147–157. doi: 10.1084/jem.183.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chu CQ, Wittmer S, Dalton DK. Failure to suppress the expansion of the activated CD4 T cell population in interferon gamma-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192:123–128. doi: 10.1084/jem.192.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oppmann B, Lesley R, Blom B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 35.McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL23-IL17 immune pathway. Trends Immunol. 2006;27(1):17–23. doi: 10.1016/j.it.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Langrish CL, McKenzie BS, Wilson NJ, de Waal Malefyt R, Kastelein RA, Cua DJ. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol Rev. 2004;202:96–105. doi: 10.1111/j.0105-2896.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 37.Wilson NJ, Boniface K, Chan JR, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8(9):950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 38.Chan JR, Blumenschein W, Murphy E, et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med. 2006;203:2577–2587. doi: 10.1084/jem.20060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee E, Trepicchio WL, Oestreicher JL, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125–130. doi: 10.1084/jem.20030451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Presky DH, Yang H, Minetti LJ, et al. A functional interleukin 12 receptor complex is composed of two β type cytokine receptor subunits. Proc Natl Acad Sci USA. 1996;93:14002–14007. doi: 10.1073/pnas.93.24.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lighvani AA, Frucht DM, Jankovic D, et al. T-bet is rapidly induced by interferon-γ in lymphoid and myeloid cells. Proc Natl Acad Sci U S A. 2001;98(26):15137–15142. doi: 10.1073/pnas.261570598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Afkarian M, Sedy JR, Yang J, et al. T-bet is a STAT1-induced regulator of IL-12 receptor expression in naïve CD4+ T cells. Nat Immunol. 2002;3(6):549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 43.Grohmann U, Frucht DM, Jankovic D, et al. IL-12 acts directly on DC to promote nuclear localization of NF-κβ and primes DC for IL-12 production. Immunity. 1998;9:315–323. doi: 10.1016/s1074-7613(00)80614-7. [DOI] [PubMed] [Google Scholar]
- 44.Parham C, Chirica M, Timans J, et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rβ1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168(11):5699–5708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- 45.McGovern D, Powrie F. The IL23 axis plays a key role in the pathogenesis of IBD. Gut. 2007;56(10):133–136. doi: 10.1136/gut.2006.115402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Belladonna ML, Renauld JC, Bianchi R, et al. IL-23 and IL-12 have overlapping, but distinct, effects on murine dendritic cells. J Immunol. 2002;168:5448–5454. doi: 10.4049/jimmunol.168.11.5448. [DOI] [PubMed] [Google Scholar]
- 47.Magram J, Connaughton SE, Warrier RR, et al. IL-12-deficient mice are defective in IFN-γ production and type I cytokine responses. Immunity. 1996;4(5):471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- 48.Wu C, Ferrante J, Gately MK, Magram J. Characterization of IL-12 receptorβ1 chain (IL-12Rβ1)-deficient mice: IL-12Rβ1 is an essential component of the functional mouse IL-12 receptor. J Immunol. 1997;159(4):1658–1665. [PubMed] [Google Scholar]
- 49.Camoglio L, Juffermans NP, Peppelenbosch M, et al. Contrasting roles of IL-12p40 and IL-12p35 in the development of hapten-induced colitis. Eur J Immunol. 2002;32(1):261–269. doi: 10.1002/1521-4141(200201)32:1<261::AID-IMMU261>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 50.Lieberman LA, Cardillo F, Owyang AM, et al. IL-23 provides a limited mechanism of resistance to acute toxoplasmosis in the absence of IL-12. J Immunol. 2004;173(3):1887–1893. doi: 10.4049/jimmunol.173.3.1887. [DOI] [PubMed] [Google Scholar]
- 51.Becher B, Durell BG, Noelle RJ. Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J Clin Invest. 2002;110:493–497. doi: 10.1172/JCI15751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee E, Trepicchio WL, Oestericher JL, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125–130. doi: 10.1084/jem.20030451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teunissen MB, Koomen CW, de Waal Malefyt R, Wierenga EA, Bos JD. Interleukin-17 and interferon-γ synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol. 1998;111:645–649. doi: 10.1046/j.1523-1747.1998.00347.x. [DOI] [PubMed] [Google Scholar]
- 54.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 55.Wynn TA. T(H)-17: a giant step from T(H)1 and T(H)2 [comment] Nat Immunol. 2005;6:1069–1070. doi: 10.1038/ni1105-1069. [DOI] [PubMed] [Google Scholar]
- 56.Liang SC, Tan XY, Luxenberg DP, et al. Interleukin (IL)-22 and IL-17 are co-expressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolk K, Witte E, Wallace E, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Imunnol. 2006;36:1309–1323. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
- 58.Kikly K, Liu L, Na S, Sedgwick JD. The IL-23/Th(17) axis: therapeutic targets for autoimmune inflammation. Curr Opin Immunol. 2006;18(6):670–675. doi: 10.1016/j.coi.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 59.Asarch A, Barak O, Loo D, Gottlieb A. Th17 cells: a new paradigm for cutaneous inflammation. J Dermatolog Treat. 2008;19:259–266. doi: 10.1080/09546630802206686. [DOI] [PubMed] [Google Scholar]
- 60.Zheng Y, Danilenko DM, Valdez P, et al. Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 61.Kreymborg K, Böhlmann U, Becher B. IL-23: changing the verdict on IL-12 function in inflammation and autoimmunity. Expert Opin Ther Targets. 2005;9(6):1123–1136. doi: 10.1517/14728222.9.6.1123. [DOI] [PubMed] [Google Scholar]
- 62.Brok HP, van Meurs M, Blezer E, et al. Prevention of experimental autoimmune encephalomyelitis in common marmosets using an anti-IL-12p40 monoclonal antibody. J Immunol. 2002;169:6554–6563. doi: 10.4049/jimmunol.169.11.6554. [DOI] [PubMed] [Google Scholar]
- 63.Kasper LH, Everitt D, Leist T, et al. Safety, pharmacokinetic, and biological response profile of an IL-12p40 antagonist in MS: A phase I clinical trial. Proc Congress Eur Committee Treatment Res Multiple Sclerosis. 2004;107 [Google Scholar]
- 64.Leonard JP, Waldburger KE, Goldman SJ. Prevention of experimental autoimmune encephalomyelitis by antibodies against interleukin 12. J Exp Med. 1995;181(1):381–386. doi: 10.1084/jem.181.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Malfait AM, Butler DM, Presky DH, Maini RN, Brennan FM, Feldmann M. Blockade of IL-12 during the induction of collagen-induced arthritis (CIA) markedly attenuates the severity of the arthritis. Clin Exp Immunol. 1998;111(2):377–383. doi: 10.1046/j.1365-2249.1998.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brok HP, van Meurs M, Blezer E, et al. Prevention of experimental autoimmune encephalomyelitis in common marmosets using an anti-IL-12p40 monoclonal antibody. J Immunol. 2002;169(11):6554–6563. doi: 10.4049/jimmunol.169.11.6554. [DOI] [PubMed] [Google Scholar]
- 67.Hong K, Chu A, Ludviksson BR, Berg EL, Ehrhardt RO. IL-12, independently of IFN-γ, plays a crucial role in the pathogenesis of a murine psoriasis-like skin disorder. J Immunol. 1999;162:7480–7491. [PubMed] [Google Scholar]
- 68.Segal BM, Constantinescu CS, Raychaudhuri A, Kim L, Fidelus-Gort R, Kasper LH. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomized, dose-ranging study. Lancet Neurol. 2008;7(9):796–804. doi: 10.1016/S1474-4422(08)70173-X. [DOI] [PubMed] [Google Scholar]
- 69.Sandborn WJ, Feagan BG, Fedorak RN, et al. A randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate to severe Crohn’s disease. Gastroenterology. 2008;135(4):1130–1141. doi: 10.1053/j.gastro.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 70.Kauffman CL, Aria N, Toichi E, et al. A phase I study evaluating the safety, pharmacokinetics, and clinical response of a human IL-12 p40 antibody in subjects with plaque psoriasis. J Invest Dermatol. 2004;123:1037–1044. doi: 10.1111/j.0022-202X.2004.23448.x. [DOI] [PubMed] [Google Scholar]
- 71.Gottlieb AB, Cooper KD, McCormick TS, et al. A phase 1, double-blind, placebo-controlled study evaluating single subcutaneous administration of a human interleukin-12/23 monoclonal antibody in subjects with plaque psoriasis. Curr Med Res Opin. 2007;23:1081–1092. doi: 10.1185/030079907x182112. [DOI] [PubMed] [Google Scholar]
- 72.Toichi E, Torres G, McCormick TS, et al. An anti-IL-12p40 antibody down-regulates type 1 cytokines, chemokines, and IL12/IL23 in psoriasis. J Immunol. 2006;177(7):4917–4926. doi: 10.4049/jimmunol.177.7.4917. [DOI] [PubMed] [Google Scholar]
- 73.Toichi E, McCormick TS, Kaufmann CL, et al. Quantitation of cutaneous cytokine mRNA levels following anti-IL-12 psoriasis therapy: identification of a psoriasis subset. J Invest Dermatol. 2003;121:1249. [Google Scholar]
- 74.Krueger GC, Langley RG, Leonardi C, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580–592. doi: 10.1056/NEJMoa062382. [DOI] [PubMed] [Google Scholar]
- 75.Leonardi C, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX1) Lancet. 2008;371:1665–1674. doi: 10.1016/S0140-6736(08)60725-4. [DOI] [PubMed] [Google Scholar]
- 76.Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX1) Lancet. 2008;371:1675–1684. doi: 10.1016/S0140-6736(08)60726-6. [DOI] [PubMed] [Google Scholar]
- 77.Niemann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand JM. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol. 2006;55:829–835. doi: 10.1016/j.jaad.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 78.Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:155–168. doi: 10.1016/s1359-6101(01)00032-6. [DOI] [PubMed] [Google Scholar]
- 79.Overwijk WW, de Visser KE, Tirion FH, et al. Immunological and antitumor effects of IL-23 as a cancer vaccine adjuvant. J Immunol. 2006;176:5213–5222. doi: 10.4049/jimmunol.176.9.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tan ZY, Bealgey KW, Fang Y, Gong YM, Bao S. Interleukin-23: immunological roles and clinical implications. Int J Biochem Cell Biol. 2008 doi: 10.1016/j.biocel.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 81.Ghiladri N, Klajavin N, Chen Q, Lucas S, Gurney AL, Sauvage FJ. Compromised humoral and delayed-type hypersensitivity responses in IL-23 deficient mice. J Immunol. 2004;172(5):2827–2833. doi: 10.4049/jimmunol.172.5.2827. [DOI] [PubMed] [Google Scholar]
- 82.de Jong R, Altare F, Haagen IA, et al. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science. 1998;280(5368):1435–1438. doi: 10.1126/science.280.5368.1435. [DOI] [PubMed] [Google Scholar]
- 83.Doffinger R, Dupuis S, Picard C, et al. Inherited disorders of IL-12 and IFN-γ-mediated immunity: a molecular genetics update. Mol Immunol. 2002;38(12–13):903–909. doi: 10.1016/s0161-5890(02)00017-2. [DOI] [PubMed] [Google Scholar]
- 84.Leonardi CL, Powers JL, Matheson RT, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349:2014–2022. doi: 10.1056/NEJMoa030409. [DOI] [PubMed] [Google Scholar]
- 85.Lebwohl M, Christophers E, Langley R, Ortonne JP, Roberts J, Griffiths CE. An international, randomized, double-blind, placebo-controlled phase 3 trial of intramuscular alefacept in patients with chronic plaque psoriasis. Arch Dermatol. 2003;139:719–727. doi: 10.1001/archderm.139.6.719. [DOI] [PubMed] [Google Scholar]
- 86.Lebwohl M, Tyring SK, Hamilton TK, et al. A novel targeted T-cell modulator, efalizumab, for plaque psoriasis. N Engl J Med. 2003;349:2004–2013. doi: 10.1056/NEJMoa030002. [DOI] [PubMed] [Google Scholar]
- 87.Giezen TJ, Mantel-Teeuwisse AK, Straus SM, Schellekens H, Leufkens HG, Egberts A. Safety-related regulatory actions for biologicals approved in the United States and the European Union. JAMA. 2008;300(16):1887–1896. doi: 10.1001/jama.300.16.1887. [DOI] [PubMed] [Google Scholar]