Abstract
Visual system development utilizes global and local cues to assemble a topographic map of the visual world, arranging synaptic connections into columns and layers. Recent genetic studies have provided new insights into the mechanisms that underlie these processes. In flies, a precise temporal sequence of neural differentiation provides a global organizing cue; in vertebrates, gradients of ephrin-mediated signals, acting with neurotrophin co-receptors and neural activity, play crucial roles. In flies and mice, neural processes tile into precise arrays through homotypic, repulsive interactions, autocrine signals, and cell-intrinsic mechanisms. Laminar targeting specificity is achieved through temporally regulated cell–cell adhesion, as well as combinatorial expression of specific adhesion molecules. Future studies will define the interactions between these global and local cues.
Introduction
The visual world is represented in the brain by circuits that define separate, parallel streams of information. These circuits segregate early, occupy distinct brain regions, and monitor the visual field while maintaining the spatial relationships between parts of visual space. Thus, these circuits are organized into topographic maps composed of reiterated arrays of columns that have identical neural circuitry and differ only in the area of the visual space to which they are devoted. Within each column, neurons of the same type make synapses in specific layers, forming laminated structures. Here we summarize recent progress made in defining the molecular mechanisms that underlie the cues that assemble these structures.
Finding places in the target field
Temporal gradients and local interactions map the fly visual system
Photoreceptor axons in the fly visual system project directly into the brain, where they make retinotopic connections with target neurons. Along the anteroposterior (A-P) axis, photoreceptors differentiate and extend their axons in a precise temporal order, preserving this timing sequence [1] (Figure 1). Each incoming axon induces the proliferation and differentiation of their associated target neurons [1–3]. Thus, there is a gradient of time that creates topography along this axis. Less well understood is how axons choose targets along the dorsoventral axis. Regionally restricted expression of a member of the Wnt family is required for photoreceptor axons to appropriately innervate the dorsal versus the ventral part of the target field [4]. These global mapping cues then appear to be reinforced by local, repulsive signals between R cell axons [5]. Retinotopic map formation in the fly is complicated by the optics of the compound eye, which cause neighboring photoreceptors to look at non-neighboring parts of visual space [6]. As a result, a complex set of photoreceptor axon projections reconstructs the appropriate topographic map in the lamina [7](Figure 2). These connections form using interactions among afferent axons to direct individual photoreceptor axons to appropriate target columns [8,9]. At least some of these interactions are mediated by the non-classical cadherin Flamingo, which uses differences in the levels of homophillic adhesion between R cell growth cones to influence their trajectory [10,11•]. Anterograde signals using the receptor tyrosine kinase ALK also play an important role in directing individual R cell axons to appropriate targets, probably regulating the expression of adhesion molecules on target neurons [12].
Figure 1.
Topographic map formation in Drosophila. Map formation along the antero-posterior axis is driven by a precise temporal sequence of differentiation, extension, and induction. Photoreceptors differentiate following a wave that begins first at the posterior edge of the retina and spreads anteriorly. They then extend their axons into the lamina following their birth order and induce the differentiation and assembly of target neurons into a columnar array. As each incoming bundle of photoreceptor axons induces its own target column, the number of target columns is precisely matched to the number of photoreceptors that differentiation, and map formation along the A-P axis is ensured by the precise sequence of differentiation in the retina. Dwnt4 is expressed in the ventral lamina, and is regulates dorso-ventral R cell projections.
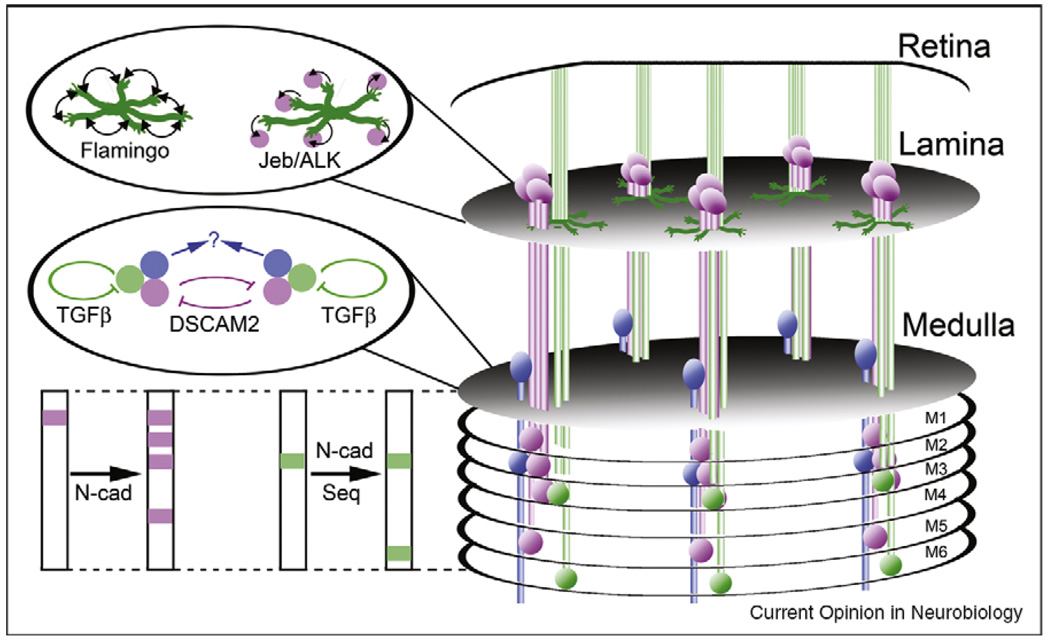
Figure 2.
The anatomy of ganglia, columns and layers in the Drosophila visual system. Photoreceptor axons (green) extend into the brain from the retina, with R1-R6 axons terminating in the lamina, and R7 and R8 axons terminating in the medulla. Within the lamina, R1-R6 axons choose targets arranged in a precise relative pattern, and make synapses with lamina neurons (magenta) arranged in an array of columns. These targeting choices are dependent upon afferent–afferent interactions mediated by the non-classical cadherin Flamingo, as well as by anterograde signals transmitted by Jellybelly (Jeb) and its cognate receptor, the tyrosine kinase ALK. Within the medulla, R7 and R8 axons make synaptic connections with medulla neurons (blue; only one type is shown for simplicity) arranged in columns. Projections from lamina neurons and photoreceptors are restricted to specific columns, and are laminated. Inhibitory interactions mediated by DSCAM2 serve to restrict a specific subset of lamina neurons to their target columns, while autocrine signals provided by TGFβ play an important role in tiling R7 terminals. As-yet unknown mechanisms presumably tile medulla neuron processes. The layer-specific connections of lamina neurons in the medulla are dependent upon the classical cadherin N-cadherin (N-cad), and the layer-specific connections of R7 and R8 axons are dependent upon a temporal control mechanism that utilizes a dynamic pattern of expression of the transcription factor Sequoia (Seq) to regulate the responsivity of growth cones to N-cadherin.
Vertebrates use gradients and competition to specify position
Vertebrates use graded labels in the retina and its targets to specify synaptic partners (Figure 3). Recent results using the nasal-temporal (N-T) retinal projection to the anterior-posterior axis of the superior colliculus (SC, or its lower vertebrate equivalent, the tectum) as a model have begun to elucidate the mechanisms by which gradients control the projection map, and to identify other mechanisms that are used with gradient cues [13••]. In vertebrates, there are complementary gradients of expression of EphAs and ephrin-As along the N-T axis of the visual system, such that areas of high EphA expression project to areas with low ephrin-A expression, and areas of high ephrin-A expression project to areas of low EphA expression (Figure 3). Recent experiments have analyzed the topography of visual maps in the SC of mice lacking ephrin-As using anatomical tracing and physiology. In ephrin-A2/A3/A5 triple mutant mice, both temporal and nasal axons make mapping errors, although traces of a map remain [14]. Intrinsic optical imaging of these mice finds that the functional map is discontinuous, with patches of SC responding to visual signals from topographically incorrect locations. Thus, in these mutants, large groups of neurons project together to topographically inappropriate locations. These clusters disappear in mice that are deficient in both ephrin-As and structured neural activity in the retina, suggesting that activity-dependent cues are used to group axons [15••].
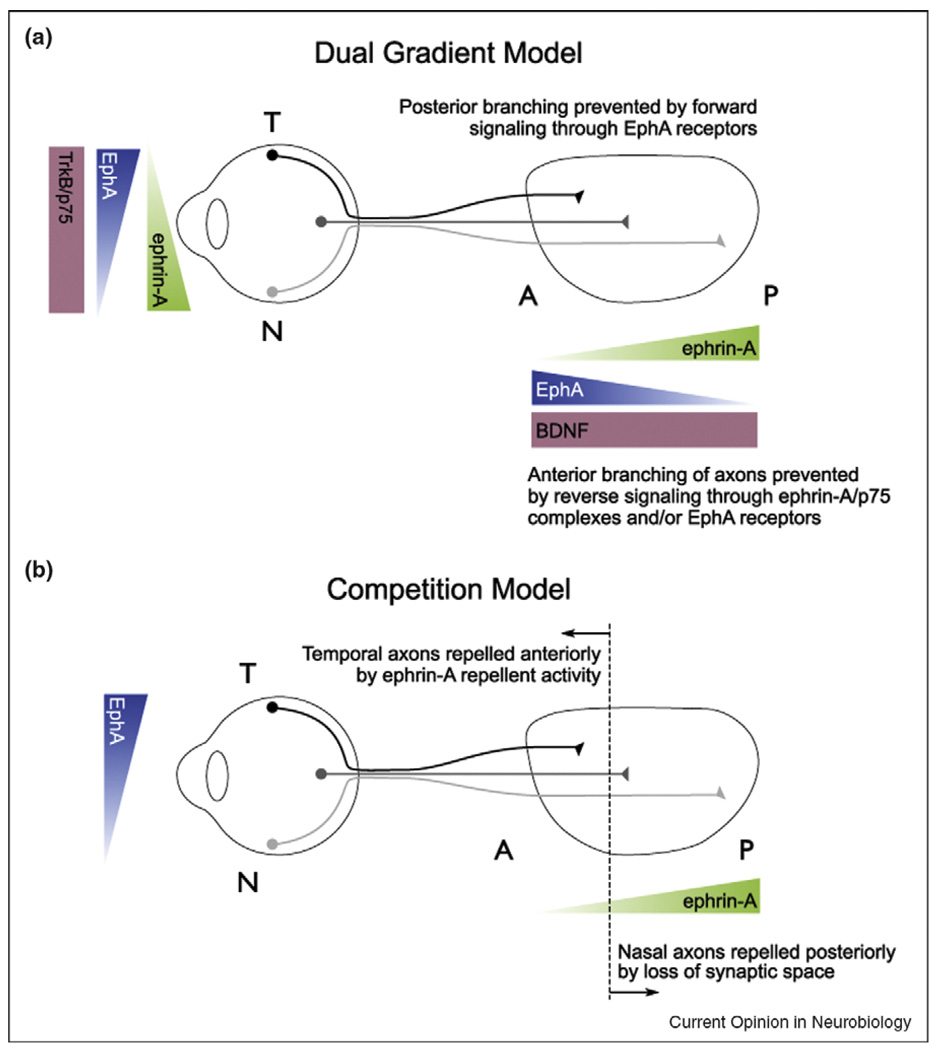
Figure 3.
Topographic map formation in vertebrates, (a) Complimentary gradients of expression of EphAs and ephrin-As along the N-T mapping axis are generated such that in the retina EphAs are expressed in a temporal (high) to nasal (low) gradient, whereas ephrin-As are expressed in the opposite, nasal (high) to temporal (low) gradient. In the SC/tectum, ephrin-As are expressed in a posterior (high) to anterior (low) gradient, whereas EphAs are expressed in an opposite anterior (high) to posterior (low) gradient. Temporal axons map to anterior SC, because they are repelled by ephrin-As. Nasal axons map to areas of the SC that contain high ephrin-A concentrations. (b and c) Different models have been developed to explain the mapping of nasal axons. (b) Bi-directional signaling models propose that ephrin-A/p75 containing nasal axons are repelled to posterior SC by EphAs expressed there, or that SC derived EphAs prevent TrkB/ephrin-A branch induction by SC-derived BDNF. (c) Competition models propose that nasal axons are competed away from anterior SC and go to posterior SC where competition is diminished. Note that these models are not mutually exclusive.
While the mapping errors seen in temporal axons in these mutants are consistent with ephrin-As acting as SC-derived repellent molecules toward EphA-bearing axons, why nasal axons make mapping errors in these mutants is unclear. Recent experiments have tested the hypothesis that SC-derived EphAs can act as repellent ligands for ephrin-As expressed on retinal ganglion cell (RGC) axons. Indeed, in vitro studies demonstrate that axons are repelled by surface-coated EphA receptors, and in vivo studies show that nasal axons in mice lacking EphA7 shift to the anterior SC [16]. Such models postulate the existence of a ‘co-receptor’ because ephrin-As are glycosylphosphatidylinosital (gpi)-linked proteins lacking intracellular signaling domains. Two groups have identified different neurotrophin receptors that may play this role. In particular, the p75 receptor physically associates with ephrin-As, RGCs derived from p75 mutant mice are not repelled by surface bound EphA7 in vitro, and retina-specific removal of p75 causes nasal axons to shift anteriorly. This leads to a model whereby ephrin-A/p75 receptors on axons are repelled by SC-derived EphAs (Figure 3). However, the nasal mapping defects in p75 mutant mice are not as dramatic as those of ephrin-A mutants, which suggest that nasal axons also use p75-independent mechanisms [17•]. Intriguingly, signaling by the TrkB receptor (which is normally activated by BDNF) is also enhanced by ephrin-A5 co-expression, and suppressed by exogenous EphA7, leading to a model whereby SC-derived EphAs suppress BDNF dependent branching anterior to the termination zone [18•] (Figure 3). These experiments are exciting because BDNF has been implicated in many activity-dependent processes, and thus may link ephrin-A signaling to the activity-dependent processes required for map formation.
It is important to note that a strict matching of dual gradients cannot explain the results demonstrating that the relative, rather than the absolute levels of EphA signaling are important for mapping [19]. One solution is that axon–axon competition for limiting positive factors in the SC sort axons based on their relative abilities to obtain this factor. Strict dual gradient models predict that nasal axons would project to their normal location in the absence of competition, while competition-based models would predict that nasal axons would shift anteriorly because of the availability of positive factors. To test this notion, Gosse et al. created zebrafish that contained only one RGC, and found that this RGC had increased branching in more anterior areas of the SC, with the most distal branch projecting to a similar position as those in WT retina [20]. This suggests that the distal tips of RGCs are directed to their final position independent of competition, perhaps using dual gradients, but that competition is also important for creating topography.
Forming columns, tiling axons and dendrites
Overview
Once incoming axons have been directed to appropriate portions of the visual field, additional mechanisms ‘discretize’ the continuous map of visual space represented by these projections into columns. These tiling mechanisms ensure that the visual field is completely and efficiently sampled by the processes of each neuron type. Therefore, the problem of restricting axons and dendrites is conceptually identical: interactions with or among processes from cells of the same type must restrict lateral growth. Recent work has shed light on the molecular mechanisms that underlie these lateral interactions.
Axon tiling in the fly visual system uses lateral interactions and autocrine signaling
Two very different mechanisms by which the processes of specific subsets of cells achieve tiling in flies have been described. The first of these studies found that the Immunoglobulin super family (IgSF) member, DSCAM2, mediates repulsive interactions between axons of a specific lamina neuron, LI [21••] (Figure 2). Each L1 axon normally innervates a single column; however, in DSCAM2 mutants, L1 axons enter multiple, neighboring columns. DSCAM2 is expressed on L1 axons, binds homophilically in vitro, and these DSCAM-DSCAM interactions are required for tiling in vivo. A second study found that mutations that disrupt TGF-β signaling cause R7 photoreceptor axons to innervate multiple columns. These axons secrete activin (a TGF β ligand) that acts in autocrine fashion, influencing transcription of genes that reduce growth cone motility. Thus, once R7 growth cones reach the appropriate target layer, their exploratory activity is reduced, restricting them to a single column [22••] (Figure 2). As DSCAM2 and activin only affect subsets of neurons in the medulla, other mechanisms of columnar restriction remain to be identified.
Both lateral interactions and cell-intrinsic mechanisms tile the vertebrate retina
Recent work toward understanding tiling mechanisms in vertebrates has focused on retinal circuits. The retina performs a wide range of visual processing tasks, such as detecting motion, discriminating color, and adapting to changes in luminance. To accomplish this, retinal cell types are morphologically and physiologically specialized (Figure 4). Cells of the same type form morphological and functional mosaics, tiling dendritic processes such that overlap between neighboring cells is uniform, and dendrites from each individual cell rarely cross. Recent work has provided evidence that mosaic formation emerges from extrinsic and intrinsic factors. One extrinsic mechanism has been identified: amacrine cell tiling is dependent upon the function of a DSCAM2 homolog [23••]. In DSCAM mutant mice, the dendritic processes of specific amacrine cell types fail to respect their boundaries, overlapping with those from other cells and those from the same cell. Other experiments suggest that intrinsic mechanisms also regulate dendrite growth. For example, mice lacking the transcription factor Brn3b have a retina that contains only 20% of the normal number of RGCs. In these mutants, while RGC cell bodies tile the retina evenly, their dendritic arbors do not completely fill the excess space [24]. Thus, the extent of arbor growth is influenced by their fate. Other retinal cell types, such as horizontal cells, have cell soma that tile the retina but dendrites that significantly overlap. Therefore, dendritic repulsion cannot account for these projection patterns. Work in fish has provided one answer to this puzzle: horizontal cells first elaborate vertical neurites that repel one another homotypically (and which tile) before dendrite elaboration, thus establishing the tiling pattern of the cell bodies [25].
Figure 4.
Tiling and column formation in the vertebrate retina. The retina develops into a laminated structure with the cell bodies, axons, and dendrites of specific cells segregated to specific layers. The IPL is further subdivided into multiple lamina labeled S1–S5 as done in [35••]. In general, the dendrites of cells of the same type do not overlap and the cell somas are evenly space, forming a mosaic that tiles the retina. (Top) In classes of ACs this is due to a repellent activity of DSCAM. (Middle) HCs have evenly spaced cell soma, although the dendrites significantly overlap. Huckfeldt et al. show that HCs make vertical, transient projections that repel each other such that they become evenly spaced. (Bottom) The chick IPL can be divided into five layers. Lock and Key models for lamination predict that each pre-synaptic and post-synaptic cell expresses complementary adhesion molecules. Homotypic adhesions are expressed in specific lamina and have been shown to be necessary and sufficient for proper lamination. These four proteins cannot explain all of lamination; other mechanisms remain to be elucidated. (ONL: Outer Nuclear Layer; OPL Outer Plexiform Layer; INL: Inner Nuclear Layer; IPL: Inner Plexiform Layer; GCL: Ganglion Cell Layer; PRs: photoreceptors; HCs: horzontal cells; BPCs: bipolar cells; ACs: amacrine cells; RGCs: retinal ganglion cells). Bottom left adapted from [35••].
Laminating the brain
Overview
In addition to their highly columnar structure, visual systems also exhibit a strikingly laminar organization of axons and dendrites (Figure 2 and Figure 4). As the choice of target lamina correlates strongly with the choice of synaptic partner, innervating the appropriate layer is an important step in circuit assembly. Three classes of models have been proposed to explain how lamina specificity is achieved. In one view, each layer is defined by a ‘lock and key’ mechanism where pre-synaptic and post-synaptic cells express cell adhesion molecules (or combinations of adhesion molecules) unique to the layer. Then, through either homophilic or heterophilic interactions, only contacts within the appropriate layer are stabilized. Alternatively, sophisticated temporal control of axonal and dendritic responsiveness, combined with the dynamic expression of relatively few cell-adhesion molecules, allows stable contacts to form only at particular developmental times, causing individual layers to assemble in sequence. Finally, layer-specific targeting could use mechanisms that rely heavily on competition between neural processes in which a relatively imprecise initial projection pattern is discretized into functional units. Recent work has provided evidence in favor of all three mechanisms.
Temporally regulated adhesive interactions program layer-specific targeting
In the fly visual system, the temporal expression pattern of the nuclear protein sequoia is crucial for the layer-specific targeting of R7 and R8 axons. Normally, R8 axons enter the target field first, and innervate a relatively shallow layer followed by R7 axons that innervate a deeper layer. Sequoia expression is first expressed in R8, and then in R7. Loss of sequoia causes R7 axons to innervate the R8 layer, while synchronizing sequoia expression in R7 and R8 causes the ‘opposite phenotype’, directing R8 to the R7 layer. Sequoia activity is dependent on the expression of the broadly expressed cell surface molecule N-cadherin [26••], which had previously been implicated in R7 layer-specific targeting [27]. These experiments argue that dynamic regulation of the R7 and R8 growth cone’s competence to respond to N-cadherin-mediated adhesion is essential for layer-specific targeting. Strikingly, dynamic regulation of N-cadherin activity is also crucial for layer-specific target choices of L neurons, suggesting that temporal control of targeting competence is of broad importance [28]. This temporal control of targeting specificity appears to be coordinated with cell fate determination in R7 targeting [29], and appears to lie upstream of mechanisms that stabilize layer-specific connections after they form [12,30].
Using combinatorial adhesion codes to pattern the vertebrate retina
In vertebrates both the retina and its targets are highly laminated. Some reports have shown a role for neural activity in refining synapses to their proper layer [31,32]; however, recent reports have suggested that synaptic lamination can occur when activity patterns are perturbed [13••,33]. Consistent with this latter notion, many RGC types show direct dendritic growth to their proper lamina, defined by pre-patterned plexuses of amacrine cells, suggesting that guidance molecules supplied by amacrine cells may direct lamination of RGCs [34]. Some of these guidance molecules are likely to be homophilic cell adhesion molecules. Indeed, recent studies in chick have suggested that a cell adhesion code using IgSF members can explain layer-specific targeting [35••]. While the identification of such a code has been hindered by the lack of cell type specific markers in the retina, a number of genetically labeled lines of mice that express GFP in specific subsets of cells have recently been described [13••,36,37]. For example, a mouse line expressing GFP in a mosaic of a class of OFF RGCs discovered that these cells project to columns within a discrete lamina in the SC, a pattern that was not known to exist [13••].
Conclusions
One could envision two broadly different mechanisms used to organize visual circuits into topographic maps. At one level, global signals, in the form of molecular or temporal gradients, could provide ‘top-down’ cues, guiding axons or dendrites to appropriate positions within the target field. At another level, strictly local interactions among and between axons and dendrites could provide ‘bottom-up’ cues that determine the relative positioning of each element in the map. Recent work has shown that both mechanisms are used to different extents in different species. Defining the mechanisms by which these systems interact, and the circumstances under which one mode of development dominates over the other is a central challenge to the field.
Acknowledgements
We would like to thank Ben Stafford for help with figures, and Andy Huberman, Jason Triplett, Alexander Sher, Jena Yamada, Flynn Hermanson for critical reading of the manuscript. D Feldheim is supported by NIH R01 EYO14689; T Clandinin is supported by NIH R01 EY015231.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Huang Z, Kunes S. Hedgehog, transmitted along retinal axons, triggers neurogenesis in the developing visual centers of the Drosophila brain. Cell. 1996;86:411–422. doi: 10.1016/s0092-8674(00)80114-2. [DOI] [PubMed] [Google Scholar]
- 2.Huang Z, Shilo BZ, Kunes S. A retinal axon fascicle uses spitz, an EGF receptor ligand, to construct a synaptic cartridge in the brain of Drosophila. Cell. 1998;95:693–703. doi: 10.1016/s0092-8674(00)81639-6. [DOI] [PubMed] [Google Scholar]
- 3.Umetsu D, Murakami S, Sato M, Tabata T. The highly ordered assembly of retinal axons and their synaptic partners is regulated by Hedgehog/Single-minded in the Drosophila visual system. Development. 2006;133:791–800. doi: 10.1242/dev.02253. [DOI] [PubMed] [Google Scholar]
- 4.Sato M, Umetsu D, Murakami S, Yasugi T, Tabata T. DWnt4 regulates the dorsoventral specificity of retinal projections in the Drosophila melanogaster visual system. Nat Neurosci. 2006;9:67–75. doi: 10.1038/nn1604. [DOI] [PubMed] [Google Scholar]
- 5.Tomasi T, Hakeda-Suzuki S, Ohler S, Schleiffer A, Suzuki T. The transmembrane protein Golden goal regulates R8 photoreceptor axon-axon and axon-target interactions. Neuron. 2008;57:691–704. doi: 10.1016/j.neuron.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Kirschfeld K. The projection of the optical environment on the screen of the rhabdomere in the compound eye of the Musca. Exp Brain Res. 1967;3:248–270. doi: 10.1007/BF00235588. [DOI] [PubMed] [Google Scholar]
- 7.Braitenberg V. Patterns of projection in the visual system of the fly. 1. Retina-lamina projections. Exp Brain Res. 1967;3:271–298. doi: 10.1007/BF00235589. [DOI] [PubMed] [Google Scholar]
- 8.Clandinin TR, Zipursky SL. Afferent growth cone interactions control synaptic specificity in the Drosophila visual system. Neuron. 2000;28:427–436. doi: 10.1016/s0896-6273(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 9.Hiesinger PR, Zhai RG, Zhou Y, Koh TW, Mehta SQ, Schulze KL, Cao Y, Verstreken P, Clandinin TR, Fischbach KF, et al. Activity-independent prespecification of synaptic partners in the visual map of Drosophila. Curr Biol. 2006;16:1835–1843. doi: 10.1016/j.cub.2006.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee RC, Clandinin TR, Lee CH, Chen PL, Meinertzhagen IA, Zipursky SL. The protocadherin Flamingo is required for axon target selection in the Drosophila visual system. Nat Neurosci. 2003;6:557–563. doi: 10.1038/nn1063. [DOI] [PubMed] [Google Scholar]
- 11. Chen PL, Clandinin TR. The cadherin Flamingo mediates level-dependent interactions that guide photoreceptor target choice in Drosophila. Neuron. 2008;58:26–33. doi: 10.1016/j.neuron.2008.01.007. The non-classical cadherin Flamingo is required for R1-R6 axons to choose appropriate target columns within the developing Drosophila lamina. Using a series of somatic mosaic studies, this work demonstrates that Flamingo mediates cell-cell interactions between growth cones within the same ommatidial bundle, and that relative differences in Flamingo levels between neighboring growth cones determine subsequent outgrowth trajectory.
- 12.Bazigou E, Apitz H, Johansson J, Loren CE, Hirst EM, Chen PL, Palmer RH, Salecker I. Anterograde Jelly belly and Alk receptor tyrosine kinase signaling mediates retinal axon targeting in Drosophila. Cell. 2007;128:961–975. doi: 10.1016/j.cell.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 13. Huberman AD, Manu M, Koch SM, Susman MW, Lutz AB, Ullian EM, Baccus SA, Barres BA. Architecture and activity-mediated refinement of axonal projections from a mosaic of genetically identified retinal ganglion cells. Neuron. 2008;59:425–438. doi: 10.1016/j.neuron.2008.07.018. A transgenic line of mice that expresses GFP in a complete mosaic of transient OFF-alpha RGCs is described. This class of RGCs projects exclusively to the SC and LGN and are restricted to a specific lamina in each of these targets. In addition, the axons are also organized into columns in the SC. Column structure, but not lamination, become blurred when spontaneous activity patterns are disrupted suggesting that lamina choice is independent of activity for this cell type. This is the first example of a study examining the development of a genetically identified visual projection in mammals.
- 14.Pfeiffenberger C, Yamada J, Feldheim DA. Ephrin-As and patterned retinal activity act together in the development of topographic maps in the primary visual system. J Neurosci. 2006;26:12873–12884. doi: 10.1523/JNEUROSCI.3595-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cang J, Wang L, Stryker MP, Feldheim DA. Roles of ephrin-as and structured activity in the development of functional maps in the superior colliculus. J Neurosci. 2008;28:11015–11023. doi: 10.1523/JNEUROSCI.2478-08.2008. A method of Fourier imaging of intrinsic signals was used to visualize the functional maps in the mouse SC in wild type, ephrin-Atko mice, and mice missing ephrin-As plus the β2 subunit of the nACh receptor (the mice have altered patterns of spontaneous retinal activity). In the absence of ephrin-As, functional maps are disrupted selectively along the N-T mapping axis, and are discontinuous, with patches of SC responding to topographically incorrect locations. Removal of ephrin-As and β2 results in a near absence of map, selectively along the N-T axis. These results are consistent with a computational model that includes graded guidance cues, axon–axon competition and activity-dependent mechanisms in mapping.
- 16.Rashid T, Upton AL, Blentic A, Ciossek T, Knoll B, Thompson ID, Drescher U. Opposing gradients of Ephrin-As and EphA7 in the superior colliculus are essential for topographic mapping in the mammalian visual system. Neuron. 2005;47:57–69. doi: 10.1016/j.neuron.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 17. Lim YS, McLaughlin T, Sung TC, Santiago A, Lee KF, O’Leary DD. p75(NTR) mediates ephrin-A reverse signaling required for axon repulsion and mapping. Neuron. 2008;59:746–758. doi: 10.1016/j.neuron.2008.07.032. The low affinity neurotrophin receptor, p75, was identified as a co-receptor required for ephrin-A reverse signaling by retinal ganglion cells. Retinal axons require p75 for EphA7 repulsion in vitro and nasal axons make topographic mapping errors in p75 mutant mice.
- 18. Marler KJ, Becker-Barroso E, Martinez A, Llovera M, Wentzel C, Poopalasundaram S, Hindges R, Soriano E, Comella J, Drescher U. A TrkB/EphrinA interaction controls retinal axon branching and synaptogenesis. J Neurosci. 2008;28:12700–12712. doi: 10.1523/JNEUROSCI.1915-08.2008. A novel cis interaction between ephrin-A5 and TrkB on RGC axons was discovered. Data presented suggest that this interaction contributes to the control of axon branching of RGC axons and is also involved in branching and synaptogenesis of hippocampal neurons.
- 19.Brown A, Yates PA, Burrola P, Ortuno D, Vaidya A, Jessell TM, Pfaff SL, O’Leary DDM, Lemke G. Topographic mapping from the retina to the midbrain is controlled by relative but not absolute levels of EphA receptor signaling. Cell. 2000;102:77–88. doi: 10.1016/s0092-8674(00)00012-x. [DOI] [PubMed] [Google Scholar]
- 20.Gosse NJ, Nevin LM, Baier H. Retinotopic order in the absence of axon competition. Nature. 2008;452:892–895. doi: 10.1038/nature06816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Millard SS, Flanagan JJ, Pappu KS, Wu W, Zipursky SL. Dscam2 mediates axonal tiling in the Drosophila visual system. Nature. 2007;447:720–724. doi: 10.1038/nature05855. A member of the Ig Superfamily, DSCAM2, was knocked out and shown to play a role in restricting the axons of a specific subtype of lamina neuron to single columns in the medulla. Biochemical analysis demonstrated that this protein mediates homophillic interactions, and single-cell somatic mosaic analysis determined that this protein has both cell-autonomous and non-autonomous effects on neighboring terminals. This paper proposed that homophillic, repulsive interactions between neighboring growth cones underlies the phenomenon of axon tiling in this context.
- 22. Ting CY, Herman T, Yonekura S, Gao S, Wang J, Serpe M, O’Connor MB, Zipursky SL, Lee CH. Tiling of r7 axons in the Drosophila visual system is mediated both by transduction of an activin signal to the nucleus and by mutual repulsion. Neuron. 2007;56:793–806. doi: 10.1016/j.neuron.2007.09.033. Activin-mediated signals are required to restrict the terminals of R7 photoreceptors to single columns in the medulla. Genetic studies of multiple signaling components reveal that this pathway functions in autocrine fashion, requires transmitting a signal from the growth cone to the nucleus, and acts by reducing growth cone motility. This work thus demonstrates the importance of both cell-intrinsic and extrinsic cues in achieving axon tiling.
- 23. Fuerst PG, Koizumi A, Masland RH, Burgess RW. Neurite arborization and mosaic spacing in the mouse retina require DSCAM. Nature. 2008;451:470–474. doi: 10.1038/nature06514. A DSCAM mutant mouse was identified and shown to have tiling defects consistent with the idea that DSCAM is a homophillic repellent molecule. DSCAM is normally expressed in subsets of neurons in the retina including two types of amacrine cells. In DSCAM mutants these cells have altered spacing and dendritic morphologies. This is the first example of a genetic perturbation that affects tiling in the vertebrate visual system.
- 24.Lin B, Wang SW, Masland RH. Retinal ganglion cell type, size, and spacing can be specified independent of homotypic dendritic contacts. Neuron. 2004;43:475–485. doi: 10.1016/j.neuron.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Huckfeldt RM, Schubert T, Morgan JL, Godinho L, Di Cristo G, Huang ZJ, Wong RO. Transient neurites of retinal horizontal cells exhibit columnar tiling via homotypic interactions. Nat Neurosci. 2009;12:35–43. doi: 10.1038/nn.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Petrovic M, Hummel T. Temporal identity in axonal target layer recognition. Nature. 2008;456:800–803. doi: 10.1038/nature07407. Mutations in the transcription factor sequoia disrupt the layer-specific targeting decisions made by R7 and R8 photoreceptor axons. Sequoia is expressed in a temporally dynamic pattern as these cells differentiate, being expressed first in R8, and then in R7. Altering this temporal sequence of expression, causing R7 and R8 axons to express sequoia simultaneously directs R7 and R8 axons to the same layer, an effect that is dependent on the classical cadherin, N-cadherin. This work suggests that dynamic regulation of competence to respond to a relatively generic adhesive factor can drive lamination.
- 27.Lee CH, Herman T, Clandinin TR, Lee R, Zipursky SL. N-cadherin regulates target specificity in the Drosophila visual system. Neuron. 2001;30:437–450. doi: 10.1016/s0896-6273(01)00291-4. [DOI] [PubMed] [Google Scholar]
- 28.Nern A, Zhu Y, Zipursky SL. Local N-cadherin interactions mediate distinct steps in the targeting of lamina neurons. Neuron. 2008;58:34–41. doi: 10.1016/j.neuron.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morey M, Yee SK, Herman T, Nern A, Blanco E, Zipursky SL. Coordinate control of synaptic-layer specificity and rhodopsins in photoreceptor neurons. Nature. 2008;456:795–799. doi: 10.1038/nature07419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shinza-Kameda M, Takasu E, Sakurai K, Hayashi S, Nose A. Regulation of layer-specific targeting by reciprocal expression of a cell adhesion molecule, capricious. Neuron. 2006;49:205–213. doi: 10.1016/j.neuron.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 31.Liu X, Grishanin RN, Tolwani RJ, Renteria RC, Xu B, Reichardt LF, Copenhagen DR. Brain-derived neurotrophic factor and TrkB modulate visual experience-dependent refinement of neuronal pathways in retina. J Neurosci. 2007;27:7256–7267. doi: 10.1523/JNEUROSCI.0779-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tian N, Copenhagen DR. Visual stimulation is required for refinement of ON and OFF pathways in postnatal retina. Neuron. 2003;39:85–96. doi: 10.1016/s0896-6273(03)00389-1. [DOI] [PubMed] [Google Scholar]
- 33.Nevin LM, Taylor MR, Baier H. Hardwiring of fine synaptic layers in the zebrafish visual pathway. Neural Dev. 2008;3:36. doi: 10.1186/1749-8104-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mumm JS, Williams PR, Godinho L, Koerber A, Pittman AJ, Roeser T, Chien CB, Baier H, Wong RO. In vivo imaging reveals dendritic targeting of laminated afferents by zebrafish retinal ganglion cells. Neuron. 2006;52:609–621. doi: 10.1016/j.neuron.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yamagata M, Sanes JR. Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature. 2008;451:465–469. doi: 10.1038/nature06469. Four closely related IGG super family members are expressed in overlapping and distinct subsets of interneurons and RGCs in the retina. Loss and gain of function perturbations resulted in predictable changes in retinal lamination that are consistent with these molecules acting as homophilic adhesion molecules. This work suggests that there is a combinatorial cell-adhesion code that is used to direct pre- and postsynaptic partners to their proper lamina
- 36.Yonehara K, Shintani T, Suzuki R, Sakuta H, Takeuchi Y, Nakamura-Yonehara K, Noda M. Expression of SPIG1 reveals development of a retinal ganglion cell subtype projecting to the medial terminal nucleus in the mouse. PLoS ONE. 2008;3:e1533. doi: 10.1371/journal.pone.0001533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim IJ, Zhang Y, Yamagata M, Meister M, Sanes JR. Molecular identification of a retinal cell type that responds to upward motion. Nature. 2008;452:478–482. doi: 10.1038/nature06739. [DOI] [PubMed] [Google Scholar]