Abstract
TMS is increasingly used in Cognitive Neuroscience to study functional contributions of a stimulated brain region to cognitive and perceptual processing. TMS-related behavioural effects are often interpreted as reflecting selective disruption of processing primarily within the stimulated region itself. This approach is now being extended by studies that combine TMS with concurrent neuroimaging measures, such as fMRI. We discuss some recent combined TMS-fMRI studies and their implications for TMS investigations of cognition and perception. An emerging theme is that TMS does not affect only the stimulated region, but can also influence remote brain areas interconnected with the stimulation site. Such ‘network’ effects of TMS can be anatomically specific, but also context-dependent, changing with the current functional state of the targeted network rather than simply reflecting just fixed, context-invariant anatomical connectivity. Perceptual and behavioural effects of TMS may correspondingly involve TMS influences on remote interconnected brain regions, not solely on the stimulated region itself. Thus, TMS can now be used to study the consequences of functional interactions between the stimulated region and other parts of the network. This may lead beyond strictly modular views of brain function, that emphasize functional properties of single brain areas, towards new perspectives on how functional interactions between remote but interconnected brain regions may support perception and cognition.
Keywords: top-down, concurrent TMS-fMRI, connectivity, motor systems, visual cortex
Introduction
Over the last two decades, TMS has become an established technique in cognitive neuroscience, and is now routinely used to study the possible involvement of stimulated brain regions in cognitive and perceptual functions (for reviews see e.g., Chambers and Mattingley 2005; Pascual-Leone et al., 1999; Walsh and Cowey 2000). The growing popularity of TMS may relate to it filling a gap left open by methodical limitations of other techniques for studying the human brain non-invasively. Functional neuroimaging methods such as fMRI or EEG are useful for detecting changes in brain activity correlated with performance of a cognitive task, but can leave unclear whether such activations are necessary - in a causal sense - for specific cognitive functions. On the other hand, while studies of brain-lesioned patients can provide evidence for causal involvement of damaged brain areas in impaired cognitive functions, damage to particular areas of interest can be rare or variable, and long-term neural reorganisation may arise. TMS can elegantly circumvent such problems, as it can be applied to any healthy volunteer to directly manipulate neural activity with respectable spatial resolution and excellent temporal specificity (see e.g., Wagner et al., 2007). This has made TMS a tool of choice for testing the cognitive necessity of brain areas implicated indirectly by neuroimaging studies; or to examine and refine the temporal characteristics of functional contributions from cortical areas implicated by lesion studies.
However, current use of TMS also has limitations that may render interpretation of behavioural TMS effects more complicated. For instance, while it is often argued that TMS induces some form of ‘neuronal noise’ (or task-irrelevant signal) that interferes with task-relevant neural activity (e.g., Walsh and Cowey, 2000), the exact nature of TMS impacts on neural processing is still intensely debated (e.g., Harris, Clifford, and Miniussi 2008; Siebner et al., in this issue). Behavioural TMS results are often interpreted as if TMS selectively affects processing in just (or primarily) the stimulated region, creating a form of reversible ‘virtual lesion’ (Pascual-Leone et al., 1999). However, TMS may also affect processing in remote brain regions, either directly via neural interconnections, or indirectly due to compensatory mechanisms (e.g., Lomber, 1999). Moreover, such inter-regional considerations are necessitated by results of double-coil TMS studies, showing that behavioural effects of TMS applied to one cortical area (e.g., hand twitches for M1 TMS, or phosphenes for TMS to visual cortex) can be modulated by preceding ‘conditioning’ TMS pulses to a second, potentially interconnected region (e.g., Koch et al., 2008; O’Shea et al., 2007; Silvanto et al., 2006). To date, such double-coil protocols have been restricted to pairs of cortical sites where one member produces measurable behavioural output (as for M1-induced twitches, or visual areas generating phosphenes).
Such considerations have triggered interest in recent methodological advances that make it possible to combine TMS with recording of neural activity changes throughout the brain, as for PET (Fox et al., 1997; Paus et al., 1997; Siebner et al., 2001), EEG (Ilmoniemi et al., 1997), or fMRI (Bohning et al., 1998). These combinations, sometimes described as “perturb- and-measure” approaches (Paus, 2005), allow for direct assessment of how TMS affects neural processing both locally and in remote interconnected brain regions. Such combined approaches may provide a window on functional interactions between different brain areas in extended networks, and their relation to cognition and perception. Here we briefly discuss a few recent illustrative studies that combined TMS with fMRI, in order to study inter-regional interactions in the human brain and the possible functional consequences. We mainly focus on studies using concurrently combined TMS and fMRI here, but we note that ‘off-line’ TMS imaging approaches can be used to study more medium-term changes in the brain (over several minutes; see e.g., O’Shea et al., 2007). The ‘online’ combination described here seems potentially well-suited for relating behavioural and neural effects of TMS on a trial-by-trial basis, and to study immediate effects of TMS on cortical networks with the anatomical specificity afforded by fMRI.
Combining fMRI and TMS can characterise functional interplay between cortical regions
The technical feasibility of combining TMS and fMRI on-line was first demonstrated by Bohning and colleagues (Roberts et al., 1997, Bohning et al., 1998). Several studies applied this new combination to investigate brain responses to TMS over M1 (e.g., Bohning et al., 1998; Baudewig et al., 2001; Bestmann et al., 2004). The focus on M1 may reflect the ease with which M1 can be localised, and the substantial complementary literature on electrophysiological effects of M1-TMS (e.g., Siebener et al., this issue). These motor-system TMS-fMRI studies already found that TMS can affect BOLD signal not only at the stimulation site but also in remote brain structures interconnected with M1, such as dorsal premotor cortex (PMd), supplementary motor area, and subcortical structures (e.g. Bestmann et al., 2004; Denslow et al., 2005). Similar results arose when combining TMS with PET, with rTMS pulse-trains to M1 (Fox et al., 1997) or premotor cortex (Paus et al., 1997) eliciting rCBF changes in some distant brain areas. These convergent findings led to proposals that combining TMS with fMRI or PET may be a valuable tool to directly probe cortical connectivity.
But these initial studies also highlighted some of the difficulties in disentangling ‘direct’ TMS effects on neural activity, both locally and in remote cortical areas, from those due to peripheral effects of TMS. High-intensity M1-TMS can produce hand twitches, so that re-afferent feedback from peripheral muscles (rather than neural stimulation of M1 by TMS per se) may cause some of the BOLD signal changes. However, remote activity changes can also be observed in the absence of hand twitches, for instance when M1-TMS is given at intensities below motor threshold (Bestmann et al., 2004), or for TMS to pre-motor structures (Paus et al., 1997, Bestmann et al., 2005). A more general issue is that the sound and tactile scalp sensation produced by TMS pulses may trivially account for some remote activity changes. Several of the studies mentioned above found BOLD changes in auditory/somatosensory areas (see Bohning et al., 1998; Baudewig et al., 2001; Bestmann et al., 2004; Denslow et al., 2005). These findings already indicated that - as for purely behavioural TMS studies -appropriate control conditions (e.g. control stimulation sites, monitoring of peripheral effects) may be essential for inferring inter-regional interactions with certainty from TMS-fMRI.
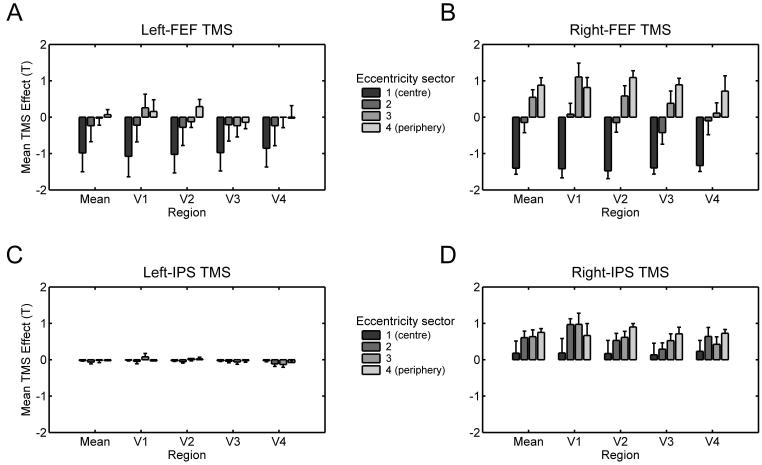
More recently, several concurrent TMS-fMRI studies have moved beyond the motor system, using this methodical combination to test hypotheses about functional interactions between fronto-parietal and visual cortical areas. It has often been proposed in the literature on attention and eye-movement control that processing in retinotopic visual cortex may be influenced in a top-down manner, by a network of frontal and parietal areas (e.g., Kastner and Ungerleider, 2000; Serences and Yantis 2006). But such causal influences have rarely been shown directly in the human brain, and it remains unclear to what degree such influences might differ for different regions, in the left or right cortical hemisphere. To address this, Ruff et al. (2006, 2008, in press) used concurrent TMS-fMRI to study how stimulation of FEF or intra-parietal sulcus (IPS) affects BOLD signal in retinotopic visual areas V1-V4 and in V5/MT+. These studies thus used TMS-fMRI to characterise and compare functional influences from the stimulated regions upon remote but interconnected visual cortex. The same TMS protocol (short bursts of parametrically varied intensity) was applied over FEF or IPS of the same participants, for either the right or left cortical hemisphere. This elicited retinotopically specific patterns of BOLD signal changes in visual cortex, which differed significantly for stimulation of the different sites (see Figure 1).
Figure 1. TMS to different regions in left and right fronto-parietal cortex causes distinct spatiotopic patterns of activity changes in remote retinotopic visual cortex.
A-D show the spatial patterns of TMS effects on BOLD signal (correlations of BOLD with TMS intensity, quantified as T-value) in visual areas V1-V4, and averaged across these areas (‘Mean’). Dark-to-light grey bars represent the cortical representation of different eccentricities, ranging from the central visual field (on the left of each region-specific plot) to increasingly more eccentric representations (when moving towards the right in each plot). Whiskers represent s.e.m. A-B In all visual areas, TMS to both left and right FEF led to similar BOLD signal decreases for the central visual field, but BOLD signal increases in peripheral visual field representations were found only for right-FEF TMS. This pattern differed significantly from the effects of IPS TMS (C-D). When no visual stimuli were present (see main text), right-IPS TMS elicited BOLD signal increases throughout all visual field representations in V1-V4 (D), whereas left-IPS TMS did not affect BOLD signal in any visual area (C). Adapted in parts from Ruff et al. (in press).
The results by Ruff et al. (2006, 2008) establish two notable points about the nature of remote activity changes due to TMS. First, applying TMS to one region can affect activity in remote cortical areas with high spatial specificity, supporting the notion that TMS may influence interconnected brain regions not just diffusely, but via topographically organised anatomical tracts (see also Taylor et al., 2007a and Silvanto et al., 2006 for double-coil and EEG evidence on the timing of FEF-visual interactions; or Strafella et al., 2001 for PET evidence for specific neuropharmacological effects of TMS at a remote site). Right-FEF TMS had opposite effects on representations of the central visual field (BOLD decreases) vs peripheral field (BOLD increases; see Figure 1B). This appears broadly consistent with anatomical findings of separate tracts linking the FEF with visual areas involved in central vs more peripheral vision (Schall et al., 1995). Second, Ruff et al. (2008) confirm that remote BOLD effects can be very different for different sites considered to be within the same overall network. The effects of right-FEF TMS described above contrasted with those of right-IPS TMS, which led to a significantly different pattern of activity increases in V1-V4 (see Figure 1D, and the next section for a more detailed description). Such findings indicate that concurrent TMS-fMRI can identify distinct functional contributions of different cortical regions, by virtue of their distinct influence on distant components within a network of interconnected regions.
Cortical interactions probed by TMS-fMRI depend on the functional state of connections
Many lines of evidence now suggest that the impact of TMS is not context-invariant, but can depend strongly on current neuronal excitability. Electrophysiological studies have shown that tasks that pre-activate the motor system (e.g., tonic contraction) can enhance descending motor volleys elicited by M1-TMS (see Siebner et al., this issue). Perhaps analogously, contextual factors that modulate neural excitability in visual cortex, such as spatial attention, can alter the intensities required for occipital TMS to elicit phosphene sensations (Bestmann et al., 2007). Such findings of the context-dependence of putatively local TMS effects (see also Silvanto et al., in press) raise the question of whether inter-regional interactions, and thus remote effects, may also be context-dependent.
Such changes in ‘effective connectivity’ as a function of cognitive or sensory state have recently been shown by TMS-EEG studies (Massimini et al., 2005; Taylor et al., 2007b), as well as by double-coil TMS of the motor system (Koch et al., 2008; O’Shea et al., 2007). Concurrent TMS-fMRI now makes it possible to investigate context-dependence of functional interactions within extended networks of remote but interconnected brain areas, while varying stimulation or task-state. Such approaches can provide well-matched internal controls for possible non-specific effects of TMS (constant between different task conditions), and permit assays of how different cognitive, perceptual, or motor states may relate to changing interactions between different nodes in a network of interconnected brain regions.
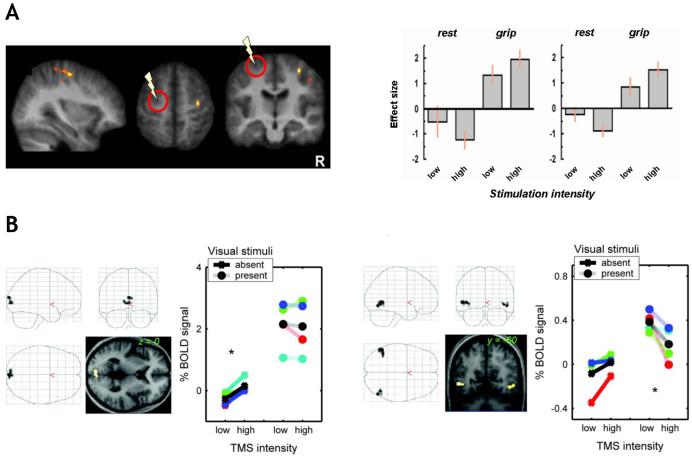
Bestmann et al., (2008) used such an approach to assess how functional interactions of PMd with interconnected brain structures may change with different activation states of the motor system. Brief bursts of rTMS were applied to left PMd during fMRI, while participants either rested (passive condition) or engaged in a simple motor task (active motor task; brief isometric contractions of the left hand). High- vs low-intensity PMd TMS specifically affected BOLD signal in PMd and M1 of the opposite (right) hemisphere, providing direct evidence for inter-hemispheric interactions in a circumscribed cortical motor network. Crucially, these remote TMS effects differed significantly between the active and the passive task conditions (even though rTMS did not affect measurable grip parameter, that might otherwise have introduced a behavioural confound). Increased intensity of TMS over left PMd led to BOLD signal increases in right PMd and M1 specifically during left-hand grip trials, but elicited BOLD signal decreases instead in these regions during the passive rest condition (see Figure 2A). These results support the view that engagement in active motor behaviour modifies inter-hemispheric interactions between (pre-)motor structures in opposite hemispheres, so that neural activity elicited in one of these structures (via TMS) is transmitted to other interconnected regions of the network currently engaged in implementing the motor behaviour, in a different way when acting than when in a passive state.
Figure 2. Effects of TMS on remote but interconnected regions can depend on task- or stimulus-context.
A (left graph) Standard brain renderings of regions in right PMd and M1 where BOLD signal changes due to TMS over PMd in the opposite (left) hemisphere depended on task context. BOLD signal estimates extracted from both these regions (A, right graph) show that increased intensity of TMS to left PMd (area indicated by the circle and flash symbol) elicited BOLD increases in right PMd/M1 specifically during the active motor task (‘grip’), while producing BOLD decreases in these regions during passive rest instead. Adapted from Bestmann et al. (2008). B Brain regions in visual cortex where BOLD signal changes due to right-IPS TMS depended on visual stimulus context. The brain renderings and signal plots on the left show a region in medial occipital cortex (confirmed in retinotopic analyses to correspond to areas V1-V4) where right-IPS TMS elicited BOLD increases only when no visual stimulus was presented concurrently. The right plots shows a different pattern of context-dependence, as right-IPS TMS elicited BOLD signal decreases in a region confirmed to correspond to V5/MT+ only when moving visual stimuli were presented to drive this visual region. Adapted from Ruff et al. (2008).
For the visual system, the studies by Ruff et al. (2006, 2008, in press) also assessed possible context-dependence, now for fronto-parietal TMS influences on retinotopic visual cortex. Rather than using a task manipulation to manipulate the endogenous state of the stimulated regions (FEF or IPS), instead the ‘baseline’ activation level in occipital visual areas was varied by means of visual stimuli (present or absent during TMS). This manipulation of stimulus-context allowed for a direct test of whether influences of FEF or IPS on processing in visual cortex may apply in an additive fashion with respect to current bottom-up input, or may instead interact (i.e., have a different impact) with different visual contexts. Clear differences were found in this respect between effects of frontal versus parietal stimulation. FEF TMS effects on occipital BOLD signals were unaffected by visual context. By contrast, right IPS-TMS led to BOLD signal increases in V1-V4 only when no visual stimuli were presented; but to BOLD signal decreases for area V5/MT+ only when moving visual stimuli were present to drive this visual region (see Figure 2B). These results show that functional interactions of parietal and visual cortex may change with differences in ‘bottom-up’ driving input to visual cortex, whereas circuits involving frontal regions may exert influences on visual cortex in a more ‘top-down’ manner regardless of current visual input.
Taken together, all these results demonstrate that context can changes functional interactions between brain areas, as probed by concurrent TMS-fMRI. Such contextual-modulations of remote TMS effects can be expressed specifically in structures involved in implementing the current task (as for PMd-M1 interactions; Bestmann et al., 2008; see also Sack et al. 2007, discussed in the next section); and for sensory areas can vary with stimulus context in a site-specific manner, i.e., affecting V1-V4 in a very different fashion than V5/MT+, and only for parietal but not frontal TMS (Ruff et al., 2008). This underlines that remote effects elicited by TMS do not simply reflect fixed anatomical connectivity per se, but rather functional interactions within cortical networks that can vary with context. This raises the question of how such changes in functional coupling may contribute to perception, cognition, and behaviour.
Remote effects due to TMS can have functional significance for perception and cognition
The relation between neural and behavioural changes due to TMS was recently examined by Sack et al. (2007) using concurrent TMS-fMRI. TMS was applied over left or right parietal cortex, during a spatial task (angle judgements) or a non-spatial control task (colour judgement). Right but not left parietal TMS affected reaction times and neural processing specifically during the spatial task. TMS-related BOLD signal decreases were found not only in the (stimulated) right parietal cortex, but also in a portion of the right medial frontal gyrus thought to be interconnected with the stimulation site, more so for the spatial than non-spatial task. This finding of a task-specific (and hence state-dependent) effect of parietal TMS on remote frontal cortex appears in broad agreement with the points made in the previous section. Since the critical TMS in Sack et al (2007) disrupted behaviour for the spatial task, one might argue that the remote effects on frontal cortex could either reflect propagation of TMS-induced activity, or rapid adaptation to the disrupting effects of TMS on performance (see also O’Shea et al, 2007). In fact TMS-related BOLD signal changes in both right parietal and frontal cortex correlated with the behavioural effects, confirming a brain-behaviour relation. Sack et al. (2007) concluded that behavioural effects of right-hemisphere parietal TMS on spatial performance may relate not only to local changes in the stimulated region, but rather to changes across a network of interconnected regions specifically involved in the task.
The possible functional significance of TMS effects on neural areas remote from the stimulation site was also considered by Ruff et al. (2006, and in press). Motivated by proposals of a right-hemisphere dominance for the top-down control of visual attention and perception (e.g. Karnath et al., 2002), Ruff et al (in press) directly compared the impact of right- versus left-hemisphere TMS to FEF or IPS on BOLD signal in remote but interconnected visual cortex. Right-hemisphere TMS had much stronger effects on neural processing in visual cortex, with this lateralisation being most evident for the parietal site (compare Figure 1A-B, and Figure 1C-D). These data demonstrate directly that right-hemisphere fronto-parietal structures can exert remote influences upon visual cortex that left-hemisphere analogues appear unable to exert. This could offer a new type of explanation (i.e., in terms of remote physiological effects upon visual cortex) for why TMS or lesions to right-hemisphere fronto-parietal cortex often affect performance for visual tasks in a more pronounced fashion than corresponding left-hemisphere disruptions.
Ruff et al. (2006) tested whether the spatial pattern of BOLD effects in retinotopic visual areas V1-V4 due to right-FEF TMS may affect perception in a corresponding way. Based on their concurrent TMS-fMRI findings (that right FEF-TMS leads to BOLD increases for peripheral visual field representations, but BOLD decreases for the central visual field), it was predicted that right-FEF TMS should enhance perceptual salience of peripheral visual stimuli, relative to central, in both visual hemifields. This prediction was confirmed in a psychophysical experiment, where perceived contrast was enhanced for peripheral relative to central Gabor gratings during right-FEF TMS (versus ineffective vertex TMS). These results indicate that remote physiological effects of TMS can be used to generate new predictions for behavioural TMS effects. This may call into question the notion that TMS effects on perception and cognition solely reflect local cortical effects underneath the TMS coil.
Conclusions and outlook
Recent combinations of TMS with neuroimaging have shown that TMS does not simply influence the stimulated brain region alone, but can also affect remote brain areas interconnected with the stimulation site (as also implied by some dual-coil TMS studies). Such remote effects of TMS can be highly spatially specific, but do not appear to reflect fixed spread of neural activity along anatomical tracts. Instead, they can systematically depend on context and thus the current functional state within the network of interconnected regions. There is growing evidence that behavioural effects of TMS may involve such remote effects on neural processing in interconnected regions, rather than merely the local impact of TMS on just the stimulated region.
Does this mean that all previous demonstrations of behavioural TMS effects may require re-interpretation? Any behavioural effect due to TMS still clearly indicates some causal involvement of the targeted brain area in performance of the current task. But behavioural data alone may not reveal whether this involvement reflects task-related processes instantiated only in the stimulated region, or influences of this region upon remote but interconnected areas that are also critically involved. Addressing this issue will require some concurrent measure of neural activity, and we hope to have illustrated that combining TMS with neuroimaging may help to refine how interactions between components of extended networks may support cognition, perception and behaviour.
The distinction between local and remote effects of TMS may also provide a fresh perspective on the long-standing contrast between functional specialisation vs functional integration (see e.g., Friston 2002). That debate has concerned whether the brain should best be thought of as a set of specialised cortical processing modules that each make unique contributions to cognition, or as comprising distributed networks that act in an integrated manner to support cognition, perception and behaviour. TMS has traditionally been used to provide evidence for functional specialisation. We suggest that combining TMS with neuroimaging methods may extend that perspective, potentially disambiguating whether causal contributions of a specific brain area to task performance may reflect mostly local ‘modular’ processes, or rather functional interactions with interconnected cortical regions.
Abbreviations
- TMS
Transcranial magnetic stimulation
- fMRI
Functional magnetic resonance imaging
- PET
Positron emission tomography
- EEG
Electro-encephalography
- NIRS
Near-infrared spectropscopy
- FEF
Frontal eye-fields
- IPS
Intra-parietal sulcus
References
- Armstrong KM, Moore T. Rapid enhancement of visual cortical response discriminability by microstimulation of the frontal eye field. Proceedings of the National Academy of Sciences U.S.A. 2007;104:9499–9504. doi: 10.1073/pnas.0701104104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudewig J, Siebner HR, Bestmann S, Tergau F, Tings T, Paulus W, Frahm J. Functional MRI of cortical activations induced by transcranial magnetic stimulation (TMS) Neuroreport. 2001;12:3543–3548. doi: 10.1097/00001756-200111160-00034. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J. BOLD MRI responses to repetitive TMS over human dorsal premotor cortex. Neuroimage. 2005;28:22–29. doi: 10.1016/j.neuroimage.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J. Functional MRI of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits. European Journal of Neuroscience. 2004;19:1950–1962. doi: 10.1111/j.1460-9568.2004.03277.x. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Ruff CC, Blakemore C, Driver J, Thilo KV. Spatial attention changes excitability of human visual cortex to direct stimulation. Current Biology. 2007;17:134–139. doi: 10.1016/j.cub.2006.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann S, Swayne O, Blankenburg F, Ruff CC, Haggard P, Weiskopf N, Josephs O, Driver J, Rothwell JC, Ward NS. Dorsal premotor cortex exerts state-dependent causal influences on activity in contralateral primary motor and dorsal premotor cortex. Cerebral Cortex. 2008;18:1281–1291. doi: 10.1093/cercor/bhm159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohning DE, Shastri A, Nahas Z, Lorberbaum JP, Andersen SW, Dannels WR, Haxthausen EU, Vincent DJ, George MS. Echoplanar BOLD fMRI of brain activation induced by concurrent transcranial magnetic stimulation. Investigative Radiology. 1998;33:336–340. doi: 10.1097/00004424-199806000-00004. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Mattingley JB. Neurodisruption of selective attention: insights and implications. Trends in Cognitive Sciences. 2005;9:542–550. doi: 10.1016/j.tics.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Denslow S, Lomarev M, George MS, Bohning DE. Cortical and subcortical brain effects of transcranial magnetic stimulation (TMS)-induced movement: an interleaved TMS/functional magnetic resonance imaging study. Biological Psychiatry. 2005;57:752–760. doi: 10.1016/j.biopsych.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Fox P, Ingham R, George MS, Mayberg H, Ingham J, Roby J, Martin C, Jerabek P. Imaging human intra-cerebral connectivity by PET during TMS. Neuroreport. 1997;8:2787–2791. doi: 10.1097/00001756-199708180-00027. [DOI] [PubMed] [Google Scholar]
- Friston K. Functional integration and inference in the brain. Progess in Neurobiology. 2002;68:113–143. doi: 10.1016/s0301-0082(02)00076-x. [DOI] [PubMed] [Google Scholar]
- Harris JA, Clifford CW, Miniussi C. The functional effect of transcranial magnetic stimulation: signal suppression or neural noise generation? Journal of Cognitive Neuroscience. 2008;20:734–740. doi: 10.1162/jocn.2008.20048. [DOI] [PubMed] [Google Scholar]
- Ilmoniemi RJ, Virtanen J, Ruohonen J, Karhu J, Aronen HJ, Naatanen R, Katila T. Neuronal responses to magnetic stimulation reveal cortical reactivity and connectivity. Neuroreport. 1997;8:3537–3540. doi: 10.1097/00001756-199711100-00024. [DOI] [PubMed] [Google Scholar]
- Karnath H-O, Milner AD, Vallar G. The cognitive and neural bases of spatial neglect. Oxford University Press; Oxford: 2002. [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annual Review of Neuroscience. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Koch G, Fernandez Del Olmo M, Cheeran B, Schippling S, Caltagirone C, Driver J, Rothwell JC. Functional interplay between posterior parietal and ipsilateral motor cortex revealed by twin-coil transcranial magnetic stimulation during reach planning toward contralateral space. Journal of Neuroscience. 2008;28:5944–5953. doi: 10.1523/JNEUROSCI.0957-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomber SG. The advantages and limitations of permanent or reversible deactivation techniques in the assessment of neural function. Journal of Neuroscience Methods. 1999;86:109–117. doi: 10.1016/s0165-0270(98)00160-5. [DOI] [PubMed] [Google Scholar]
- Massimini M, Ferrarelli F, Huber R, Esser SK, Singh H, Tononi G. Breakdown of cortical effective connectivity during sleep. Science. 2005;309:2228–2232. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- O’Shea J, Johansen-Berg H, Trief D, Gobel S, Rushworth MF. Functionally specific reorganization in human premotor cortex. Neuron. 2007;54:479–490. doi: 10.1016/j.neuron.2007.04.021. [DOI] [PubMed] [Google Scholar]
- O’Shea J, Sebastian C, Boorman ED, Johansen-Berg H, Rushworth MF. Functional specificity of human premotor-motor cortical interactions during action selection. European Journal of Neuroscience. 2007;26:2085–2095. doi: 10.1111/j.1460-9568.2007.05795.x. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Bartres-Faz D, Keenan JP. Transcranial magnetic stimulation: studying the brain-behaviour relationship by induction of ‘virtual lesions’. Philosophical Transactions of the Royal Society London B. 1999;354:1229–1238. doi: 10.1098/rstb.1999.0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Inferring causality in brain images: a perturbation approach. Philosophical Transactions of the Royal Society London B. 2005;360:1109–1114. doi: 10.1098/rstb.2005.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Jech R, Thompson CJ, Comeau R, Peters T, Evans AC. Transcranial magnetic stimulation during positron emission tomography: a new method for studying connectivity of the human cerebral cortex. Journal of Neuroscience. 1997;17:3178–3184. doi: 10.1523/JNEUROSCI.17-09-03178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DR, Vincent DJ, Speer AM, Bohning DE, Cure J, Young J, George MS. Multi-modality mapping of motor cortex: comparing echoplanar BOLD fMRI and transcranial magnetic stimulation. Short communication. Journal of Neural Transmission. 1997;104:833–843. doi: 10.1007/BF01285552. [DOI] [PubMed] [Google Scholar]
- Ruff CC, Bestmann S, Blankenburg F, Bjoertomt O, Josephs O, Weiskopf N, Deichmann R, Driver J. Distinct causal influences of parietal versus frontal areas on human visual cortex: evidence from concurrent TMS-fMRI. Cerebral Cortex. 2008;18:817–827. doi: 10.1093/cercor/bhm128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff CC, Blankenburg F, Bjoertomt O, Bestmann S, Freeman E, Haynes JD, Rees G, Josephs O, Deichmann R, Driver J. Concurrent TMS-fMRI and psychophysics reveal frontal influences on human retinotopic visual cortex. Current Biology. 2006;16:1479–1488. doi: 10.1016/j.cub.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Ruff CC, Blankenburg F, Bjoertomt O, Bestmann S, Weiskopf N, Driver J. Hemispheric differences in frontal and parietal influences on human occipital cortex: Direct confirmation with concurrent TMS-fMRI. Journal of Cognitive Neuroscience. doi: 10.1162/jocn.2009.21097. in the press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack AT, Kohler A, Bestmann S, Linden DE, Dechent P, Goebel R, Baudewig J. Imaging the Brain Activity Changes Underlying Impaired Visuospatial Judgments: Simultaneous fMRI, TMS, and Behavioral Studies. Cerebral Cortex. 2007;17:2841–52. doi: 10.1093/cercor/bhm013. [DOI] [PubMed] [Google Scholar]
- Schall JD, Morel A, King DJ, Bullier J. Topography of visual cortex connections with frontal eye field in macaque: convergence and segregation of processing streams. Journal of Neuroscience. 1995;15:4464–4487. doi: 10.1523/JNEUROSCI.15-06-04464.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences JT, Yantis S. Selective visual attention and perceptual coherence. Trends in Cognitive Sciences. 2006;10:38–45. doi: 10.1016/j.tics.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Takano B, Peinemann A, Schwaiger M, Conrad B, Drzezga A. Continuous transcranial magnetic stimulation during positron emission tomography: a suitable tool for imaging regional excitability of the human cortex. Neuroimage. 2001;14:883–890. doi: 10.1006/nimg.2001.0889. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Hartwigsen G, Kassuba T, Rothwell JC. How does transcranial magnetic stimulation modify neuronal activity in the brain? Implications for studies of cognition. Cortex. doi: 10.1016/j.cortex.2009.02.007. in the press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvanto J, Lavie N, Walsh V. Stimulation of human frontal eye fields modulates sensitivity of extrastriate visual cortex. Journal of Neurophysiology. 2006;96:941–945. doi: 10.1152/jn.00015.2006. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Muggleton N, Walsh V. State dependency in Brain Stimulation Studies of Perception and Cognition. Trends in Cognitive Sciences. doi: 10.1016/j.tics.2008.09.004. in the press. [DOI] [PubMed] [Google Scholar]
- Strafella AP, Paus T, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. Journal of Neuroscience. 2001;21:RC157. doi: 10.1523/JNEUROSCI.21-15-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PC, Nobre AC, Rushworth MF. FEF TMS Affects Visual Cortical Activity. Cerebral Cortex. 2007a;17:391–399. doi: 10.1093/cercor/bhj156. [DOI] [PubMed] [Google Scholar]
- Taylor PC, Nobre AC, Rushworth MF. Subsecond changes in top down control exerted by human medial frontal cortex during conflict and action selection: a combined transcranial magnetic stimulation electroencephalography study. Journal of Neuroscience. 2007b;27:11343–11353. doi: 10.1523/JNEUROSCI.2877-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner T, Valero-Cabre A, Pascual-Leone A. Non-invasive human brain stimulation. Annual Review of Biomedical Engineering. 9:527–565. doi: 10.1146/annurev.bioeng.9.061206.133100. [DOI] [PubMed] [Google Scholar]
- Walsh V, Cowey A. Transcranial magnetic stimulation and cognitive neuroscience. Nature Reviews Neuroscience. 2000;1:73–79. doi: 10.1038/35036239. [DOI] [PubMed] [Google Scholar]