Abstract
Introduction
In the classic neurological model of language, the human inferior parietal lobule (IPL) plays an important role in visual word recognition. The region is both functionally and structurally heterogeneous, however, suggesting that subregions of IPL may differentially contribute to reading. The two main sub-divisions are the supramarginal (SMG) and angular gyri, which have been hypothesized to contribute preferentially to phonological and semantic aspects of word processing, respectively.
Methods
Here we used single-pulse TMS to investigate the functional specificity and timing of SMG involvement in reading. Participants performed two reading tasks that focused attention on either the phonological or semantic relation between two simultaneously presented words. A third task focused attention on the visual relation between pairs of consonant letter strings to control for basic input and output characteristics of the paradigm using non-linguistic stimuli. TMS to SMG was delivered on every trial at 120, 180, 240 or 300 msec post-stimulus onset.
Results
Stimulation at 180 msec produced a reliable facilitation of reaction times for both the phonological and semantic tasks, but not for the control visual task.
Conclusion
These findings demonstrate that SMG contributes to reading regardless of the specific task demands, and suggests this may be due to automatically computing the sound of a word even when the task does not explicitly require it.
Keywords: reading, phonology, semantics, chronometric TMS, inferior parietal lobe, neurological model of reading
1. Introduction
In the classic neurological model of language, visual word recognition involves a distributed set of left hemisphere brain regions. The written word first activates visual cortices that send information to the inferior parietal lobule (IPL) where visual word forms are recognized (Dejerine, 1891, 1892). These are then linked to auditory word forms in Wernicke's area and then to articulatory motor patterns located in Broca's area. Damage to either IPL or the visual pathway linking occipital cortex to IPL can result in preferential impairments for reading by destroying or disconnecting one's memory for visual word forms (Damasio and Damasio, 1983; Dejerine, 1891, 1892; Philipose et al., 2007; Warrington and Shallice, 1980). Functional neuroimaging studies confirm the importance of IPL for reading, but question its specific contribution (Price and Mechelli, 2005; Pugh et al., 2001). Several authors, for instance, attribute IPL activation to the process of converting spelling-to-sound rather than to stored visual word forms and suggest that dysfunction in this region is the cause of poor reading skills in developmental dyslexics (Bookheimer et al., 1995; Horwitz et al., 1998; Pugh et al., 2000; Shaywitz et al., 2002). Currently there is no clear consensus regarding the functional role of IPL in reading.
Anatomically, IPL is a heterogeneous region comprised of several distinct cytoarchitectonic fields (von Bonin and Bailey, 1947) with the two largest, Brodmann areas 40 and 39, roughly corresponding to the supramarginal (SMG) and angular (ANG) gyri, respectively (Rushworth et al., 2005). Functionally, these two regions are also heterogeneous and contribute to a wide range of tasks, not just reading (e.g. Gobel et al., 2001; Schacter et al., 2007). Within the visual word recognition literature, studies have shown increased SMG activation when participants focus on the sound of a word which contrasts with increased ANG activation when participants focus on its meaning (Demonet et al., 1994; Devlin et al., 2003; Mummery et al., 1998; Price et al., 1997). This double dissociation corresponds with the distinct patterns of connectivity for each region. SMG is connected to auditory association regions of posterior supratemporal plane and to a region of posterior inferior frontal gyrus (Catani et al., 2005), both of which are involved in phonological processing (Gough et al., 2005; Hickok and Poeppel, 2000; Poldrack et al., 1999). In contrast, ANG sits at the posterior end of the inferior longitudinal fasciculus which links it with temporal lobe regions implicated in semantic memory (Catani et al., 2002; Hodges et al., 1995; Spitsyna et al., 2006). In other words, it may be possible to establish a more fine grained understanding of IPL contributions to reading by separately considering its two major sub-divisions.
Here, we investigated the specific contributions of supramarginal involvement in visual word recognition using chronometric TMS (Walsh and Pascual-Leone, 2003). We hypothesized that stimulating SMG would disrupt phonological more than semantic processing based on previous imaging studies. In addition, we hypothesized this disruption would be strongest approximately 200-300 msec after the onset of the word based on previous ERP and MEG findings (Khateb et al., 1999; Pammer et al., 2004). Participants performed three different tasks emphasizing phonological, semantic, or visual aspects of the stimuli while single pulses of TMS were delivered to SMG at 120, 180, 240, or 300 msec post-stimulus onset.
2. Methods
2.1 Subjects
22 healthy, right-handed volunteers (13 female, aged 19-34) participated in the study. All subjects were native English speakers who were free of any neurological deficits and screened for TMS exclusion criteria. Prior to the experiment, subjects gave their informed written consent in accordance with the Declaration of Helsinki. Subjects were paid for participation and free to leave the experiment any time. The study was approved by the NHS Oxfordshire Research Ethics Committee.
2.2 TMS parameters
TMS was carried out with a MagStim Rapid2 stimulator (Magstim, Whitland, UK) using a 70-mm figure-of-eight coil. The stimulation intensity was set to 55% of the maximum stimulator output for all subjects. During the localiser task, trains of three pulses were delivered at 100, 200 and 300 msec following stimulus onset in a third of all trials. During the main task, a single pulse was delivered at 120, 180, 240, or 300 msec post-stimulus onset in every trial. Stimulation parameters were well within international safety guidelines (Wassermann, 1998).
2.3 Neuronavigation
In order to accurately target SMG in our participants, we employed a two-stage localisation procedure. The first used the BrainSight frameless stereotaxy system (Rogue Research, Montreal, Canada) to anatomically identify potential stimulation targets within each participant's left SMG. For each subject, a high-resolution MRI scan was either already available or collected on a Siemens (Erlangen, Germany) 1.5 T Sonata scanner (FLASH sequence, TR = 10 msec, TE = 4.75msec, flip angle = 19°, resolution = 1×1×1 mm). Prior to the TMS session, a single target site was marked on the individual's structural scan by placing a marker at the end of the posterior ascending ramus of the Sylvian fissure. A set of eight additional markers were placed around this site, generating a 3 × 3 grid of testing locations within SMG (Figure 1a). These sites were then tested in a second stage to determine whether stimulation of any of them disrupted phonological processing.
Figure 1.
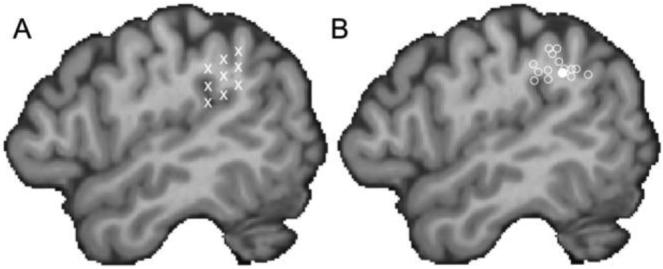
Stimulation sites. A) In each subject, the termination of the posterior ascending ramus of the Sylvian fissure was identified and marked using a frameless stereotaxy system. Then a 3 × 3 grid of markers was overlaid to label nine possible SMG testing sites. B) In the main experiment, the final testing site for all 14 participants is shown (open circles) as well as the mean group location (filled circle). Both panels are shown on a para-saggital plane through a single participant's brain after normalizing to the standard MNI152 space with an affine registration (Jenkinson and Smith, 2001).
The aim of the first part of the TMS experiment was to find a stimulation site where short bursts of rTMS interfered with phonological processing. A rhyme judgement task was used to focus attention on the sounds of the words. A trial began with a centrally presented fixation cross which remained on the screen for 1000 msec before two words appeared, above and below the cross, for a further 500 msec. Participants had to decide whether or not the words rhymed (e.g. chair – pear) and indicate their responses by pressing a button with either their index or middle finger. There was a 2500 msec inter-trial interval before the next trial began and each run consisted of 30 trials presented in a random order, for a total duration of 2 mins per run. One third of the trials had concurrent rTMS (10Hz for 300 msec) starting 100 msec after the onset of the word pair. Reaction times (RTs) were recorded from the onset of the stimulus and only correct responses were analysed. When the median RT for TMS trials was numerically greater than for non-TMS trials on two separate runs at the same location, then that site was used for testing in the main experiment. In short, a TMS-based functional localiser was employed to identify the main testing site (cf. Ashbridge et al., 1997; Devlin et al., 2003; Gough et al., 2005).
Testing began with a practice run where no TMS was delivered. When participants were comfortable with the task, then TMS was introduced by placing the coil on the scalp such that the line of maximum magnetic flux intersected one of the anatomically marked SMG sites. All participants tolerated TMS with no discomfort, although in a few cases there was peripheral stimulation of the temporalis muscle. After each run, RTs were analysed and if TMS led to faster RTs (i.e. facilitation), the next site was tested. When there was inhibition, the site was re-tested to ensure the effect was repeatable. Inhibition effects of more than 100 msec were considered physiologically unlikely and were re-tested. If after 10 runs we were unable to identify a suitable testing site, the experiment ended.
2.4 Procedure
The main experiment involved three different tasks: i) deciding whether two words sounded the same (e.g. cite – sight); ii) deciding whether two words meant the same thing (e.g. idea – notion); and iii) deciding whether two consonant letters strings were visually identical (e.g. shqwy – shqwy). In all cases, there were equal numbers of matching and non-matching trials (i.e. “yes” and “no” responses). The first two tasks were designed to focus attention on either the sound of the word or its meaning while the third was used as a non-linguistic control task, which shared visual and response characteristics of the main tasks without any linguistic component. The tasks were blocked to minimize switching costs and the order was counter-balanced across participants. A block began with a short instruction screen (2s) followed by 13 trials using the same presentation characteristics as the localiser task (1000 msec fixation, 500 msec stimulus duration) but a shorter ITI (1500 msec). The first trial in each block was excluded from analyses to help minimize contamination from task switching. In total there were 8 blocks (i.e. 96 trials) per task. On each trial a single pulse of TMS was delivered at 120, 180, 240 or 300 msec after the onset of the stimulus and these were balanced so that there were 24 trials per task per TMS condition. Participants first practiced the main experiment with TMS to familiarise themselves with the experience of single pulse stimulation and the fact that it occurred on all trials. None of the stimuli used in the practice were repeated in the main experiment.
2.5 Stimuli
The words used in the TMS localiser task ranged in length from 3-8 letters. TMS and non-TMS trials were matched within and across runs for British English written word frequency (Baayen and Pipenbrook, 1995), for rated familiarity, and imageability (Coltheart, 1981), and for number of letters and syllables (all p>0.1). Note that the rimes of word pairs always had different spellings (e.g. razor – laser) so that rhyme judgements could not be performed solely on the orthography of the words. The words in the main experiment were 3-10 letters long and were matched for familiarity and written word frequency. Due to the limited numbers of homophones in English, we were unable to match the sets for the remaining factors, with items in the phonological task being less imageable (500 versus 547), having fewer letters (4.9 versus 5.3) and fewer syllables (1.3 versus 1.6, all p<0.05). Importantly, there were no significant differences between the four TMS conditions (120, 180, 240, 300) nor Task × TMS interactions. Nonetheless, a behavioural pre-test was conducted with a different set of 22 native English speakers (15 female; age ranged: 17-47 years) to determine whether any putative TMS effects could be due to insufficient matching on psycholinguistic factors in the absence of TMS. That is, the items were classified as being in one of the four TMS conditions (120, 180, 240 or 300 msec) even though no stimulation occurred in this experiment. This allowed us to determine whether our stimuli matching was sufficient by looking for significant differences in RTs across conditions even without TMS. Any such difference would indicate insufficient matching. An item analysis confirmed a highly significant effect of Task (F(3,184) = 35.1, p<0.001) with faster responses for the phonological than semantic task. Crucially, however, there was no significant main effect of TMS (F(3,184)=0.02, p=0.996) nor a significant Task × TMS interaction (F(3,184) = 0.15, p=0.895). In short, although the two tasks have different RTs, the conditions within tasks were well balanced. No words from the practice or localiser task appeared in the main experiment and no words were repeated within the experiment. Finally, the consonant strings were matched in length to the lexical stimuli.
2.6 Analyses
There were no significant effects of TMS on accuracy in either experiment so all the analyses focus on RTs. To minimize the effect of outliers, the median RT of correct responses per condition per subject was used when calculating group results. Paired t-tests were used to compare RTs on the TMS and no-TMS trials in the localiser task. For the main experiment, a repeated measures 3 × 4 ANOVA used Task (phonological, semantic, visual) and TMS (120, 180, 240, and 300 msec) as the independent factors. In addition, a second analysis compared each TMS condition to the task mean to normalize for overall task performance. This has the advantage of providing a statistically unbiased measure which can reveal relative facilitation and inhibition effects (Drager et al., 2004).
3. Results
3.1 Localisers
One participant was slower by approximately 400 msec across tasks than the others and was therefore removed from all analyses leaving 21 participants. In 7 of these we failed to find a consistent TMS-induced slow down at any of the SMG sites within 10 TMS localiser runs. In the remaining 14 participants, TMS clearly interfered with rhyme judgements. In these participants, TMS produced a significant inhibition effect of 51msec (t(13)=5.9, p<0.001). When normalized to reflect between-subject variability in overall RT, this equated to a 7.9% slow-down in individuals. In contrast, stimulation of the other SMG sites produced a (non-significant) facilitation effect of 16msec which, when normalized, was equivalent to a 2.7% speed-up in RTs. The sites where stimulation led to a slow-down for rhyme judgements are shown in Figure 1B. The mean coordinate in standard space defined by the MNI152 template brain was X= −52, Y= −45, Z= +39 and is shown with a filled circle. The sites for the individuals are shown as open circles and these sites were used as the test sites for the main experiment.
3.2 Main experiment
Participants responded correctly to 93% of the homophone judgements (phonological task), 90% of the synonym judgements (semantic task), and 92% of visual judgements (control task). A 3 × 4 repeated measures ANOVA with Task and TMS as independent factors revealed no significant main effects nor interactions (all F<1.8, all p>.12), demonstrating that accuracy was unaffected by either Task or TMS.
Reaction times for all three tasks are shown in Figure 2. In the left column, RTs measured from the onset of the visual stimulus are shown for each of the four TMS conditions. An ANOVA confirmed a significant effect of Task (F(2,26)=55.6, p<0.001) indicating that semantic decisions (956 msec) were performed more slowly than phonological decisions (876 msec) which were slower than purely visual decisions (738 msec). The main effect of TMS was non-significant (F(2,26)=2.2, p=0.10) with mean RTs per TMS time window of 858, 844, 859, and 865 msec, respectively. The Task × TMS interaction term was not significant (F(2,26)=0.9, p=0.51).
Figure 2.
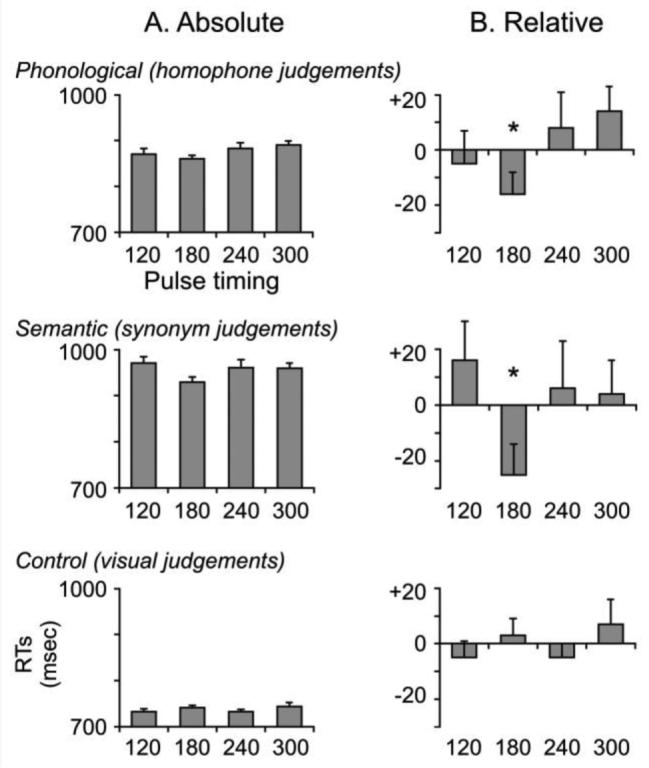
Reaction times for all three tasks in the main experiment. A. The left column displays the absolute RTs from the onset of the visual stimulus for each of the four stimulation timings. The dashed line represents the mean RT across the four stimulation conditions. Note the scale of the y-axes are not identical due to the main effect of Task with visual < phonological < semantic decisions. B) The right column displays the timings relative to the task mean and reveals significant facilitation effects at 180 msec post-stimulus onset for both phonological and semantic judgements. The numbers above or below the bar plots are the effect sizes (in msec) from the mean. Error bars reflect standard error of the mean adjusted to correctly reflect the variance in the within-subject design (Loftus and Masson, 1994).
Our second analysis investigated TMS effects within task by comparing each condition to its overall task mean (cf. Drager et al., 2004). For phonological decisions, the only inhibitory effects occurred at 240 and 300 msec and were non-significant (both t(13)<1.6, p>0.137) but there was a 16msec facilitation effect at 180 msec post-stimulus onset (t(13)=2.16, p=0.050). Similarly, for semantic decisions there was a significant 25 msec facilitation effect at 180 msec (t(13)=2.41, p=0.031) but no other reliable differences. When normalized for overall RTs per task, both the phonological and semantic TMS effects represented a 2% decrease in RTs relative to the overall mean. Finally, there were no significant effects of TMS during the visual control task.
Discussion
Despite identifying the testing site in each individual as one where rTMS interfered with rhyme judgements, single-pulse TMS of the same area facilitated homophone judgements, but only when applied at 180 msec post-stimulus onset. This temporal specificity was also present for synonym judgements but not for the visual control task. These findings were surprising for three reasons. First, we anticipated the effect would be specific to phonological decisions. Second, both repetitive and single pulse TMS were expected to interfere with processing. Third, we expected TMS to have its effect later than 180 msec, based on previous ERP, MEG, and TMS findings (Devlin et al., 2003; Khateb et al., 1999; Pammer et al., 2004). Here we consider a number of potential explanations for these surprising results.
Previous neuroimaging studies have reported increased activation in SMG when directly comparing phonological to semantic decisions (Demonet et al., 1994; Devlin et al., 2003; Mummery et al., 1998) and an earlier TMS study demonstrated that rTMS to SMG significantly disrupted verbal working memory (Romero et al., 2006). In contrast, semantic decisions are more typically associated with the angular gyrus (Demonet et al., 1994; Devlin et al., 2003; Mummery et al., 1998; Price, 2000) so it was surprising that TMS at 180 msec facilitated responses for both tasks. One possibility is that direct comparisons of activation levels in phonological and semantic tasks hide the fact that the area is activated by both tasks but to different extents. Preliminary evidence supporting this hypothesis comes from an imaging study comparing activation levels in a sentence completion task (e.g. “I like to play on a warm summer's ____”) where participants either chose a response that made the sentence rhyme (e.g. “day”) or just completed the sentence sensibly (e.g. “evening”; Gough, Nobre, and Devlin, in preparation). Although rhyme completions led to significantly greater activation in several cortical areas including SMG, both tasks significantly activated SMG above baseline levels. This common activation may reflect the fact that all words require phonological processing at some level which would explain why in the current study, TMS to SMG affected semantic as well as phonological decisions. Clearly these results raise new questions, however, and these can be explored with further TMS experiments. If our hypotheses are correct, then a more robust chronometric stimulation technique such as paired pulses delivered 40 msec apart (Juan and Walsh, 2003) should disrupt processing in SMG and interfere with both phonological and semantic decisions, presumably at a slightly later time window.
The other surprising findings were that stimulation facilitated, rather than interfered with, response times in the main experiment and that this effect occurred earlier than one might expect. One possibility that can be ruled out is that this was due to the specific task used in the main experiment, particularly as inhibition was observed at the same site in the localiser task. Rhyme and homophone decisions were chosen because both focus attention on the sound of the words and because a previous study found that rTMS to left pars opercularis significantly impaired both types of decisions (Gough et al., 2005). Therefore, there is no reason to expect the difference in task to drive the divergent findings. A more likely candidate is the difference in stimulation paradigms. Short bursts of repetitive TMS disrupt processing in a region for the length of the stimulation train whereas a single pulse has its effect for roughly 40-60 msec (Amassian et al., 1989; Brasil-Neto et al., 1992). As a result, rTMS typically has a larger effect than single pulses and is most commonly used to induce virtual lesions (Devlin and Watkins, 2007; Pascual-Leone et al., 1999). In fact, several studies have shown facilitated responses following single-pulse stimulation (Grosbras and Paus, 2003; Pascual-Leone et al., 1992; Pulvermuller et al., 2005; Topper et al., 1998). For instance, Topper and colleagues (1998) found that single pulses of TMS delivered to Wernicke's area decreased picture naming latencies while Pulvermuller et al. (2005) demonstrated that single pulses of stimulation delivered to either hand or leg areas of primary motor cortex facilitated lexical decisions to either hand- or leg-related action words, respectively. Interestingly, a common factor in these studies was that the pulse was delivered early in the time course of a trial – before activation in the region was expected. This is consistent with the current results where the only significant facilitation occurred at 180 msec post-stimulus onset – roughly 70-120 msec before SMG was expected to contribute to the tasks (Devlin et al., 2003; Khateb et al., 1999; Pammer et al., 2004). In other words, the fact that single TMS pulse facilitated, rather than inhibited, responses and that this occurred relatively early in time is at least consistent with some previous studies. These facilitation effects are often explained as TMS “priming” the region to respond (Pulvermuller et al., 2005), although the physiological basis of the effect remains unknown and further studies using state-dependent effects of TMS (Silvanto and Muggleton, 2008a, b) would be necessary to evaluate the validity of this hypothesis.
Acknowledgements
MCS was supported by the European Commission (Marie Curie Fellowship 25413) and the British Academy (small research grant SG-43944). This work was supported by the Wellcome Trust (JTD).
References
- Amassian VE, Cracco RQ, Maccabee PJ, Cracco JB, Rudell A, Eberle L. Suppression of visual perception by magnetic coil stimulation of human occipital cortex. Electroencephalogr Clin Neurophysiol. 1989;74:458–462. doi: 10.1016/0168-5597(89)90036-1. [DOI] [PubMed] [Google Scholar]
- Ashbridge E, Walsh V, Cowey A. Temporal aspects of visual search studied by transcranial magnetic stimulation. Neuropsychologia. 1997;35:1121–1131. doi: 10.1016/s0028-3932(97)00003-1. [DOI] [PubMed] [Google Scholar]
- Baayen RH, Pipenbrook R, Linguistic Data Consortium . The Celex Lexical Database. University of Pennsylvania; Philadelphia, PA: 1995. [Google Scholar]
- Bookheimer SY, Zeffiro TA, Blaxton T, Gaillard W, Theodore W. Regional cerebral blood flow during object naming and word reading. Hum Brain Mapp. 1995;3:93–106. [Google Scholar]
- Brasil-Neto JP, McShane LM, Fuhr P, Hallet M, Cohen LG. Topographic mapping of the human motor cortex with magnetic stimulation: Factors affecting accuracy and reproducability. Journal of Electroencephalography and Clinical Neurophysiology. 1992;85:9–16. doi: 10.1016/0168-5597(92)90095-s. [DOI] [PubMed] [Google Scholar]
- Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17:77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Coltheart M. The MRC Psycholinguistics database. Quarterly Journal of Experimental Psychology. 1981;33A:497–505. [Google Scholar]
- Damasio A, Damasio H. The anatomic basis of pure alexia. Neurology. 1983;33:1573–1583. doi: 10.1212/wnl.33.12.1573. [DOI] [PubMed] [Google Scholar]
- Dejerine J. Sur un cas de cecite verbale avec agraphie, suivi d'autopsie. Memoires Societe Biologique. 1891;3:197–201. [Google Scholar]
- Dejerine J. Contribution a l'etude anatomoclinique et clinique des differentes varietes de cecite verbal. CR Hebdomadaire des Sceances et Memories de la Societe de Biologie. 1892;4:61–90. [Google Scholar]
- Demonet JF, Price C, Wise R, Frackowiak RS. Differential activation of right and left posterior sylvian regions by semantic and phonological tasks: a positron-emission tomography study in normal human subjects. Neurosci Lett. 1994;182:25–28. doi: 10.1016/0304-3940(94)90196-1. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Matthews PM, Rushworth MF. Semantic processing in the left inferior prefrontal cortex: a combined functional magnetic resonance imaging and transcranial magnetic stimulation study. J Cogn Neurosci. 2003;15:71–84. doi: 10.1162/089892903321107837. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Watkins KE. Stimulating language: insights from TMS. Brain. 2007;130:610–622. doi: 10.1093/brain/awl331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drager B, Breitenstein C, Helmke U, Kamping S, Knecht S. Specific and nonspecific effects of transcranial magnetic stimulation on picture-word verification. Eur J Neurosci. 2004;20:1681–1687. doi: 10.1111/j.1460-9568.2004.03623.x. [DOI] [PubMed] [Google Scholar]
- Gobel S, Walsh V, Rushworth MF. The mental number line and the human angular gyrus. Neuroimage. 2001;14:1278–1289. doi: 10.1006/nimg.2001.0927. [DOI] [PubMed] [Google Scholar]
- Gough PM, Nobre AC, Devlin JT. Dissociating linguistic processes in the left inferior frontal cortex with transcranial magnetic stimulation. J Neurosci. 2005;25:8010–8016. doi: 10.1523/JNEUROSCI.2307-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosbras MH, Paus T. Transcranial magnetic stimulation of the human frontal eye field facilitates visual awareness. Eur J Neurosci. 2003;18:3121–3126. doi: 10.1111/j.1460-9568.2003.03055.x. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Towards a functional neuroanatomy of speech perception. Trends Cogn Sci. 2000;4:131–138. doi: 10.1016/s1364-6613(00)01463-7. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Graham N, Patteron K. Charting the progression in semantic dementia: Implications for the organization of semantic memory. Memory. 1995;3:463–495. doi: 10.1080/09658219508253161. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Rumsey JM, Donohue BC. Functional connectivity of the angular gyrus in normal reading and dyslexia. Proc Natl Acad Sci U S A. 1998;95:8939–8944. doi: 10.1073/pnas.95.15.8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Juan CH, Walsh V. Feedback to V1: a reverse hierarchy in vision. Exp Brain Res. 2003;150:259–263. doi: 10.1007/s00221-003-1478-5. [DOI] [PubMed] [Google Scholar]
- Khateb A, Annoni J-M, Landis T, Pegna AJ, Custodi M-C, Fonteneau E, Morand SM, Michel CF. Spatio-temporal analysis of electric brain activity during semantic and phonological word processing. International Journal of Psychophysiology. 1999;32:215–231. doi: 10.1016/s0167-8760(99)00017-3. [DOI] [PubMed] [Google Scholar]
- Loftus GR, Masson MEJ. Using confidence-intervals in within-subject designs. Psychon Bull Rev. 1994;1:476–490. doi: 10.3758/BF03210951. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Hodges JR, Price CJ. Functional neuroanatomy of the semantic system: Divisible by what? J Cogn Neurosci. 1998;10:766–777. doi: 10.1162/089892998563059. [DOI] [PubMed] [Google Scholar]
- Pammer K, Hansen PC, Kringelbach ML, Holliday I, Barnes G, Hillebrand A, Singh KD, Cornelissen PL. Visual word recognition: the first half second. Neuroimage. 2004;22:1819–1825. doi: 10.1016/j.neuroimage.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Bartres-Faz D, Keenan JP. Transcranial magnetic stimulation: studying the brain-behaviour relationship by induction of ‘virtual lesions’. Philosophical Transactions of the Royal Society London B. 1999;354:1229–1238. doi: 10.1098/rstb.1999.0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Sole J, Wassermann EM, Brasil-Neto J, Cohen LG, Hallett M. Effects of focal transcranial magnetic stimulation on simple reaction time to acoustic, visual and somatosensory stimuli. Brain. 1992;115(Pt 4):1045–1059. doi: 10.1093/brain/115.4.1045. [DOI] [PubMed] [Google Scholar]
- Philipose LE, Gottesman RF, Newhart M, Kleinman JT, Herskovits EH, Pawlak MA, Marsh EB, Davis C, Heidler-Gary J, Hillis AE. Neural regions essential for reading and spelling of words and pseudowords. Ann Neurol. 2007;62:481–492. doi: 10.1002/ana.21182. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: Contributions from fuctional neuroimaging. Journal of Anatomy. 2000;197:335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Mechelli A. Reading and reading disturbance. Curr Opin Neurobiol. 2005;15:231–238. doi: 10.1016/j.conb.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Price CJ, Moore CJ, Humphreys GW, Wise RJ. Segregating semantic from phonological processes during reading. J Cogn Neurosci. 1997;9:727–733. doi: 10.1162/jocn.1997.9.6.727. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, Shaywitz SE, Shaywitz BA. Neurobiological studies of reading and reading disability. J Commun Disord. 2001;34:479–492. doi: 10.1016/s0021-9924(01)00060-0. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Shaywitz BA, Shaywitz SE, Fulbright RK, Constable RT, Skudlarski P, Marchione KE, Jenner AR, Fletcher JM, Liberman AM, Shankweiler DP, Katz L, Lacadie C, Gore JC. The angular gyrus in developmental dyslexia: task-specific differences in functional connectivity within posterior cortex. Psychol Sci. 2000;11:51–56. doi: 10.1111/1467-9280.00214. [DOI] [PubMed] [Google Scholar]
- Pulvermuller F, Hauk O, Nikulin VV, Ilmoniemi RJ. Functional links between motor and language systems. Eur J Neurosci. 2005;21:793–797. doi: 10.1111/j.1460-9568.2005.03900.x. [DOI] [PubMed] [Google Scholar]
- Romero L, Walsh V, Papagno C. The neural correlates of phonological short-term memory: a repetitive transcranial magnetic stimulation study. J Cogn Neurosci. 2006;18:1147–1155. doi: 10.1162/jocn.2006.18.7.1147. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE, Johansen-Berg H. Connection Patterns Distinguish 3 Regions of Human Parietal Cortex. Cereb Cortex. 2005 doi: 10.1093/cercor/bhj079. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: the prospective brain. Nat Rev Neurosci. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Mencl WE, Fulbright RK, Skudlarski P, Constable RT, Marchione KE, Fletcher JM, Lyon GR, Gore JC. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biol Psychiatry. 2002;52:101–110. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Muggleton NG. New light through old windows: moving beyond the “virtual lesion” approach to transcranial magnetic stimulation. Neuroimage. 2008a;39:549–552. doi: 10.1016/j.neuroimage.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Muggleton NG. Testing the validity of the TMS state-dependency approach: targeting functionally distinct motion-selective neural populations in visual areas V1/V2 and V5/MT+ Neuroimage. 2008b;40:1841–1848. doi: 10.1016/j.neuroimage.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Spitsyna G, Warren JE, Scott SK, Turkheimer FE, Wise RJ. Converging language streams in the human temporal lobe. J Neurosci. 2006;26:7328–7336. doi: 10.1523/JNEUROSCI.0559-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topper R, Mottaghy FM, Brugmann M, Noth J, Huber W. Facilitation of picture naming by focal transcranial magnetic stimulation of Wernicke's area. Experimental Brain Research. 1998;121:371–378. doi: 10.1007/s002210050471. [DOI] [PubMed] [Google Scholar]
- von Bonin G, Bailey P. The Neocortex of Macaca Mulatta. University of Illinois Press; Urbana, IL: 1947. [Google Scholar]
- Walsh V, Pascual-Leone A. Transcranial magnetic stimulation: A neurochronometrics of mind. MIT Press; London: 2003. [Google Scholar]
- Warrington EK, Shallice T. Word form dyslexia. Brain. 1980;103:99–112. doi: 10.1093/brain/103.1.99. [DOI] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: Report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5-7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]


