Abstract
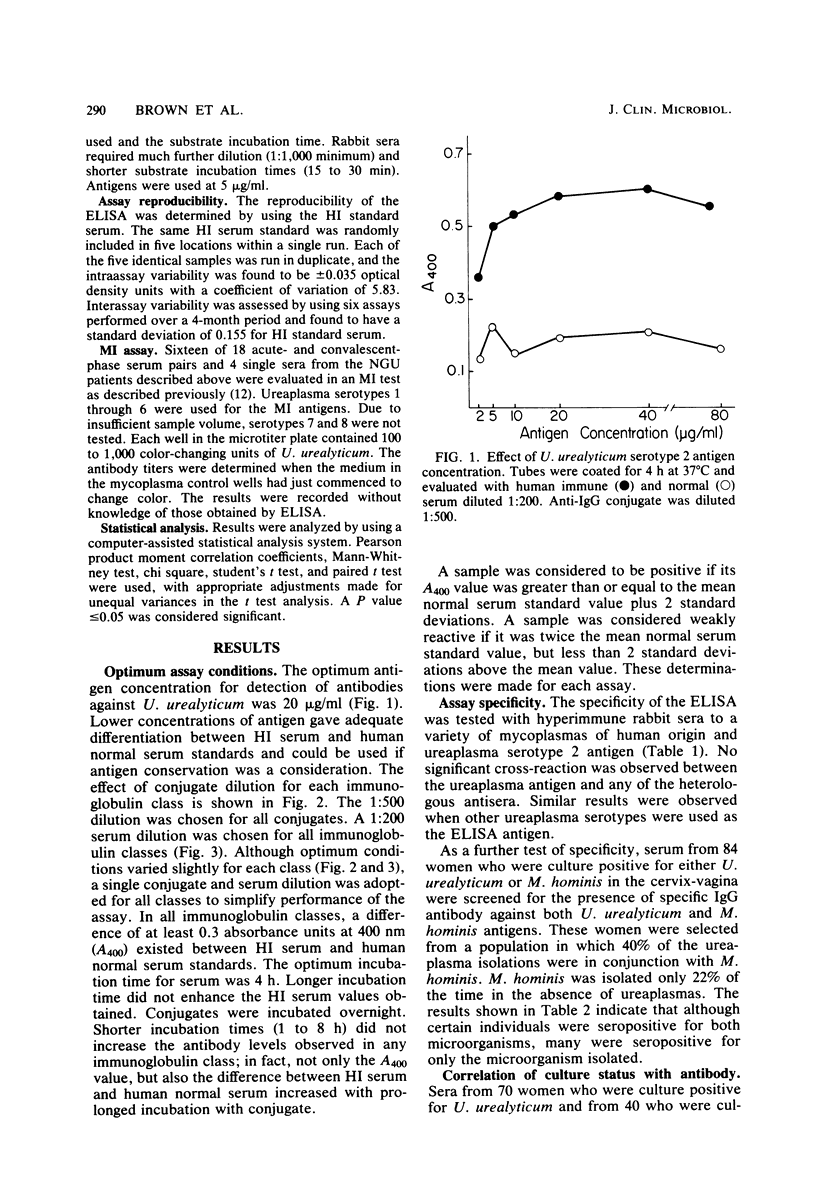
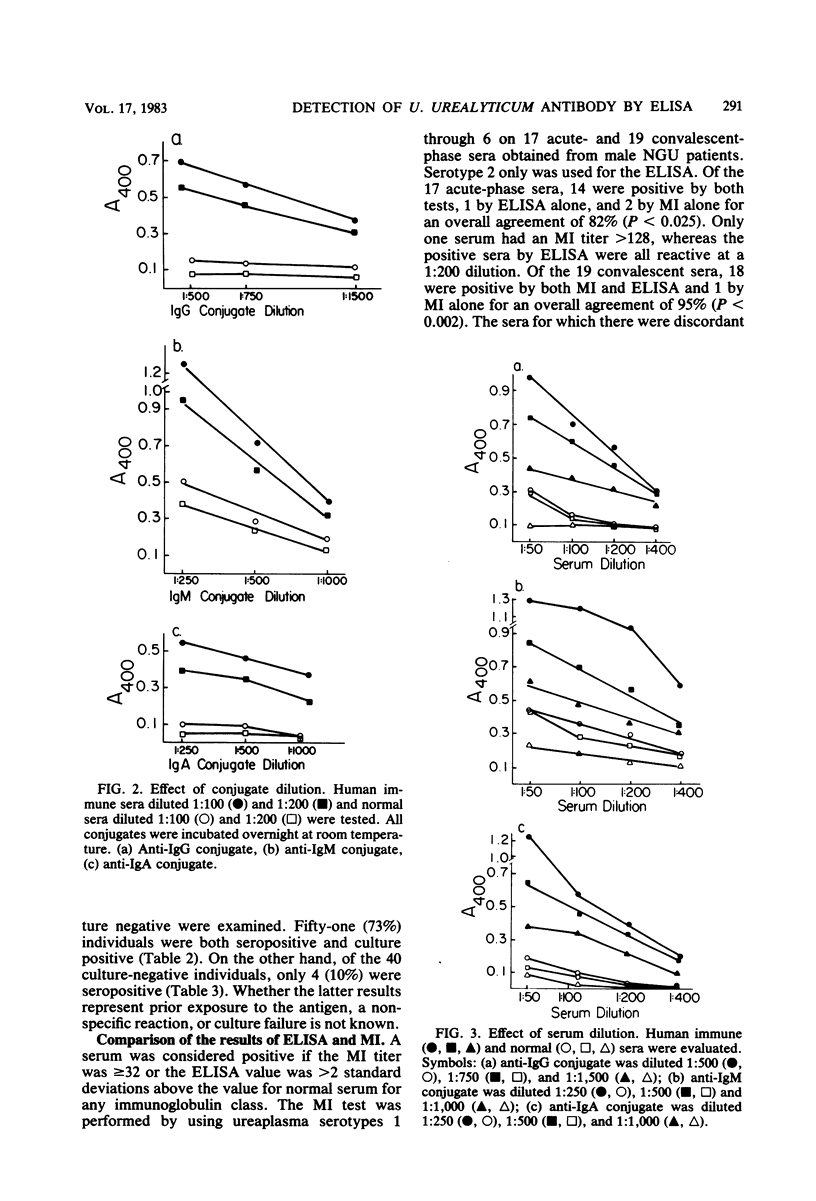
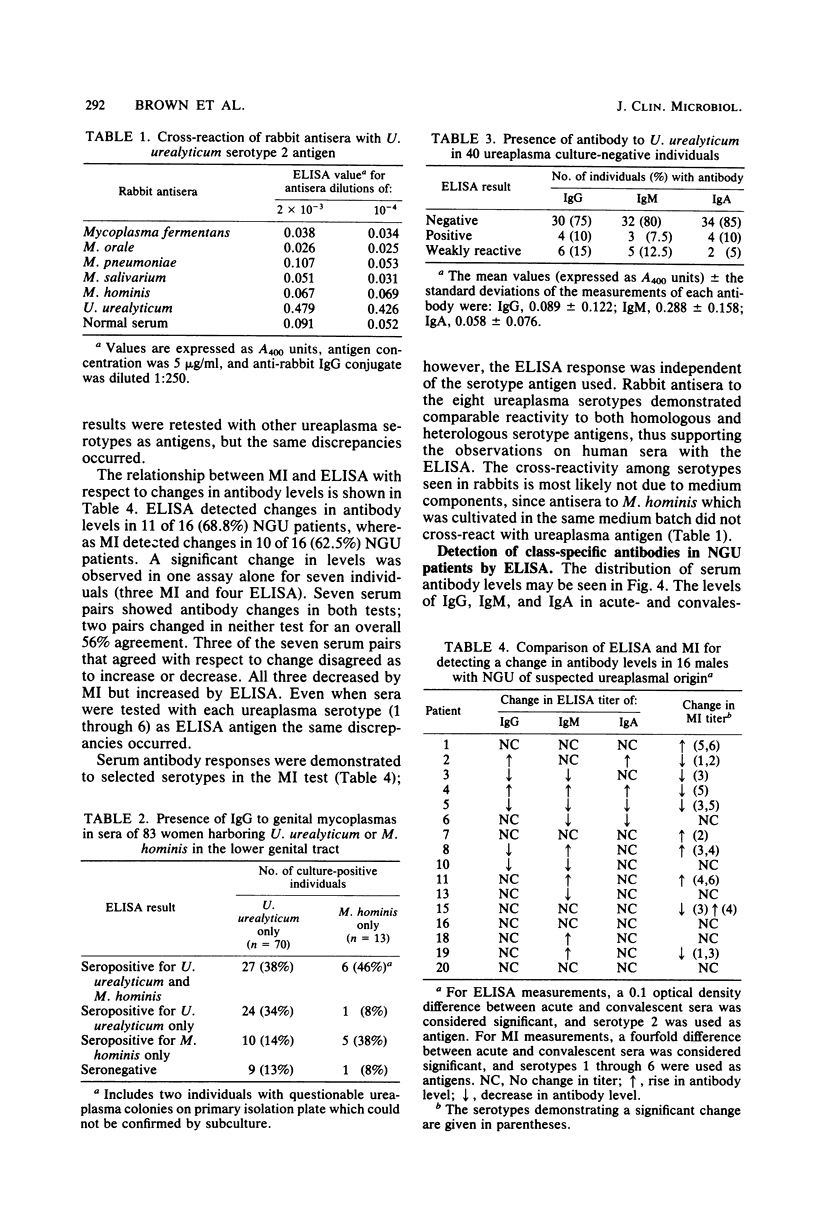
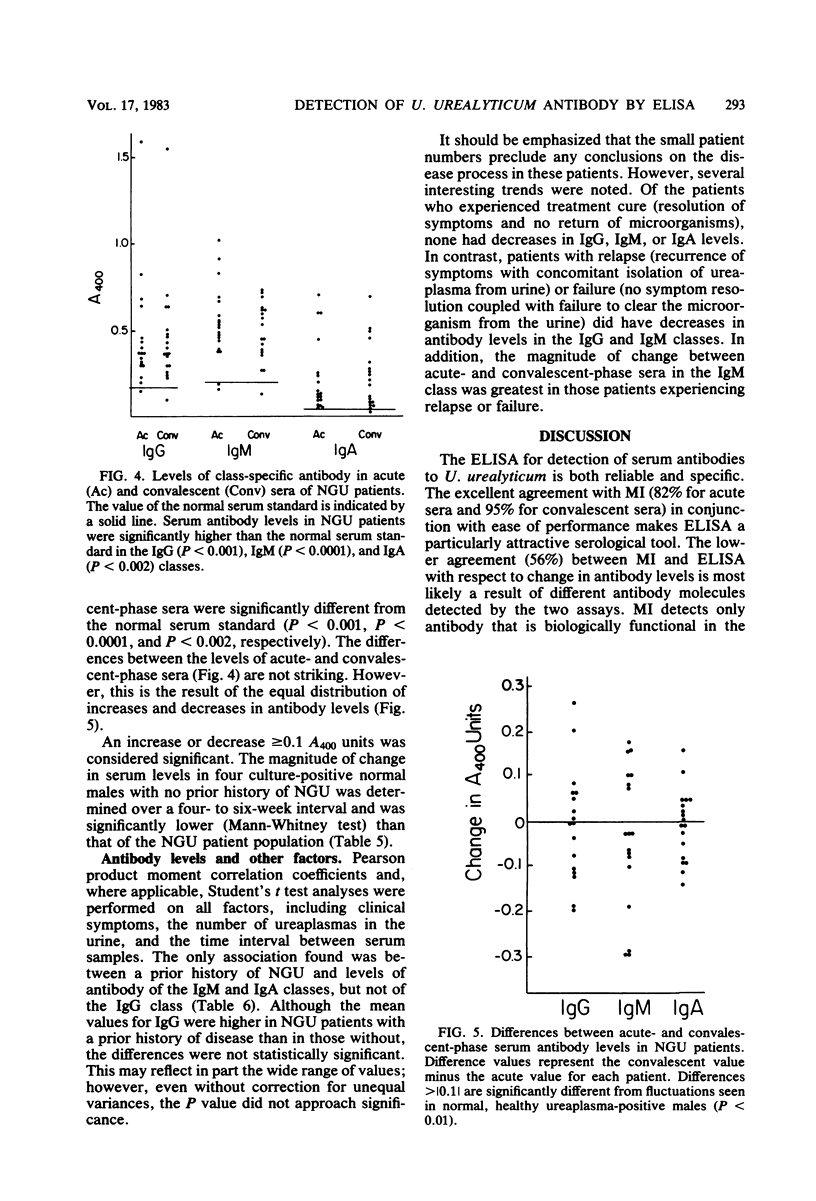
The optimum conditions for the detection of human immunoglobulin G (IgG), IgM, and IgA antibodies to Ureaplasma urealyticum by an enzyme-linked immunosorbent assay (ELISA) were established by using a cell lysate antigen and commercially available alkaline phosphatase conjugates. No significant cross-reactions were observed among rabbit antisera to a variety of mycoplasmas of human origin and ureaplasma antigen, thus demonstrating the specificity of the ELISA. All human sera were assayed at a 1:200 dilution. Antigen was used at 20 μg of protein/ml and conjugates were diluted 1:500. Presence of IgG antibody to U. urealyticum was significantly associated with isolation of U. urealyticum (P < 0.001) in 110 women. Seventeen acute-phase and 19 convalescent-phase sera from male nongonococcal urethritis (NGU) patients were tested for the presence of antibody by both the metabolism inhibition assay and by ELISA, with overall agreements of 82 and 95% for acute- and convalescent-phase sera, respectively. Serum antibody responses were demonstrated to selected serotypes in the metabolism inhibition test, but the response as measured by the ELISA was independent of the serotype of the antigen used. Serum antibody levels in NGU patients were significantly higher (P < 0.002) than the normal serum standard in the IgG, IgM, and IgA classes. Additionally, the magnitude of change between acute- and convalescent-phase sera was greater for NGU patients than for normal asymptomatic ureaplasma-positive male controls. A significant change in antibody levels of one or more antibody classes was detected for 12 of 18 (67%) NGU patients by ELISA. Ten of the 12 (83%) individuals had a change in the IgM class, which is suggestive of an active infectious process. The ELISA is advantageous in that it requires only a single serotype antigen, uses one serum dilution, is class specific, and allows quantitative detection of differences between acute- and convalescent-phase sera.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammar A. M., Heitmann J., Kirchhoff H. Untersuchung von Stutenseren auf Antikörper gegen Acholeplasmen und Mykoplasmen mit dem Enzyme Linked Immunosorbent Assay (ELISA). Zentralbl Bakteriol A. 1980;247(4):517–525. [PubMed] [Google Scholar]
- Berntsson E., Broholm K. A., Kaijser B. Serological diagnosis of pneumococcal disease with enzyme-linked immunosorbent assay (ELISA). Scand J Infect Dis. 1978;10(3):177–181. doi: 10.3109/inf.1978.10.issue-3.04. [DOI] [PubMed] [Google Scholar]
- Boothby J. T., Jasper D. E., Rollins M. H., Thomas C. B. Detection of Mycoplasma bovis specific IgG in bovine serum by enzyme-linked immunosorbent assay. Am J Vet Res. 1981 Jul;42(7):1242–1247. [PubMed] [Google Scholar]
- Bruggmann S., Keller H., Bertschinger H. U., Engberg B. Quantitative detection of antibodies to Mycoplasma suipneumoniae in pigs' sera by an ezyme-linked immunosorbent assay. Vet Rec. 1977 Aug 6;101(6):109–111. doi: 10.1136/vr.101.6.109. [DOI] [PubMed] [Google Scholar]
- Busolo F., Tonin E., Conventi L. Enzyme-linked immunosorbent assay for detection of Mycoplasma pneumoniae antibodies. J Clin Microbiol. 1980 Jul;12(1):69–73. doi: 10.1128/jcm.12.1.69-73.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell G. H., Cole B. C. Mycoplasmas as agents of human disease. N Engl J Med. 1981 Jan 8;304(2):80–89. doi: 10.1056/NEJM198101083040204. [DOI] [PubMed] [Google Scholar]
- Horowitz S. A., Cassell G. H. Detection of antibodies to Mycoplasma pulmonis by an enzyme-linked immunosorbent assay. Infect Immun. 1978 Oct;22(1):161–170. doi: 10.1128/iai.22.1.161-170.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. S., Radnay K., Kendrick M. I., Rosner B., Kass E. H. Serologic studies of human genital mycoplasmas: distribution of titers of mycoplasmacidal antibody to Ureaplasma urealyticum and Mycoplasma hominis in pregnant women. J Infect Dis. 1978 Mar;137(3):266–273. doi: 10.1093/infdis/137.3.266. [DOI] [PubMed] [Google Scholar]
- Møller B. R., Mårdh P. A., Ahrons S., Nüssler E. Infection with Chlamydia trachomatis, Mycoplasma hominis and Neisseria gonorrhoeae in patients with acute pelvic inflammatory disease. Sex Transm Dis. 1981 Jul-Sep;8(3):198–202. [PubMed] [Google Scholar]
- Nicolet J., Paroz P., Bruggmann S. Tween 20 soluble proteins of Mycoplasma hyopneumoniae as antigen for an enzyme linked immunosorbent assay. Res Vet Sci. 1980 Nov;29(3):305–309. [PubMed] [Google Scholar]
- Onoviran O., Taylor-Robinson D. Detection of antibody against Mycoplasma mycoides subsp mycoides in cattle by an enzyme-linked immunosorbent assay. Vet Rec. 1979 Aug 25;105(8):165–166. doi: 10.1136/vr.105.8.165. [DOI] [PubMed] [Google Scholar]
- Purcell R. H., Taylor-Robinson D., Wong D., Chanock R. M. Color test for the measurement of antibody to T-strain mycoplasmas. J Bacteriol. 1966 Jul;92(1):6–12. doi: 10.1128/jb.92.1.6-12.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Räisänen S. M., Suni J. I., Leinikki P. Serological diagnosis of Mycoplasma pneumoniae infection by enzyme immunoassay. J Clin Pathol. 1980 Sep;33(9):836–840. doi: 10.1136/jcp.33.9.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard M. C., Lunceford C. D. Serological typing of Ureaplasma urealyticum isolates from urethritis patients by an agar growth inhibition method. J Clin Microbiol. 1978 Nov;8(5):566–574. doi: 10.1128/jcm.8.5.566-574.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson D., Csonka G. W., Prentice M. J. Human intra-urethral inoculation of ureplasmas. Q J Med. 1977 Jul;46(183):309–326. [PubMed] [Google Scholar]
- Taylor-Robinson D., McCormack W. M. The genital mycoplasmas (first of two parts). N Engl J Med. 1980 May 1;302(18):1003–1010. doi: 10.1056/NEJM198005013021805. [DOI] [PubMed] [Google Scholar]
- Thomsen A. C. Mycoplasmas in human pyelonephritis: demonstration of antibodies in serum and urine. J Clin Microbiol. 1978 Aug;8(2):197–202. doi: 10.1128/jcm.8.2.197-202.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


