Abstract
An important enabling factor for realising integrated micro fluidic analysis instruments for medical diagnostics purposes is front-end sample preparation. Dielectrophoresis is a method that offers great potential for cell discrimination and isolation for sample processing, and here we have applied it to the problem of isolating malaria-infected cells from blood. During development of the malarial pathogen, Plasmodium falciparum, increases occur in the ionic permeability of the plasma membrane of infected erythrocytes. When challenged by suspension in a low conductivity medium, infected cells lose internal ions while uninfected cells retain them. The resultant dielectric differences between infected and uninfected cells were exploited by dielectrophoretic manipulation in spatially inhomogeneous, travelling electrical fields produced by two types of microelectrode arrays. Parasitised cells of ring form or later stage from cultures and clinical specimens were isolated by steric dielectric field-flow-fractionation, focused at the centre of a spiral electrode array, and identified and counted. The dielectrophoretic methods require only a few micro litres of blood, and should be applicable to the production of small, low-cost automated devices for assessing parasite concentrations with potential applicability to drug sensitivity studies and the diagnosis of malaria. By simple adjustment of the electrical field parameters, other cell subpopulations that characterise disease, such as residual cancer cells in blood, can be similarly isolated and analysed.
Introduction
Although great progress has been made in the development of micro fluidic molecular analysis methods, the immediate applicability of such methods is limited by the absence of adequate methods to prepare samples. Indeed, sample preparation is a key requirement for the realisation of widely useful labon-a-chip devices. Several laboratories have studied a number of dielectrophoretic techniques for cell manipulation and separation and it appears that dielectrophoresis may provide an ideal tool for interfacing clinical samples to the micro fluidic domain.1-15 In the present study, we have applied dielectrophoresis to malaria, which is estimated to infect between 300 and 500 million people worldwide and to kill between 1.5 and 2.7 million annually.16 The definitive diagnostic indicator of malaria is the presence of parasitised erythrocytes in the blood.17,18 Microscopic inspection of thick and thin blood smears for parasitised cells after Wright-Giemsa staining remains the most commonly applied malaria detection method because it demands no more than a microscope, staining supplies, and a trained technician.17 Several alternative detection methods, such as those using immunological dipsticks and the polymerase chain reaction, are now available.19-24 However, because malaria is most prevalent in rural areas of developing countries, reusable diagnostic instruments that are small, robust and automatic, that are objective rather than subjective, that do not depend upon trained technicians, and that offer very low cost per test would be of great benefit. Such automated devices would, in addition, enable more rapid and cost effective testing of the efficacy and modes of action of antimalarial drugs in the laboratory and thereby facilitate basic malaria research. Micro fluidic approaches would seem to be ideal for these applications.
Here, we report a new approach to the isolation of malaria-parasitised cells that not only can meet these needs but also is applicable for other diseases characterised by the presence of abnormal cells in the blood. Cells having dissimilar dielectric properties will tend to move to a position of minimum dielectric energy if their suspending medium is subjected to an inhomogeneous electrical field. The energy gradient away from this minimum, which is called the dielectrophoretic (DEP) force,25-29 is comprised of two components, F̄DEP = F̄lev + F̄trav, that arise from interactions of field-induced charge polarization in the cells with field inhomogeneneity and field travel, respectively. F̄lev is a force component that acts to attract or repel cells to or from electrode edges, while F̄trav carries cells parallel to electrode surfaces. The DEP force can be approximated in terms of dipolar effects as
| (1) |
where the first and second terms correspond to F̄lev and F̄trav, respectively, V is the particle volume, εm is the electrical permittivity of the suspending medium, and E2 and φx (x = x, y, z) are the magnitude and phase of each field component in a Cartesian co-ordinate frame.27-29 The Claussius-Mossotti charge polarization factor for a spherical particle, given by
| (2) |
approximates the electrical polarization of the cell with respect to its suspending medium. Normal erythrocytes have been modelled using an oblate spheroid model, but as explained previously, this spherical approximation is appropriate for parasitised cell populations in which considerable cell geometry variations occur.30 ε*cell and ε*medium are the complex permittivities of the cell and its medium, respectively.31,32 These take the form ε*x = εx - iσx/2πf where εx is the real dielectric permittivity (dielectric constant), σx is the conductivity, f is the frequency of the applied electrical field, and i = (-1)-1 The real and imaginary components of fCM, Re[fCM] and Im[fCM], couple with the spatial inhomogeneity and travelling components of the applied electrical field to create the DEP forces. These forces depend not only on the geometrical configuration and excitation scheme of the electrode array but also on the dielectric properties of the cell and of its suspending medium.
To manipulate cells, DEP forces are typically produced by applying alternating (AC) electric fields of 10 kHz to 100 MHz in frequency to microfabricated electrode arrays. Fields in this frequency range obviate electrochemical effects that are potentially deleterious to cells33 as well as electroconvective effects that can confound cell separations.34 The polarizability factor, fCM, of cells depends on their specific membrane capacitance and conductivity properties and their internal conductivities. Therefore, the magnitude, direction and frequency dependencies of cellular DEP responses depend on the composition, conformation and barrier function of the cell plasma membranes.35,36 Because cells of different types and of different physiological and pathologic states have unique morphological and structural properties, it is possible to discriminate between them and differentially manipulate them by exploiting their DEP AC field frequency dependencies.1,3,4,37-40 We and others have shown that the electrical conductivity of erythrocyte membranes increases sharply when they become hosts to malarial parasites,30,41 an effect that may be related to the introduction of new membrane permeation pathways42-48 to membrane peroxidation damage,49-51 and to changes in membrane fluidity52-55 following infection. In the present study, these properties were exploited in order to permit the discrimination and isolation of parasitised cells from uninfected cells.
Experimental
Micro fluidic chambers were made by sandwiching a 100 mm thick, moulded silicon-rubber gasket, having a slot in the centre to form a fluid channel, between 50 mm × 25 mm glass slides. Tubes through the chamber tops at opposite ends of the fluid channel allowed sample injection, perfusion with cell-free suspending medium, or flushing. Electrodes on the bottom glass slide were produced photolithographically of 0.2μm thick gold over a 0.1μm titanium seed layer.
To exploit the cellular differences between infected and uninfected cells for detecting malaria by DEP, we used two microelectrode designs. The first was an interdigitated array (Fig. 1A) that was energised by a single-phase sinusoidal generator operated within the frequency range 1 kHz to 5 MHz and up to 5 V peak-to-peak. This design trapped cells having a positive (attractive) DEP response at the electrode tips and repelled cells having a negative (repulsive) DEP response to bay regions between the electrode tips where they were free to be swept away by flow of suspending medium.
Fig. 1.
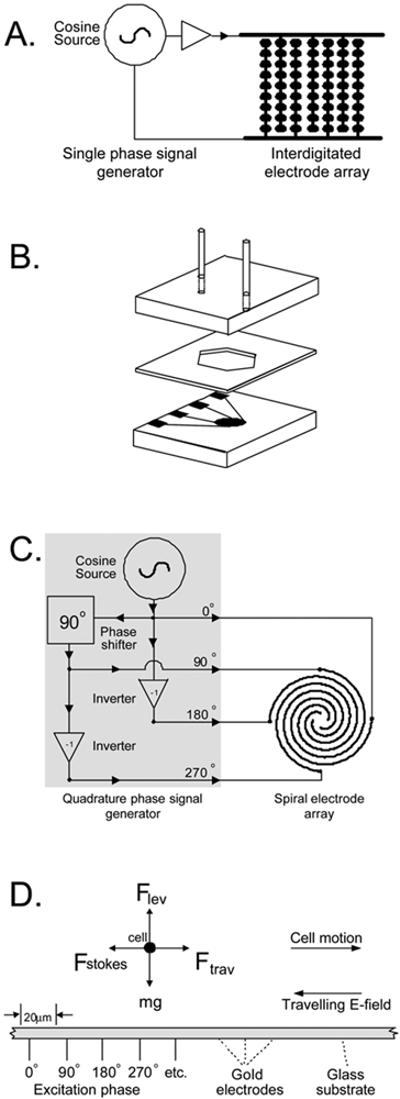
(A) The interdigitated electrode array, of 500 mm2 active area, was comprised of 124 parallel elements having fingers with a characteristic dimension of 100 μm. Alternate elements were connected to bus lines on opposite edges of the chamber. The volume of the interdigitated section of the array was 50 μL. (B) Chamber construction for the DEP measurements. (C) The spiral microelectrode array, of 2 mm2 area, was comprised of 5 complete turns of four parallel spiral elements 20 μm in width and spacing. For clarity only one-and-a-half turns of the spiral array is shown in (B). The active volume of sample above the spiral was 0.2 μL. (D) A cross-section of part of the spiral array showing the dielectrophoretic, gravitational and hydrodynamic forces acting on cells on the spiral electrode.
The second electrode design (Fig. 1C) employed a spiral array56 of four parallel electrodes energised by a quadrature phase sinusoidal generator operating in the frequency range 1 kHz to 15 MHz at up to 5 V peak-to-peak. As shown in Fig. 1C, this design produced both F̄lev and F̄trav DEP force components. Because of the planar nature of the electrodes the F̄lev DEP force component resulted from electrical field inhomogeneities in the vertical dimension and acted either towards or away from high field regions of the electrode plane. At frequencies for which F̄lev was attractive, cells were trapped at electrode edges on the electrode plane; when F̄lev was repulsive, cells were levitated above the electrode plane to a height at which F̄lev was balanced by the sedimentation force, mg. Because the quadrature phase excitation also produced a travelling component of the field, a horizontal F̄trav DEP force component acted on cells paralle to the electrode plane towards or away from the center of the spiral. The velocity with which cells moved in response to F̄trav was limited by Stokes drag.57 F̄Stokes. While cells levitated by F̄lev could be moved by F̄trav, cells trapped by F̄lev could not be moved in this way.
Cell samples from erythrocyte cultures infected with a drugresistant strain of the most common and lethal malarial pathogen, Plasmodium falciparum, and from residual blood samples from a small number of malaria patients were studied. The cultured chloroquine-resistant P. falciparum strain T9/94 RC17 was derived by dilution- and subsequent micromanipulation-cloning of isolate T9 from a patient in Mae Sod, Tak Province, Thailand, by Dr Sodsri Thaithong of the WHO Collaboratory Centre for Biological Characterization of Malarial Parasites, Chulalongkorn University, Bangkok, Thailand. Parasites were cultured in normal (group `O') erythrocytes in human serum-supplemented-RPMI 1640 medium under a 95% air/5% CO2 atmosphere at 37 °C using the methods of Trager and Jensen.58 Percent parasitemia was determined from Giemsa-stained thin smears and maintained between 1% and 5% during culture.
In preparation for DEP analysis, cell cultures were diluted 100-fold with an isotonic buffer of 8.5% sucrose + 0.3% dextrose adjusted with RPMI 1640 medium to target conductivities between 20 and 55 mS m-1 and a cell concentration of 107 ml, then incubated for 10 min. Samples were pre-stained with 25 μg ml-1 3,3′-dihexyloxacarbocyanine iodide (DiOC6 (3)), a cationic membrane-permeable potentiometric probe (Molecular Probes, Eugene, OR, USA), which accumulated in parasites as a result of their trans-membrane potential, rendering them easily detectable by fluorescence microscopy.59-61
Results
Fig. 2 shows the effects of the F̄lev DEP force on normal and parasitised erythrocytes in one field from a total of approximately 5 × 106 erythrocytes injected into a chamber containing an interdigitated electrode excited by an applied AC field of 5 Vp-p at 200 kHz. The frequency is slightly above the frequency at which normal cells were trapped, but not enough to pack the normal cells so that the parasitised cells were entrapped. The normal erythrocytes and peripheral blood mononuclear cells were trapped by positive dielectrophoresis in the inhomogeneous, high field regions between facing, rounded, electrode tips while the parasitised cells were repelled into the gaps. Under these conditions, more than 99.5% of normal erythrocytes were trapped while 90% of parasitised cells remained free to move, which allowed the parasitised cell fraction to be concentrated and washed from the chamber by fluid flow. The relative concentration of parasitised to normal cells was increased between 50- and 200-fold by this process. For example, if the starting proportion of parasitised cells was 0.01%, then a final concentration of approximately 2% parasitised cells could be achieved by this trapping method.
Fig. 2.
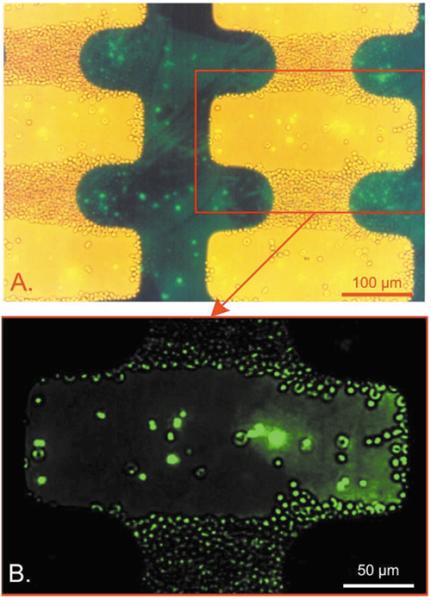
Views of a culture of strain T9/94 RC17 P. falciparum-infected erythrocytes containing approximately 1.1% parasitised cells suspended in 8.5% sucrose + 0.3% dextrose suspending medium on an interdigitated electrode with an applied field of 5 Vp-p at 200 kHz. Approximately 5 × 106 erythrocytes were injected into the chamber. Both low-level bright field and epifluorescence illumination were provided. (A) Cells containing parasites exhibited a green fluorescence due to uptake of the potentiometric dye DiOC6 (3) into the parasite interior from the suspending medium and show brightly in the figure. (B) A magnified view under epifluorescence illumination confirmed that 95% of parasitised cells were repelled from the high field regions and could be washed free by flowing suspending medium.
Fig. 3 shows a field of erythrocytes containing approximately 5% parasitised cells on a spiral electrode array under the same suspending medium and staining conditions used for the interdigitated array experiment of Fig. 2. Approximately 2000 erythrocytes settled onto the 2 mm2 spiral, a portion of which is shown in Fig. 3. Application of four-phase excitation to the arms of the spiral electrode elements of 3 Vp-p at 2 MHz caused normal erythrocytes to be strongly trapped at the electrode edges while the parasitised cells were levitated and carried towards the centre of the spiral by the travelling field. While the cell capacity of the spiral electrode is limited compared with that of the interdigitated array, which is fully scaleable, approximately 90% of the parasitised cells and < 0.1% of the normal cells focused to the centre. Thus, a concentration factor of ~ 1000 fold was achieved. The reason that a higher concentration can be achieved with the spiral configuration than with the interdigitated system is that cells on the spiral array are discriminated by both their DEP attractive and DEP travelling wave properties (see Fig. 4). These properties depend on the real and imaginary parts of the cell dielectric responses, respectively, and are functions of the membrane capacitance, membrane conductivity, and internal conductivity of the cell. Optimum collection of cells by travelling wave DEP occurs when the cells are close to the electrodes plane and the out-of-phase component of cell polarisation is high. The combined result of falling levitation height and falling out-of-phase polarisation compensated one another over a fairly wide range of frequencies for parasitised cells, and collections were performed at 2 MHz, well above frequencies where normal erythrocytes or any other cell types could move.
Fig. 3.
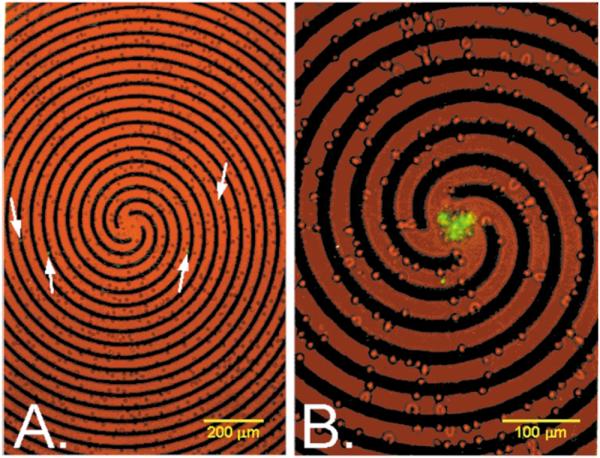
Erythrocytes containing approximately 5% parasitised cells on a spiral electrode array under the same suspending medium and staining conditions used in Fig. 2. Both low-level bright field and epifluorescence illumination were provided. (A) Prior to the application of a travelling electrical field, parasitised cells (arrows) were spread throughout the sample. (B) Application of four phase signals to the spiral electrode elements (3 Vp-p, 2 MHz) caused normal erythrocytes to be trapped at the electrode edges while parasitised cells were levitated and carried towards the centre of the spiral by the travelling field.
Fig. 4.
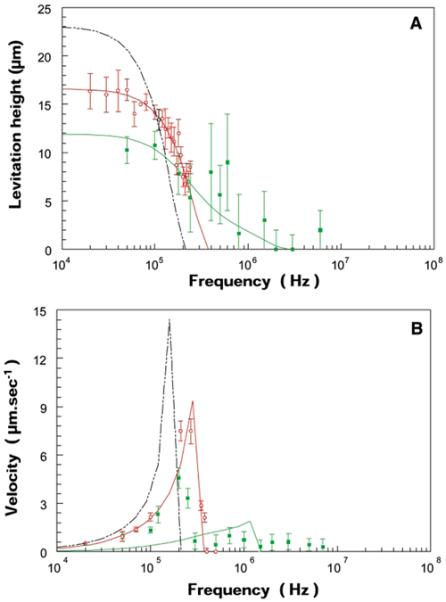
(A) The frequency response of cell levitation above the spiral electrode array was measured by microscope for (○) normal and (■) parasitised erythrocytes for stationary electrical fields (the signals applied to the four arms of the spiral electrode were phased at 0°, 180°, 0°, and 180°, respectively, so that there was no travelling wave component). (B) The frequency dependence of the cell lateral velocity induced by travelling wave DEP when four-phase excitation was established on the spiral electrode. Parasitised cells exhibited a range of frequency responses revealing a large variance in their dielectric characteristics, possibly reflecting inter-cell variations and different stages of parasite development. The solid lines show the levitation and translation behaviours predicted on the basis of the mean cellular dielectric parameters derived in Fig. 5. Spectra for T-lymphocytes (----) calculated from electro rotation data8 (Yang et al., 1999) are shown for comparison.
Owing to their much larger volumes and higher membrane capacitance values compared with erythrocytes, lymphocytes and other peripheral blood mononuclear cells exhibited positive dielectrophoretic responses and were trapped at frequencies below 100 kHz. Therefore, these cells neither emerged from the interdigitated electrode, nor collected at the centre of the spiral under our operating conditions. For this reason, mononuclear cells do not confound the effective isolation of malaria.
Discussion
In order to rationalise the cell collection behaviour in terms of cellular properties, the DEP levitation and trapping responses of normal and parasitised cells were measured as a function of applied field frequency and compared with data for the smallest mononuclear blood cells, i.e., T-lymphocytes,8 see Fig. 4. The frequency-dependent cell collection responses were analysed using shell models31,62 in which cells were approximated as one or more concentric shells each having uniform dielectric properties, with the results shown schematically in Fig. 5. Although oblate spheroid shell models63-65 have been used to approximate normal discoid erythrocytes, the simpler spherical models shown in Fig. 5 were used because of wide variations in the observed sizes and shapes of infected cells, and in the interior location and stage of parasites rendered any geometric model approximate. An iterative algorithm was used to determine the best model parameters by minimizing an error squared cost function9 for given suspension medium properties, mean cell radii, and an assumed thickness of 4.5 nm for all membranes. The electric field distribution used in the analysis was calculated previously for a parallel electrode array.6,7,9 The cell parameters shown in Fig. 5 derived from the analysis revealed that the contrasting behaviour of normal and parasitised cells arose primarily from differences in the ability to retain cytoplasmic ions and, to a much lesser extent, small dielectric contributions from the parasites. Normal cells were able to maintain a high cytoplasmic conductivity when placed in the low conductivity, isotonic suspending medium, but the cytoplasmic conductivity of parasitised cells fell to that of the medium after just 10 min. The observed dielectric differences between normal and parasitised cells were orders of magnitude greater than could be accounted for by the use of spherical rather than oblate spheroid models. For comparison, the dielectric parameters derived from the numerical analysis for normal and parasitised cells are shown in Fig. 5 alongside the experimental data.
Fig. 5.
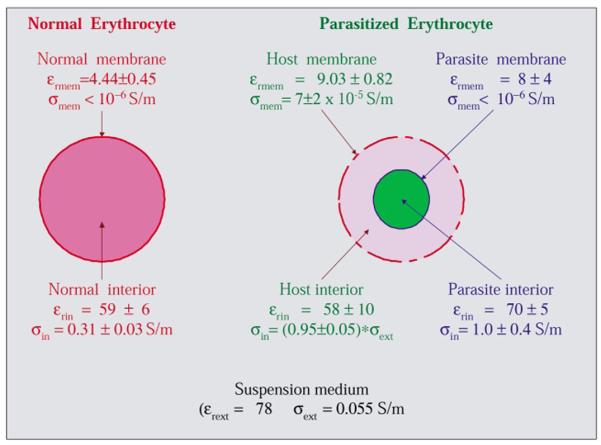
Mean dielectric parameters for normal and parasitised cells derived from iterative fitting of shell models as described in the text. Parasitised cells had a very low internal conductivity compared with normal erythrocytes, an indication they had suffered almost complete loss of ions to the low-conductivity suspending medium. The much higher membrane conductivity of parasitised cells compared with normal cells reflects the lowering of membrane barrier function that accounted for this loss. Despite the ion leakage out of their host cells, the parasites retained their internal ions as reflected by their high internal conductivity.
The mechanism of ion loss in parasitised cells may involve erythrocytic membrane pores expressed by the parasites,42-48 as well as lipid peroxidation,49-51 and fluidity changes known to occur in host cell membranes after infection.52-55 All stages of parasitised cells, with the single exception of the marginal form (a very early stage of infection in which the parasite adheres closely to the cell periphery), lost ions and were amenable to DEP separation. Parasitised cells in the peripheral blood of P. falciparum malaria patients are primarily of ring stage and, therefore, it is highly significant to diagnostic applications that this form was collected by the DEP methods.
The spiral array method is immediately applicable to anti malaria drug testing since most cultures used for this purpose have parasitemias of 0.1-10%. The potential for application to clinical diagnosis is supported by preliminary results from untreated malaria patients in Thailand. Using blood samples with parasitemias ranging from 0.1% to 9.4%, collection of parasitised cells by the spiral method was consistent with our results for cultured samples and the number of parasites collected was proportional to the degree of infection. To detect lower levels of parasitemia and to achieve a sensitivity of 20 parasites per 106 erythrocytes, the interdigitated DEP array, which is scaleable to any required number of cells, would be used to perform pre-concentration of the parasitised cells which would then be further concentrated and analysed on the spiral electrode. Using micro fabrication techniques this could be achieved with a single device having a footprint no larger than a microscope slide. The samples analysed here on the interdigitated array constituted the equivalent of ~ 1 μL of blood, so that a single drop of blood ( ~ 25 μL) is more that sufficient for a specimen. We reused the spiral and interdigitated electrodes for several months, demonstrating that although inexpensive, they are also durable and easily cleaned. The necessary electronics are straightforward and have been assembled on a single 50 mm × 50 mm board powered from a 9 V transistor battery. It is our goal to replace the microscope by an opto-electronic sensor for detecting cells collected in the centre of the spiral. This implies that the method is adaptable to a handheld, reusable instrument, operable by untrained personnel, at low cost per specimen, featuring objective discrimination of the degree of infection based upon the definitive indicator of malaria, namely the direct enumeration of parasitised cells. This sets the method apart from the accepted Wright-Giemsa staining method that is subjective and requires a skilled technician. Also, it is distinct from several new approaches for detection of malaria that can be vulnerable to limitations such as non-quantitative indication of infection and interferences by extraneous agents including residual malaria antibodies and rheumatoid factors.17,20-24
In previous studies we demonstrated that it is possible to discriminate between normal and transformed cells of the same genotype by dielectrophoresis. The basis for cell separation in that case was predominantly membrane capacitance resulting from changes in membrane morphology rather than ion loss. Either mechanism provides adequate changes in cell dielectric responses to allow cell discrimination and separation. Thus, although the focus of this article is malaria, the methods are potentially applicable to other clinical problems including the detection of residual breast cancer and leukaemia cells in blood, and the detection of cells associated with other pathologies such as bacteria in blood and yeast in urine.
Finally, the micro scale methods shown here represent a first successful approach to taking large numbers of human cells and isolating and concentrating those having diagnostic value that are present at concentrations below 1 in > 105 using a single micro device under electronic control. This DEP sample preparation capability is potentially of great value to the next generation of diagnostic devices because it will allow the incorporation, within a single device, of a front-end that executes cell preparation steps followed by chip-scale molecular analysis, e.g., by gene-chip, capillary electrophoresis or chip-scale PCR methods. Integration and automation of these functionalities within a single device under electronic control without the need for human intervention will free molecular diagnostic procedures from the current bonds of clinical infrastructure and make them available at the point of care throughout the world.
Acknowledgements
This work was supported by, and primarily conducted at, the Chulabhorn Research Institute, Bangkok, Thailand. We thank Dr S. Looareesuwan, Dean, Faculty of Tropical Medicine, Mahidol University, Bangkok, for providing samples of blood from untreated malaria patients. We are also grateful to Jaratluck Akanimanee and Jamileh Noshari for cytology and to Xiaobo Wang, Ying Huang, Jody Vykoukal and Jon Schwartz for assistance.
References
- 1.Gascoyne PRC, Huang Y, Pethig R, Vykoukal J, Becker FF. Meas. Sci. Technol. 1992;3:439. [Google Scholar]
- 2.Huang Y, Hölzel R, Pethig R, Wang X-B. Phys. Med. Biol. 1992;37:1499. doi: 10.1088/0031-9155/37/7/003. [DOI] [PubMed] [Google Scholar]
- 3.Gascoyne PRC, Wang X-B, Huang Y, Becker FF. IEEE Trans. Ind. Appl. 1997;33:670. doi: 10.1109/28.585856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker FF, Wang X, Huang Y, Pethig R, Vykoukal J, Gascoyne PRC. Proc. Natl. Acad. Sci. USA. 1995;92:860. doi: 10.1073/pnas.92.3.860. ★★ First demonstration of differential trapping of cells from a cell mixture by dielectrophoresis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X-B, Huang Y, Gascoyne PRC, Becker FF. IEEE Trans. Ind. Appl. 1997;33:660. doi: 10.1109/28.585856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X-B, Vykoukal J, Becker FF, Gascoyne PRC. Biophys. J. 1998;74:2689. doi: 10.1016/S0006-3495(98)77975-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang Y, Wang X-B, Becker FF, Gascoyne PRC. Biophys. J. 1997;73:1118. doi: 10.1016/S0006-3495(97)78144-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J, Huang Y, Wang X-J, Wang X-B, Becker FF, Gascoyne PRC. Biophys. J. 1999;76:3307. doi: 10.1016/S0006-3495(99)77483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J, Huang Y, Wang X-B, Becker FF, Gascoyne PRC. Anal. Chem. 2000;72:832. doi: 10.1021/ac990922o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talary MS, Burt JP, Pethig R. Parasitology. 1998;117(Suppl):S191. doi: 10.1017/s0031182099004126. [DOI] [PubMed] [Google Scholar]
- 11.Goater AD, Pethig R. Parasitology. 1998;117(Suppl):S177. doi: 10.1017/s0031182099004114. [DOI] [PubMed] [Google Scholar]
- 12.Fuhr G, Muller T, Baukloh V, Lucas K. Hum. Reprod. 1998;13(1):136. doi: 10.1093/humrep/13.1.136. [DOI] [PubMed] [Google Scholar]
- 13.Fiedler S, Shirley SG, Schnelle T, Fuhr G. Anal. Chem. 1998;70(9):1909. doi: 10.1021/ac971063b. [DOI] [PubMed] [Google Scholar]
- 14.Morgan H, Hughes MP, Green NG. Biophys. J. 1999;77(1):516. doi: 10.1016/S0006-3495(99)76908-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes MP, Morgan H, Rixon FJ, Burt JP, Pethig R. Biochim. Biophys. Acta. 1998;1425(1):119. doi: 10.1016/s0304-4165(98)00058-0. [DOI] [PubMed] [Google Scholar]
- 16.Butler D. Nature. 1997;386:535. doi: 10.1038/386535a0. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization Weekly Epidemiological Record. 1997;72:38285. [Google Scholar]
- 18.Lalloo D, Naraqui S. Papua New Guinea Med. J. 1992;35:243. ★ Diagnostic methods for malaria. [PubMed] [Google Scholar]
- 19.Makler MT, Palmer CJ, Ager AL. Ann. Trop. Med. Parasitol. 1998;92:419. doi: 10.1080/00034989859401. [DOI] [PubMed] [Google Scholar]
- 20.Jamjoom GA. J. Clin. Microbiol. 1983;17:717. doi: 10.1128/jcm.17.5.717-721.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howard RJ, Uni S, Aikawa M, Aley SB, Leech JH, Lew AM, Wellems TE, Rener J, Taylor DW. J. Cell. Biol. 1986;103:1269. doi: 10.1083/jcb.103.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dietze R, Perkins M, Boulos M, Luz F, Reller B, Corey GR. Am. J. Trop. Med. Hyg. 1995;52(1):45. doi: 10.4269/ajtmh.1995.52.45. [DOI] [PubMed] [Google Scholar]
- 23.Jarra W, Snounou G. Infect. Immun. 1998;66:3783. doi: 10.1128/iai.66.8.3783-3787.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiodini PL. Lancet. 1998;351:80. doi: 10.1016/S0140-6736(05)78155-1. [DOI] [PubMed] [Google Scholar]
- 25.Pohl HA. Dielectrophoresis: the Behavior of Neutral Matter in Nonuniform Electric Fields. Cambridge University Press; Cambridge and New York: 1978. ★ First comprehensive treatment of dielectrophoresis. [Google Scholar]
- 26.Wang X-B, Hughes MP, Huang Y, Becker FF, Gascoyne PRC. Biochim. Biophys. Acta. 1995;1243:185. doi: 10.1016/0304-4165(94)00146-o. [DOI] [PubMed] [Google Scholar]
- 27.Washizu M, Jones TB. J. Electrostat. 1996;37:121. [Google Scholar]
- 28.Wang XJ, Wang X-B, Gascoyne PRC. J. Electrostat. 1997;39:277. [Google Scholar]
- 29.Wang XB, Huang Y, Wang XJ, Becker FF, Gascoyne PRC. Biophys. J. 1997;72:1887. doi: 10.1016/S0006-3495(97)78834-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gascoyne PRC, Pethig R, Satayavivad J, Becker FF, Ruchirawat M. Biochim. Biophys. Acta. 1997;1323:240. doi: 10.1016/s0005-2736(96)00191-5. ★★ Presentation of dielectric properties of malarially-parasitised versus normal cells. [DOI] [PubMed] [Google Scholar]
- 31.Irimajiri A, Hanai T, Inouye A. J. Theor. Biol. 1979;78:251. doi: 10.1016/0022-5193(79)90268-6. [DOI] [PubMed] [Google Scholar]
- 32.Glaser R, Fuhr G. In: Mechanistic Approaches to Interactions of Electric and Electromagnetic Fields with Living Systems. Blank M, Findl E, editors. Plenum Press; New York: 1987. p. 251. [Google Scholar]
- 33.Wang XJ, Yang J, Gascoyne PRC. Biochem. Biphys. Acta. 1999;1426:53. [Google Scholar]
- 34.Green NG, Ramos A, Gonzalez A, Castellanos A. Phys. Rev. E: Stat. Phys. Plasmas, Fluids, Relat. Interdiscip. Top. 2000;61(4 part B):4011, 4019. doi: 10.1103/physreve.61.4011. [DOI] [PubMed] [Google Scholar]
- 35.Pethig R, Kell DB. Phys. Med. Biol. 1987;32:933. doi: 10.1088/0031-9155/32/8/001. [DOI] [PubMed] [Google Scholar]
- 36.Wang X-B, Huang Y, Gascoyne PRC, Becker FF, Holzel R, Pethig R. Biochem. Biphys. Acta. 1994;1193:330. doi: 10.1016/0005-2736(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 37.Wang X-B, Huang Y, Gascoyne PRC, Becker FF. IEEE Trans. Ind. Appl. 1997;33:660. doi: 10.1109/28.585856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Markx GH, Huang Y, Zhou XF, Pethig R. Microbiology. 1994;140:585. [Google Scholar]
- 39.Stephens M, Talary MS, Pethig R, Burnett AK, Mills KI. Bone Marrow Transplant. 1996;18:777. [PubMed] [Google Scholar]
- 40.Fuhr G, Schnelle Th., Müller T, Glasser H, Lisec Th., Wagner B. Sens. Mater. 1995;7:131. [Google Scholar]
- 41.Aceti A, Bonincontro A, Cametti C, Celestino D, Leri O. Trans. R. Soc. Trop. Med. Hyg. 1990;84:671. doi: 10.1016/0035-9203(90)90140-a. [DOI] [PubMed] [Google Scholar]
- 42.Cabantchik ZI. Blood Cells. 1990;16:421. [PubMed] [Google Scholar]
- 43.Elford BC, Cowan GM, Ferguson DJP. Biochem. J. 1995;308:361. doi: 10.1042/bj3080361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dluzewski AR, Fryer PR, Griffiths S, Wilson RJM, Gratzer WB. J. Cell Sci. 1989;92:691. doi: 10.1242/jcs.92.4.691. [DOI] [PubMed] [Google Scholar]
- 45.Kutner S, Baruch D, Ginsburg H, Cabantchik ZI. Biochim. Biophys. Acta. 1982;687:113. doi: 10.1016/0005-2736(82)90178-x. [DOI] [PubMed] [Google Scholar]
- 46.Ginsburg H, Kutner S, Krugliak M, Cabantchik ZI. Mol. Biochem. Parasitol. 1985;14:313. doi: 10.1016/0166-6851(85)90059-3. [DOI] [PubMed] [Google Scholar]
- 47.Ginsburg H. Comp. Biochem. Physiol. 1990;95A:31. [Google Scholar]
- 48.Zanner MA, Galey WR, Scaletti JV, Brahm J, Vander Jagt DL. Mol. Biochem. Parasitol. 1990;40:269. doi: 10.1016/0166-6851(90)90048-q. [DOI] [PubMed] [Google Scholar]
- 49.Mohan K, Ganguly NK, Dubey ML, Mahajan RC. Ann. Hematol. 1992;65:131. doi: 10.1007/BF01695812. [DOI] [PubMed] [Google Scholar]
- 50.Das BS, Nanda NK. Trans. R.. Soc. Trop. Med. Hyg. 1999;93:58. doi: 10.1016/s0035-9203(99)90180-3. [DOI] [PubMed] [Google Scholar]
- 51.Senok NC, Li K, Nelson EA, Arumanayagam M, Li CK. Parasitology. 1998;116:1. doi: 10.1017/s0031182097001911. [DOI] [PubMed] [Google Scholar]
- 52.Hebbel RP, Mohandas N. Biophys. J. 1991;60:712. doi: 10.1016/S0006-3495(91)82100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sugihara T, Rawicz W, Evans EA, Hebbel RP. Blood. 1991;77:2757. [PubMed] [Google Scholar]
- 54.Atamna H, Ginsburg H. Eur. J Biochem. 1997;250:670. doi: 10.1111/j.1432-1033.1997.00670.x. [DOI] [PubMed] [Google Scholar]
- 55.Postma NS, Mommers EC, Eling W, Zuidema J. Pharm. World Sci. 1996;18:121. doi: 10.1007/BF00717727. [DOI] [PubMed] [Google Scholar]
- 56.5,858,192 US Patent. 1999
- 57.Wang X-B, Huang Y, Wang X-J, Becker FF, Gascoyne PRC. Biophys. J. 1997;72:1887. doi: 10.1016/S0006-3495(97)78834-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trager W, Jensen JB. Science. 1976;193:673. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 59.Mikkelson RB, Wallach DFH, Van Doren E, Nillni E. Mol. Biochem. Parasitol. 1986;21:83. doi: 10.1016/0166-6851(86)90082-4. [DOI] [PubMed] [Google Scholar]
- 60.Mikkelson RB, Tanale K, Wallach FH. J. Cell. Biol. 1982;93:685. doi: 10.1083/jcb.93.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jacobberger JN, Horan PK, Hare JD. Cytometry. 1983;4(22):8. doi: 10.1002/cyto.990040307. [DOI] [PubMed] [Google Scholar]
- 62.Marzalek P, Zielinski JJ, Fikus M, Tsong TY. Biophys. J. 1991;59:982. doi: 10.1016/S0006-3495(91)82312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Asami K, Yonezawa T. Biochim. Biophys. Acta. 1995;1245:317. doi: 10.1016/0304-4165(95)00116-6. [DOI] [PubMed] [Google Scholar]
- 64.Kakutani T, Shibatani S, Sugai M. Bioelectrochem. Bioeng. 1993;31:131. [Google Scholar]
- 65.Miller RD, Jones TB. Biophys. J. 1993;64:1588. doi: 10.1016/S0006-3495(93)81529-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


