Abstract
Dielectrophoretic field-flow-fractionation (DEP-FFF) was used to purge human breast cancer MDA-435 cells from hematopoietic CD34+ stem cells. An array of interdigitated microelectrodes lining the bottom surface of a thin chamber was used to generate dielectrophoretic forces that levitated the cell mixture in a fluid flow profile. CD34+ stem cells were levitated higher, were carried faster by the fluid flow, and exited the separation chamber earlier than the cancer cells. Using on-line flow cytometry, efficient separation of the cell mixture was observed in less than 12 min, and CD34+ stem cell fractions with a purity >99.2% were obtained. The method of DEP-FFF is potentially applicable to many biomedical cell separation problems, including microfluidic-scale diagnosis and preparative-scale purification of cell subpopulations.
INTRODUCTION
AUTOLOGOUS STEM CELL TRANSPLANTATION for recon-stituting a cancer patient's hematopoietic system following dose-intensive chemotherapy and radiation therapy can be confounded by metastatic tumor cell contamination of the graft, which may cause disease recurrence (1-5). Currently, tumor cells are purged from autografts by pharmacologic, immunologic, or biophysical methods (6) or by positive selection of hematopoietic stem cells (HSC) with CD34 antibody-sensitized magnetic beads (7,8). Despite the high efficiency achieved with these methods, tumor cells can still be detected in the purified autografts (9,10). For this reason and for a variety of other biomedical problems, such as the purification of blood cell subpopulations, new cell separation methods are needed to improve separation resolution and cell purity. Here, we describe a dielectrophoretic field-flow-fractionation (DEP-FFF) technique in which cells are separated according to their dielectric properties, and we demonstrate that for an experimental model comprising a mixture of CD34+ stem cells and human breast cancer MDA-435 cells, DEP-FFF separation produces stem cell fractions having a purity > 99.2%.
Dielectrophoresis (DEP), the movement of cells in a nonuniform electric field, is being developed for cell manipulation and separation (11-21). DEP separation exploits the finding that cells of different types or in different physiologic states possess distinct dielectric properties, allowing them to be subjected to differential DEP forces. Three DEP separation approaches have been developed: DEP migration, DEP retention, and DEP-FFF. DEP migration uses DEP forces in opposite directions to drive different cell types to different regions of a microelectrode structure (12-14), and DEP retention exploits the competition between DEP and fluid-flow forces to selectively trap and immobilize target cells (15-19). These two approaches have been demonstrated as enriching CD34+ stem cells from mobilized blood samples and for separating leukemia, breast, and cervical carcinoma cells and Escherichia coli bacteria from normal blood cells (15-16,18-21). In DEP-FFF, cell separation is achieved by equilibrating cells at different heights in a fluid-flow profile under opposing DEP and gravitational forces. The fluid flow then carries them at velocities corresponding to the heights (22-26). Unlike DEP migration and retention, which are essentially planar phenomena prone to entrapment of dissimilar cell types, DEP-FFF exploits both horizontal and vertical forces and uses three dimensions. In this way, DEP-FFF offers potentially high discrimination for the fractionation of cell samples.
Here we show the differences in DEP-FFF responses of CD34+ stem cells and breast cancer cells, describe two separation strategies, analyze the speed and purity of separations, and discuss the basis for optimizing separation conditions.
MATERIALS AND METHODS
Cells
MDA-435 cells (27,28) were cultured in minimum essential/F12 medium containing 10% FBS, 1 mM gluta-mine, 20 mM HEPES, and 0.5% penicillin and streptomycin (15,19). Cells were harvested at 80% confluence 48 h after seeding by brief exposure to 0.25% trypsin/0.02% EDTA, washed once, and resuspended in isotonic 8.5% sucrose/0.3% dextrose (w/w) buffer at 1.5 × 106 cells/ml. The electrical conductivity of the sucrose buffer was adjusted to 10 mS/m with the culture medium.
Peripheral mononuclear cell (PMNC) samples were collected using a COBE Spectra Version 4.7 cell separator (COBE BCT, Inc., Lakewood, CO) from patients mobilized with recombinant human G-CSF (Amgen Corp., Thousand Oaks, CA) at a dose of 5 μg/kg given S.C. twice daily. CD34+ stem cells (90%-95%) were then purified by magnetic-activated cell sorting (Miltenyi Biotech, Auburn, CA) according to a Miltenyi protocol. To allow fluorescent identification, CD34+ cells were stained with PE-conjugated anti-HPCA-2 CD34 antibodies (Becton Dickinson, San Jose, CA) by adding antibodies at 20 μl per 106 cells and incubating the mixture for 30 min at 4°C in the dark. Labeled CD34+ cells were washed once and resuspended in sucrose buffer at 1.5 × 106 cells/ml. CD34+ cells and MDA-435 cells were mixed in sucrose buffer at a ratio of 3:2.
DEP-FFF system
The experimental setup is shown in Figure 1. Inter-digitated electrodes, 50 μm in width and spacing, were fabricated on 50 × 50 mm glass substrates using standard photolithography (23). Eight electrodes were glued end-to-end onto a supporting glass plate to form a long electrode that acted as the bottom wall of the separator. A Teflon spacer (H 0.42 × W 50 × L 400 mm) sandwiched between the long electrode and a top glass plate comprised the separation chamber. A slot in the spacer provided a separation channel 388 mm long from tip to tip and 25 mm wide excluding tapered ends. The micro-electrodes were connected in parallel to a laboratory-built power amplifier. Tubing was glued into 1.6-mm holes drilled in the chamber top and bottom walls to form inlet and outlet ports. A 5-cm long section of PEEK tubing (0.01 inch ID) (Upchurch Scientific, Oak Harbor, WA) served as the connection to an injection valve (Rheodyne Model 7010) (Rheodyne, Cotati, CA) that was equipped with a 50 μl loop. A digital syringe pump (Daigger, Wheeling, IL) provided continuous flow of carrier medium through the chamber. Cells exiting the bottom outlet port were fed directly into the injection needle of a flow cytometer (Bio-Rad, Hercules, CA) for detection. To reduce unnecessary cytometer flow volume and to allow DEP-FFF operation at flow rates higher than the maximum operable with the cytometer (~0.2 ml/min), a second syringe pump connected to the top outlet port withdrew 90% of the total flow through the chamber. Thus, 10% of the infused fluid, corresponding to a height of 80 μm in the parabolic flow profile in the chamber, was eluted from the bottom port to the cytometer and included all the cells because the maximum levitation for individual CD34+ and MDA-435 cells measured under the light microscope was <65 μm.
FIG. 1.

DEP-FFF experimental setup. Microfabricated electrodes on the bottom wall of the separation chamber were energized with electrical signals and provided DEP levitation forces. After being introduced to the chamber through the injection valve, the cells of different types in a mixture were levitated to different equilibrium heights under the balance of DEP and sedimentation forces. A fluid-flow profile was produced in the chamber from the injection syringe pump. The cells were transported through the chamber at different velocities corresponding to their heights, exited the chamber from the bottom outlet port, and were detected by the flow cytometer.
DEP-FFF operation
After loading the DEP-FFF chamber with sucrose buffer, a 50 μl cell mixture was injected into the chamber as described previously (25). A 4-V p-p, 10-kHz signal was applied to the electrodes during injection so that DEP forces would levitate cells and prevent them from adhering to the chamber bottom. Cells were allowed 5 min to sediment to appropriate heights under the DEP signal before the flow of the carrier medium through the chamber was initiated by starting the injection and withdrawal syringe pumps at 2 and 1.8 ml/min, respectively. Because the chamber thickness was much smaller than its width and length, an essentially vertical parabolic flow profile was established inside the chamber. With appropriate electric signals (40 kHz in a trap-and-release protocol or 15-35 kHz in a swept-frequency protocol) applied, CD34+ cells and MDA-435 cells were levitated to different heights in the flow profile and, therefore, transported at different velocities. Cells exiting the chamber were identified and counted by the cytometer, which recorded time of arrival, fluorescence, and forward and side light scatter for each cell.
RESULTS
DEP-FFF responses
Cell DEP behaviors depend sensitively on the frequency of the applied electrical field (12-19). Therefore, to establish suitable cell separation conditions, we measured the DEP-FFF responses of MDA-435 cells and CD34+ cells separately as a function of frequency. Figure 2 shows cell elution fractograms for the two populations. For MDA-435 cells at 10 kHz, cell elution spanned 3-8 min and peaked at 6 min. With increasing frequency, the elution profile rapidly broadened, and no obvious peak was observed at frequencies >15 kHz. Cell elution number decreased by ~60% at 20 kHz, indicating that many cells were trapped in the chamber by DEP forces. This was confirmed by microscopic inspection of the chamber and by the release of cells when the frequency was lowered to 5 kHz. In striking contrast to MDA-435 cells, CD34+ cells exhibited DEP-FFF fractograms with single, narrow peaks. Between 10 and 40 kHz, CD34+ cells were eluted in 4-7 min, with peaks at ~4.5 min. With increasing frequency, the elution fractogram gradually broadened, and the peak position shifted to ~8 min at 60 kHz. These differences in responses suggested that MDA-435 cells should be separable from CD34+ cells at frequencies between 20 and 50 kHz.
FIG. 2.
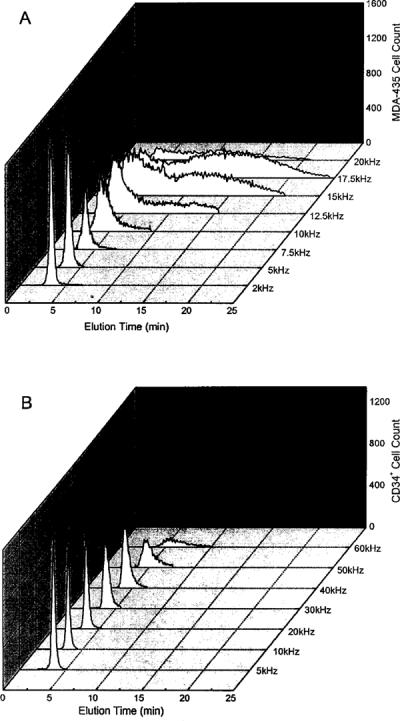
Frequency dependency of DEP-FFF elution fractograms for (A) MDA-435 human breast cancer cells and (B) CD34+ stem cells obtained by the flow cytometer. Cells were suspended at 1.5 × 106/ml in the sucrose buffer having an electrical conductivity of 10 mS/m. The applied voltage was 4 V p-p. The injection and withdrawal syringe pumps were operated at 2 and 1.8 ml/min, respectively.
Cell separations
In the trap-and-release protocol, a 40-kHz DEP field was applied when the fluid flow was started. CD34+ cells were levitated and carried by the fluid flow, whereas MDA-435 cells were trapped by DEP forces. This frequency was maintained for 7 min, during which the majority of CD34+ cells were eluted from the chamber. The frequency was then switched to 5 kHz to cause levitation and elution of the MDA-435 cells. A typical fractogram for this method is shown in Figure 3. As identified by fluorescence and light-scatter measurements in the flow cytometer, the elution peak between 4.5 and 5.5 min contained 99.5% CD34+ cells, and the peak between 9 and 12 min contained 96% MDA-435 cells.
FIG. 3.
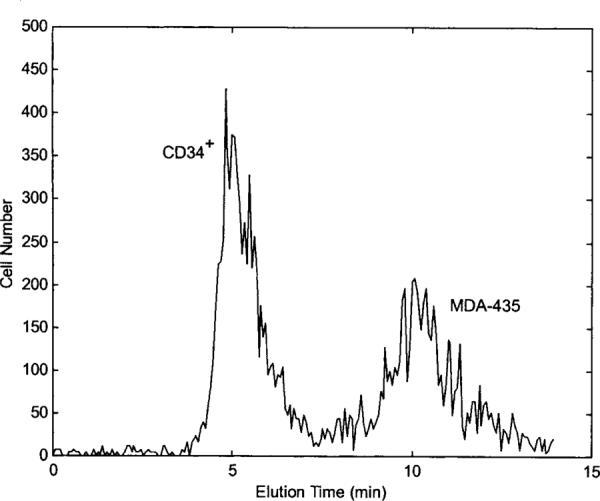
DEP-FFF fractograms for separating MDA-435 cells from CD34+ cells using the trap-and-release protocol. DEP field was operated at 40 kHz for 7 min and switched to 5 kHz for 7 min. CD34+ cells were prelabeled with PE-conjugated CD34 antibodies and were identified by flow cytometer to elute the chamber earlier than MDA-435 cells. DEP signal voltage and fluid-flow conditions were the same as those used for Figure 2.
In the swept-frequency protocol, the field frequency was swept between 15 and 35 kHz repetitively at a 5-sec period after the fluid flow was commenced. The swept-frequency was applied for 7 min, and CD34+ cells were strongly levitated and quickly eluted from the chamber. MDA-435 cells were levitated very slightly by the swept-frequency field and moved slowly through the chamber. As in the trap-and-release protocol, the frequency was finally switched to 5 kHz to elute remaining cells in the chamber. Results for this protocol are depicted in Figure 4A, which shows the separation was completed within 12 min and the elution peak difference between the two cell types was >5 min. Flow cytometry showed that the elution peak between 3 and 4 min contained 99.2% CD34+ cells and the peak between 7 and 10 min comprised 99% MDA-435 cells.
FIG. 4.

(A) DEP-FFF fractogram for separating MDA-435 cells from CD34+ cells by the swept-frequency protocol. (B) Corresponding contour plot for fluorescence level vs. elution time for cells that exited the DEP-FFF chamber. The DEP field was swept between 15 and 35 kHz for 7 min and then switched to 5 kHz for 7 min. DEP signal voltage and fluid-flow conditions were the same as those used in Figure 2.
A contour plot of fluorescence vs. time for the cells, as measured by the flow cytometer, is shown in Figure 4B. CD34+ cells, having fluorescence because of CD34-PE labeling, exited the separation chamber earlier than the unstained MDA-435 cells. The small cluster of unstained cells that exited the chamber before the CD34+ cells comprised cell debris, as revealed by their low light scattering (data not shown).
In some experiments, a fraction collector was used to collect separated fractions for further analysis. More than 95% of the cells excluded trypan blue dye, indicating that membrane integrity was maintained. This result, together with previous cell growth studies following AC field exposure (16,29), shows that cell viability is not seriously affected by DEP-FFF separation.
DISCUSSION
Cell DEP-FFF responses and membrane dielectric properties
In DEP-FFF, a balance between DEP levitation and gravitational forces positions cells at equilibrium heights within a fluid-flow profile that then determines their velocities and corresponding elution times (23,25,26). Cell elution time depends on the voltage of the applied DEP field, electrode geometry, cell dielectric polarization factor αDEP, and cell density. Because MDA-435 and CD34+ cells have similar densities (between 1.070 and 1.080 kg/L), the difference in the frequency dependency of DEP-FFF elution fractograms (Fig. 2) of these two cell types must have reflected differences in αDEP values. For CD34+ cells, the gradual increase in elution times between 10 and 60 kHz suggested a gradual drop in αDEP. On the other hand, the elution time for MDA-435 cells rapidly increased with frequency, suggesting a sharper decrease in αDEP.
To confirm this, dielectric properties of the cells were determined from electrorotation (ROT) measurements in which cells were caused to rotate by a rotating electrical field as a result of induced dielectric polarization (30,31). Rotational rates were measured as a function of frequency, and the resulting spectra (Fig. 5A) were fitted with the single-shell dielectric model (30,31) to derive their dielectric parameters (Table 1). Frequency dependencies of the polarization factor αDEP calculated from the mean dielectric parameters, are shown in Figure 5B. Whereas αDEP varied from -0.5 to -0.3 as the field frequency increased from 5 to 50 kHz for CD34+ cells, it changed from -0.4 to 0 in the frequency range of 5 to 15 kHz for MDA-435 cells. These findings support the DEP-FFF conclusions regarding cell dielectric responses.
FIG. 5.
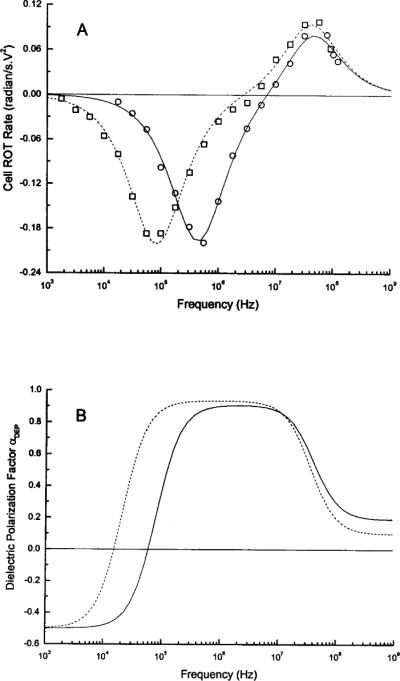
(A) Typical electrorotation spectra for CD34+ (circles) and MDA-435 (squares) cells in the sucrose buffer having a conductivity of 56 mS/m. Continuous curves show best fit of the single-shell dielectric model (30,31). (B) The frequency spectra of αDEP for CD34+ (solid) and MDA-435 (broken line) cells under separation conditions (conductivity 10 mS/m) calculated using the dielectric parameters (Table 1) derived from ROT measurements.
Table 1.
Dielectric Parameters for MDA-435 and CD34+ Cellsa
| Cell type | Cspecificb mF/m2 | σint S/m | ϵint |
|---|---|---|---|
| MDA-435 | 23.0 ± 7.1c | 0.55 ± 0.10 | 107.0 ± 29.5 |
| CD34+ | 10.2 ± 1.5 | 0.71 ± 0.11 | 141.2 ± 28.0 |
The parameters were determined for individual cells by fitting the single-shell dielectric model (30,31) to the measured electrorotation spectra. Membrane conductance values were so small that they did not contribute significantly the measured electrorotation responses and could not be determined accurately.
Cspecific, specific membrane capacitance; σint, internal conductivity; ϵmt internal permitivity
Mean ± SD for more than 30 cells for each type.
It is well established that below 1 MHz, the dielectric polarization factor αDEP is determined by electrical properties of the cell membrane (30-32). Indeed, the observed difference in the αDEP characteristics of the two cell types at frequencies below 100 kHz is caused by different specific membrane capacitances of 10.2 ±1.5 and 23.0 ± 7.1 mF/m2 for CD34+ and MDA-435 cells, respectively. We showed previously that membrane capacitance is closely associated with membrane morphologic characteristics that depend on cell types and physiologic states, such as cell cycle and transformation status (31,33,34). Therefore, the DEP-FFF separation of CD34+ and MDA-435 cells described here exploited differences in cell membrane properties.
The elution peak of MDA-435 cells also broadened much more rapidly with increasing frequency than that of CD34+ cells (Fig. 2). The spreading of elution peaks occurs when cells of the same type exhibit different DEP-FFF velocities because of the inherent population heterogeneity in the cell dielectric and density properties (23,26). MDA-435 cells were harvested in the midlog phase of growth and were, therefore, heterogeneous with respect to cell cycle phases, compared with the resting CD34+ cell population. Furthermore, it is shown that DEP-FFF velocity (and elution time) is very sensitive to cell dielectric properties when the dielectric factor αDEP is close to zero (23). The factor αDEP approached zero around 15 kHz for MDA-435 cells but remained well below zero (< -0.3) at frequencies up to 50 kHz for CD34+ cells. The combination of a large variation in membrane capacitance (Table 1) together with the fact that αDEP approached zero above 10 kHz can, therefore, account for the rapid spread of elution times for MDA-435 cells.
Separation approaches
The DEP crossover frequencies for MDA-435 and CD34+ cells were ~10 kHz and ~65 kHz, respectively (Fig. 5B). Thus, at 40 kHz, MDA-435 cells experienced positive DEP forces, tending to be trapped at the electrode edges, whereas CD34+ cells were levitated by negative DEP forces and transported by the fluid flow. After CD34+ cells were eluted, the frequency was switched to 5 kHz to release and cause fast elution of MDA-435 cells. This ability to program the DEP field for enhancing separation performance is an important advantage of DEP-FFF. Whereas CD34+ cell fractions with a purity ~99.5% were achieved with this trap-and-release protocol, MDA-435 cells were trapped at the electrode edges during the trap phase. In some applications, direct cell-electrode contact may not be ideal because some cell types might nonspecifically and irreversibly bind to the electrodes or substrate, electronically trapped cells may experience undesirably large electrical field stresses in the strong field regions close to the electrode plane, and cell population heterogeneity might result in a small untrapped fraction that moves in the fluid flow and causes broadening of the final elution peak of the initially trapped cell fraction. We, therefore, developed a swept-frequency protocol in which the DEP field was linearly swept between 15 and 35 kHz. In this case, CD34+ cells were still separated with a purity of 99.2%, but by effectively averaging the DEP forces over a broad frequency band, the protocol greatly reduced the number of MDA-435 cells that were trapped at the electrodes. Instead, most were slightly levitated and carried slowly by the fluid flow. As a result, the elution profile for the heterogeneous MDA-435 population was more compact.
Both protocols produced CD34+ cell fractions having purities >99%. We believe that the small impurity in the separated CD34+ fractions is associated with impure PMNC (5%-10%) present in the original CD34+ samples because these were prepared using magnetic-activated cell sorting with a CD34+ yield of 90%-95%. PMNC, especially lymphocytes, have similar DEP-FFF responses to CD34+ cells (35) and are expected to elute with the CD34+ cells under our conditions but be detected as CD34-.
DEP-FFF may be applied to many clinical and biomedical cell separate problems, where it has several inherent advantages over other methods. Cell discrimination ability can be electronically controlled for DEP-FFF by varying the applied electrical signals and programming them to achieve desired separation performances. Cell modification by stains or antibodies is unnecessary, making DEP-FFF particularly attractive to applications were unmodified cells are desirable or specific antibodies or markers are not available. Separated cells are viable and can be cultured for further studies, such as diagnostic analyses by molecular methods. Finally, DEP-FFF is applicable to a wide range of sample sizes from preparative-scale purification of cell subpopulations, handling several million cells at a time, to microfluidic devices that can be integrated with analytic components, such as a micro-PCR and capillary electrophoresis devices.
ACKNOWLEDGMENTS
We are grateful to J. Noshari and C. Joyce for cell culture, to T. Anderson for help in construction the DEP-FFF chamber, and to Dr. M. Donato and J. Lauppe for providing leukapheresis samples as well as helpful advice in handling and detecting CD34+ cells. We thank Drs. G. De Gasperis, M. Cristofanili, X. J. Wang, and J. Vykoukal for valuable discussions. This work is supported in part by a research contract from the Electronics Technology Office of the Defense Advanced Research Program Agency (NRaD Contract N66001-97-C-8608 under DARPA Order E934), by an NIH grant R01 DK51065-01 from the National Institute of Diabetes and Digestive and Kidney Disease, and by an Advanced Research Project grant from the Higher Education Coordinating Board of the State of Texas.
REFERENCES
- 1.Gribben JG, Freedman AS, Neuberg D, Roy DC, Blake KW, Woo SD, Grossbard ML, Rabinowe SN, Coral F, et al. Immunologic purging of marrow assessed by PCR before autologous bone marrow transplantation for B-cell lymphoma. N Engl J Med. 1991;325:1525–1533. doi: 10.1056/NEJM199111283252201. [DOI] [PubMed] [Google Scholar]
- 2.Collins RH., Jr. CD34+ selected cells in clinical transplantation. Stem Cells. 1993;12:577–585. doi: 10.1002/stem.5530120605. [DOI] [PubMed] [Google Scholar]
- 3.Ross AA, Coope BW, Lazarus HM, et al. Detection and viability of tumor cells in peripheral blood stem cell collections from breast cancer patients using immunocytochemical and clonogenic assay techniques. Blood. 1993;82:2605–2610. [PubMed] [Google Scholar]
- 4.Rummel SA, VanZant G. Future paradigm for autologous bone marrow transplantation: tumor purging and ex vivo production of normal stem and progenitor cells. J Hematother. 1994;3:213–218. doi: 10.1089/scd.1.1994.3.213. [DOI] [PubMed] [Google Scholar]
- 5.Santos GW. The role of autologous hematopoietic stem cell transplantation in hematologic malignancy. Curr Opin Oncol. 1994;6:115–121. doi: 10.1097/00001622-199403000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Crouch MA, Ross JA. Current concepts in autologous bone marrow transplantation. Semin Oncol Nurs. 1994;10:12–19. doi: 10.1016/s0749-2081(05)80041-5. [DOI] [PubMed] [Google Scholar]
- 7.Krause DS, Fackler MJ, Civin CI, May WS. CD34: structure, biology, and clinical utility. Blood. 1996;87:1–13. [PubMed] [Google Scholar]
- 8.McNiece I, Briddell R, Stoney G, Kern B, Zilm K, Recktenwald D, Miltenyi S. Large-scale isolation of CD34+ cells using the Amgen cell selection device results in high levels of purity and recovery. J Hemotother. 1997;6:5–11. doi: 10.1089/scd.1.1997.6.5. [DOI] [PubMed] [Google Scholar]
- 9.Shpall EJ, Jones RB, Bearman SI, et al. Transplantation of enriched CD34-positive autologous marrow into breast cancer patients following high-dose chemotherapy: influence of CD34-positive peripheral-blood progenitors and growth factors on engraftment. J Clin Oncol. 1994;12:28–36. doi: 10.1200/JCO.1994.12.1.28. [DOI] [PubMed] [Google Scholar]
- 10.Mapara MY, Körner IJ, Hilderbrandt M, Bargou R, Krahl D, Reichardt P, Dörken B. Monitoring of tumor cell purging after highly efficient immunomagnetic selection of CD34 cells from leukapheresis products in breast cancer patients: comparison of immunocytochemical tumor cell staining and reverse transcriptase-polymerase chain reaction. Blood. 1997;89:337–344. [PubMed] [Google Scholar]
- 11.Pohl H. Dielectrophoresis. Cambridge University Press; Cambridge, UK: 1978. [Google Scholar]
- 12.Gascoyne PRC, Huang Y, Pethig R, Vykoukal J, Becker FF. Dielectrophoretic separation of mammalian cells studied by computerized image analysis. Meas Sci Technol. 1992;3:439–445. [Google Scholar]
- 13.Wang X-B, Huang Y, Burt JPH, Markx GH, Pethig R. Selective dielectrophoretic confinement of bioparticles in potential energy well. J Physiol [D] Appl Physiol. 1993;26:1278–1285. [Google Scholar]
- 14.Markx GH, Huang Y, Zhou XF, Pethig R. Dielectrophoretic characterization and separation of microorganisms. Microbiology. 1994;140:585–591. [Google Scholar]
- 15.Becker FF, Wang X-B, Huang Y, Pethig R, Vykoukal J, Gascoyne PRC. The removal of human leukemia cells from blood using interdigitated microelectrodes. J Physiol [D] Appl Physiol. 1994;27:2659–2662. [Google Scholar]
- 16.Becker FF, Wang X-B, Huang Y, Pethig R, Vykoukal J, Gascoyne PRC. Separation of human breast cancer cells from blood by differential dielectric affinity. Proc Natl Acad Sci USA. 1995;92:860–864. doi: 10.1073/pnas.92.3.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Markx GH, Pethig R. Dielectrophoretic separation of cells: continuous separation. Biotech Bioeng. 1995;45:337–343. doi: 10.1002/bit.260450408. [DOI] [PubMed] [Google Scholar]
- 18.Stephens M, Talary MS, Pethig R, Burnett AK, Mills KI. The dielectrophoresis enrichment of CD34+ cells from peripheral blood stem cell harvests. Bone Marrow Transplant. 1996;18:777–782. [PubMed] [Google Scholar]
- 19.Gascoyne PRC, Wang X-B, Huang Y, Becker FF. Dielectrophoretic separation of cancer cells from blood. IEEE Trans Ind Appl Soc. 1997;33:670–678. doi: 10.1109/28.585856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng J, Sheldon EL, Wu L, Heller MJ, O'Connell JP. Isolation of cultured cervical carcinoma cells mixed with peripheral blood cells on a bioelectronic chip. Anal Chem. 1998;70:2321–2326. doi: 10.1021/ac971274g. [DOI] [PubMed] [Google Scholar]
- 21.Cheng J, Sheldon EL, Wu L, Uribe A, Gerrue LO, Carrino J, Heller MJ, O'Connell JP. Preparation and hybridization analysis of DNA/RNA from E. coli on microfabricated bioelectronic chips. Nature Biotechnol. 1998;16:541–546. doi: 10.1038/nbt0698-541. [DOI] [PubMed] [Google Scholar]
- 22.Gascoyne PRC, Huang Y, Wang X, Yang J, DeGasperis G, Wang X-B. Cell separation by conventional dielectrophoresis combined with field-flow-fractionation. Biophys J. 1996;70:A330. [Google Scholar]
- 23.Huang Y, Wang X-B, Gascoyne PRC, Becker FF. Introducing dielectrophoresis as a new force field for field-flow-fractionation. Biophys J. 1997;73:1118–1129. doi: 10.1016/S0006-3495(97)78144-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Markx GH, Rousselet J, Pethig R. DEP-FF: field-flow-fractionation using nonuniform electrical fields. J Liq Chrom Rel Technol. 1997;20:2857–2872. [Google Scholar]
- 25.Wang X-B, Vykoukal J, Becker FF, Gascoyne PRC. Separation of polystyrene beads using dielectrophoretic/gravitational field-flow-fractionation. Biophys J. 1998;74:2689–2701. doi: 10.1016/S0006-3495(98)77975-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang J, Huang Y, Wang X-B, Becker FF, Gascoyne PRC. Cell separation on microfabricated electrodes using dielectrophoretic/gravitational field-flow-fractionation. Anal Chem. 1999;71:911–918. doi: 10.1021/ac981250p. [DOI] [PubMed] [Google Scholar]
- 27.Cailleau R, Oliver M, Cruciger QVJ. Long-term human breast carcinoma cell lines of metastatic origin: preliminary characterization. In Vitro. 1978;14:911–915. doi: 10.1007/BF02616120. [DOI] [PubMed] [Google Scholar]
- 28.Zhang RD, Fidler IJ. Relative malignant potential of human breast carcinoma cell lines established from pleural effusions and a brain metastasis. Invasion Metastasis. 1991;11:204–215. [PubMed] [Google Scholar]
- 29.Wang XJ, Yang J, Gascoyne PRC. Role of peroxide in AC electrical field exposure effects on Friend murine erythroleukemia cells during dielectrophoretic manipulations. Biochim Biophys Acta. 1999;1426:53–68. doi: 10.1016/s0304-4165(98)00122-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnold WM. Analysis of optimum electro-rotation technique. Ferroelectrics. 1988;86:225–250. [Google Scholar]
- 31.Wang X-B, Huang Y, Gascoyne PRC, Becker FF, Hölzel R, Pethig R. Changes in Friend murine erythroleukaemia cell membranes during induced differentiation determined by electrorotation. Biochim Biophys Acta. 1994;1193:330–344. doi: 10.1016/0005-2736(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 32.Pethig R, Kell DB. The passive electrical properties of biological systems: their significance in physiology, biophysics and biotechnology. Phys Med Biol. 1987;32:933–970. doi: 10.1088/0031-9155/32/8/001. [DOI] [PubMed] [Google Scholar]
- 33.Huang Y, Wang X-B, Gascoyne PRC, Becker FF. Membrane dielectric responses of human T-lymphocytes following mitogenic stimulation. Biochim Biophys Acta. 1999;1417:51–62. doi: 10.1016/s0005-2736(98)00253-3. [DOI] [PubMed] [Google Scholar]
- 34.Yang J, Huang Y, Wang X, Wang X-B, Becker FF, Gascoyne PRC. Dielectric properties of human leukocyte subpopulations determined by electrorotation as a cell separation criterion. Biophys J. 1999;76:3307–3314. doi: 10.1016/S0006-3495(99)77483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang J, Huang Y, Wang X-B, Becker FF, Gascoyne PRC. Human leukocyte differential analysis by dielectrophoresis field-flow-fractionation. Biophys J. 1999 In press. [Google Scholar]


