Abstract
Because cladribine can increase cytarabine triphosphate levels, we tested a cladribine—cytarabine combination in the St. Jude AML97, trial in which this combination was administered before standard chemotherapy to 96 children with acute myeloid leukemia (AML) or myelodysplastic syndrome. Patients received a 5-day course of cladribine (9 mg/m2/dose) and cytarabine either as daily 2-hour infusions (500 mg/m2/dose) (arm A) or a continuous infusion (500 mg/m2/day) (arm B). Ara-CTP levels and inhibition of DNA synthesis increased from day 1 to day 2, but were not different between the two arms. In addition, the median blast percentages at day 15 did not differ between arms A and B, but patients treated in arm A had shorter intervals between the initiation of the first and second courses of therapy. Thus, although there were trends towards better CR rates and overall survival for patients treated in arm B, the reduced efficacy of arm A may have been partially compensated by more intense timing of therapy for that group. For all patients, 5-yr event-free survival and overall survival estimates were 44.1% ± 5.4 % and 50.0% ± 5.5%. Our results suggest that cladribine in combination with continuous-infusion cytarabine is effective therapy for childhood AML.
Keywords: AML, cladribine, childhood
INTRODUCTION
Recent trials for childhood acute myeloid leukemia (AML) have attempted to improve cure rates by increasing cumulative doses of drugs, number of courses of therapy, or intensity of blocks of treatment.(1, 2) However, results from most regimens are similar and novel drug combinations tried in previous trials have not improved event-free survival (EFS) rates, suggesting that a plateau in the benefit of conventional chemotherapy might have been reached.(3)
An alternative approach is to selectively combine active agents that can enhance each other’s efficacy. Cladribine (2-chlorodeoxyadenosine) — a purine analog that is intracellularly phosphorylated to its active form — inhibits DNA synthesis, causes cell death, and induces apoptosis.(4, 5) We previously demonstrated that cladribine has significant antileukemic activity in patients with AML.(6-9) Cytarabine, like cladribine, is converted to its active form by several phosphorylation steps. Cladribine increases the activity of deoxycytidine kinase, which catalyzes the first phosphorylation step. Administering cladribine before cytarabine to adults with AML increases accumulation of cytarabine triphosphate (ara-CTP) in leukemic cells.(10)
Because cladribine shows antileukemic activity as a single agent, can augment ara-CTP levels, and can be safely administered with cytarabine,(11-13) we initiated the AML97 trial, wherein the cladribine—cytarabine combination was administered before standard induction chemotherapy in children and adolescents with newly diagnosed AML. We present here the final results of this randomized clinical trial.
PATIENTS AND METHODS
Patients
From March 1997 to June 2002, 102 children with previously untreated AML or myelodysplastic syndrome (MDS) were enrolled in the St. Jude AML97 trial.(14) All patients were younger than 22 years (median, 9.03 years; range, 0.05—21 years). Patients with acute promyelocytic leukemia were excluded, but those with all other subtypes of de novo or secondary AML, as well as patients with mixed-lineage leukemia, refractory anemia with excess blasts in transformation (RAEB-T), or Down syndrome (n=4) were eligible.
Therapy
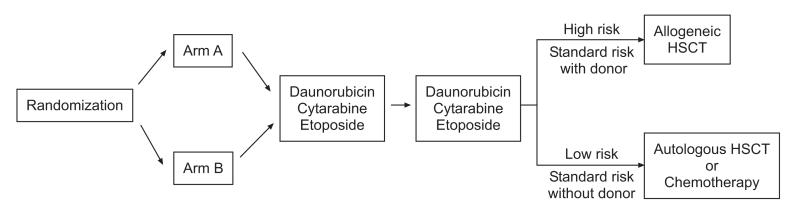
Patients who agreed to participate in the upfront “window” therapy were randomly assigned to receive either a daily short infusion of cytarabine (arm A) or a continuous infusion of cytarabine (arm B) (Fig 1).(14) Patients in arm A received 5 daily 2-hour infusions of cytarabine (500 mg/m2/day) and 5 daily 30-minute infusions of cladribine (9 mg/m2) that began 24 hours after the start of the first cytarabine infusion. There was a 2-hour interval between the end of each cladribine infusion and the start of each cytarabine infusion. Patients in arm B received cytarabine 500 mg/m2/day as a 120-hour continuous infusion and 5 daily 30-minute infusions of cladribine (9 mg/m2), which began 24 hours after the start of the cytarabine infusion.
Figure 1. AML97 treatment schema.
Patients were randomized to receive cladribine and daily 2-hour infusions (Arm A) or a continuous infusion (Arm B) of cytarabine. The second and third courses of chemotherapy consisted of daunorubicin, cytarabine, and etoposide (DAV). Further therapy was based on risk status and the availability of a matched sibling donor.
Daunorubicin (30 mg/m2/day, continuous infusion, days 1-3), cytarabine (250 mg/m2/day, continuous infusion, days 1-5), and etoposide (200 mg/m2/day, continuous infusion, days 4, 5) were the second and third courses of chemotherapy (DAV #1 and DAV #2). Patients with high-risk AML, i.e., megakaryoblastic leukemia (M7), RAEB-T, secondary AML, -7, 5q-, t(9;22), or persistent leukemia after DAV #1, were eligible for allogeneic hematopoietic stem cell transplantation (HSCT) after DAV #2. Patients with t(8;21) or inv(16) were considered to have low-risk AML and were not eligible for allogeneic HSCT. All other patients were considered to have standard-risk AML and were eligible for HSCT if a matched sibling donor was available. From March 1997 to January 1999, patients not receiving allogeneic HSCT received unpurged autologous HSCT after receiving a conditioning regimen that consisted of busulfan (1 mg/kg orally every 6 hours for 16 doses on days -9, -8, -7, -6) and cyclophosphamide (50 mg/kg/dose on days -5, -4, -3, -2). In January 1999, the protocol was amended to replace autologous HSCT with 2 courses of consolidation chemotherapy consisting of cytarabine (3 g/m2/dose every 12 hours on days 1, 2, 8, and 9) and L-asparaginase (6000 units/m2/dose after the fourth and eighth doses of cytarabine), followed by mitoxantrone (10 mg/m2/dose on days 1-5) and cytarabine (1 g/m2/dose every 12 hours on days 1-3).
Patients without central nervous system (CNS) disease received 4 monthly doses of age-adjusted intrathecal (IT) methotrexate, hydrocortisone, and cytarabine (MHA), beginning day 15 from start of chemotherapy. Patients with CNS leukemia received weekly doses of IT MHA until the cerebrospinal fluid was clear of leukemia cells (minimum 4 doses) and then 4 monthly doses. In February 1999, the protocol was amended to include 1 dose of IT cytarabine during diagnostic procedures.
Response to cladribine—cytarabine was assessed by bone marrow aspirate and biopsy at day 15 from start of chemotherapy. Complete remission (CR) was defined as trilineage hematopoietic recovery with less than 5% blasts in the marrow, platelet count greater than 30 × 109/L, and absolute neutrophil count (ANC) greater than 0.3 × 109/L. Complete response was defined as less than 5% blasts in the marrow without hematopoietic recovery. Patients with morphologic evidence of leukemia started DAV #1 immediately, whereas those without evidence of leukemia started DAV #1 when the platelet count was greater than 30 × 109/L and the ANC was greater than 0.3 × 109/L. Thus, patients with complete response at day 15 began DAV #1 when complete remission was attained.
Biology studies
In patients with ≥ 70% leukemic blasts in the bone marrow, pharmacokinetic and pharmacodynamic studies were performed as described previously.(14) Minimal residual disease (MRD) was measured by flow cytometry.(15) Briefly, leukemia-associated immunophenotypes were identified in diagnostic bone marrow specimens and marker combinations that allowed detection of 1 leukemia cell per 1000 mononuclear bone marrow cells were applied to subsequent samples. Results are reported at percentage of nucleated cells with the leukemia-associated immunophenotype. MRD negativity was defined as less than 1 leukemia cell per 1000 mononuclear bone marrow cells (i.e., <0.1%).
Statistical plan and analysis
Our primary objective was to compare the ratio of ara-CTP concentration in leukemia cells after cladribine administration to that before cladribine administration across the 2 arms. The initial design called for randomization of 80 eligible patients with evaluable ara-CTP concentrations, allowed for 1 interim analysis, and provided 80% power at the 5% level to detect a 40% difference in the change in ara-CTP concentrations between the 2 arms. In the interim analysis, we found the variance used for the initial study design to be inaccurate;(14) therefore, the planned sample size was adjusted to 52 patients per arm to give 80% power and an overall Type I error rate of 5%.
The Wilcoxon rank-sum test was used to compare pharmacokinetic and pharmacodynamic variables by arm, amendment status, or early IT therapy. The exact chi-square test was used to compare clinical features, achievement of CR, and MRD status across arms. The Kaplan-Meier method was used to compute estimates of EFS and overall survival (OS). The log-rank test was used to perform comparisons of EFS and OS distributions. EFS was defined as the time elapsed from study enrollment to study removal because of relapse, secondary malignancy, or death, with those living and event-free censored as the time of last follow-up. OS was defined as the time elapsed from study enrollment to death. Multiple-variable Cox regression models were used to explore the association of early IT therapy with EFS and OS while accounting for initial leukocyte count, age, and karyotype. Multiple-variable logistic regression models were used to explore the association of early IT therapy with achieving remission by the end of window therapy while accounting for initial leukocyte count, age, and karyotype. Cox proportional hazards modeling was used to evaluate the prognostic relevance of age at diagnosis (as a continuous variable), initial leukocyte count (as a continuous variable), cytogenetics (favorable vs. others), de novo status (de novo vs. others), and presence of minimal residual disease (as a time varying covariate) on EFS and OS. No multiple-testing adjustments of p-values were performed. All analyses were performed by using the SAS software (SAS Institute, Cary, NC), windows version 9.1.3.
RESULTS
Patient Characteristics
Table 1 shows patient characteristics overall and by upfront treatment arm. Overall, 18% had low-risk karyotypes, and 25% had 11q23 abnormalities. Eighty-two patients had de novo AML, 11 treatment-related AML, 6 MDS-related AML, and 3 mixed-lineage leukemia. Patients in the 2 arms did not differ significantly by sex, race, age, initial leukocyte count, FAB type, CNS involvement, or presence of favorable cytogenetics, although 9 patients had t(9;11) and 2 had normal karyotypes in arm A, whereas 3 patients had t(9;11) and 13 had normal karyotypes in arm B (p=0.03).
Table 1.
Patient demographics and clinical features overall and by treatment arm
| Characteristic | Overalla (N=102) |
Arm A (N=50) |
Arm B (N=46) |
P value (A vs. B) |
|---|---|---|---|---|
|
| ||||
| Age (years) | 0.2693 | |||
| Median | 9.03 | 8.77 | 9.19 | |
| Range | (0.05—21.00) | (0.31—21.00) | (0.05—20.24) | |
|
| ||||
| Race | 0.3054 | |||
| White | 64 | 35 | 25 | |
| Black | 19 | 7 | 10 | |
| Other | 19 | 8 | 11 | |
|
| ||||
| Sex | 0.0706 | |||
| Female | 52 | 21 | 28 | |
| Male | 50 | 29 | 18 | |
|
| ||||
| Cytogenetics | 0.0280 | |||
| inv(16) | 7 | 4 | 3 | |
| t(8;21) | 11 | 6 | 5 | |
| t(9;11) | 14 | 9 | 3 | |
| Other 11q23 | 12 | 7 | 5 | |
| Normal | 18 | 2 | 13 | |
| Other | 39 | 21 | 17 | |
|
| ||||
| CNS status | 0.1956 | |||
| CNS1 | 69 | 33 | 31 | |
| CNS2 | 12 | 6 | 6 | |
| CNS3 | 3 | 2 | 0 | |
| Traumatic + | 12 | 4 | 8 | |
| Traumatic - | 1 | 0 | 1 | |
|
| ||||
| FABb | 0.5809 | |||
| M0 | 3 | 3 | 0 | |
| M1 | 18 | 7 | 10 | |
| M2 | 22 | 9 | 12 | |
| M4 | 17 | 10 | 6 | |
| M4Eo | 3 | 2 | 1 | |
| M5 | 18 | 8 | 9 | |
| M7 | 16 | 9 | 6 | |
| Other | 5 | 2 | 2 | |
Six patients declined to participate in the upfront therapy and were therefore not assigned to arm A or B.
FAB, French—American—British distribution
Response to upfront and remission induction therapy
Of the 102 patients, 50 were randomly assigned to arm A and 46 to arm B; 6 patients declined to participate in the upfront window therapy. More patients experienced grade 3 or 4 toxicity during upfront therapy in arm B than in arm A (48% vs. 24%, p=0.019, Table 2). The 2 arms did not differ significantly in the number of patients experiencing toxicity during DAV #1 or DAV #2. Because we previously demonstrated that patients with monoblastic leukemia often respond well to cladribine,(9) we assessed the association between FAB and response to window therapy. Among all randomized patients, 13 of 17 (76%) of those with M5 AML achieved CR after window therapy, compared to 38 of 77 (49%) of non-M5 patients (p=0.059). The results were even more striking among randomized patients with de novo AML [11 of 13 (85%) vs. 32 of 64 (50%), p=0.031].
Table 2.
Outcome according to treatment arm for randomized patients
| All randomized patients (n=96) | De novo patients (n=78) | |||||
|---|---|---|---|---|---|---|
| Arm A | Arm B | p | Arm A | Arm B | p | |
| CR after window* | 0.026 | 0.068 | ||||
| Yes | 21 (43) | 30 (65) | 16 (43) | 27 (66) | ||
| No | 29 (57) | 16 (35) | 21 (57) | 14 (34) | ||
|
| ||||||
| MRD after window | 0.756 | 0.746 | ||||
| < 0.1% | 13 (54) | 8 (47) | 12 (57) | 8 (50) | ||
| ≥ 0.1% | 11 (46) | 9 (53) | 9 (43) | 8 (50) | ||
|
| ||||||
| Grade 3/4 toxicity | 0.019 | 0.032 | ||||
| Yes | 12 (24) | 22 (48) | 8 (22) | 19 (46) | ||
| No | 38 (76) | 24 (52) | 29 (78) | 22 (54) | ||
|
| ||||||
| CR after DAV #1 | 0.008 | 0.077 | ||||
| Yes | 38 (76) | 44 (96) | 30 (81) | 39 (95) | ||
| No | 12 (24) | 2 (4) | 7 (19) | 2 (5) | ||
|
| ||||||
| MRD after DAV #1 | 1.000 | 1.000 | ||||
| < 0.1% | 16 (67) | 11 (69) | 15 (71) | 11 (74) | ||
| ≥ 0.1% | 8 (33) | 5 (31) | 6 (29) | 4 (26) | ||
|
| ||||||
| Grade 3/4 toxicity | 1.000 | 1.000 | ||||
| Yes | 20 (40) | 16 (37) | 16 (43) | 17 (42) | ||
| No | 30 (60) | 27 (63) | 21 (57) | 24 (58) | ||
|
| ||||||
| 5-yr EFS±SE | 40.0% ± 6.8% 52.2% ± 7.4% 0.216 | 48.6% ± 8.0% 48.8% ± 7.8 0.933 | ||||
|
| ||||||
| 5-yr OS±SE | 40.0% ± 6.8% 60.9% ± 7.2% 0.069 | 48.6% ± 8.0% 58.8% ± 7.7 0.561 | ||||
The upfront window therapy consisted of daily cladribine with daily 2-hour infusions of cytarabine (arm A) or with a continuous infusion of cytarabine (arm B).
Numbers in parentheses represent percentages.
Intracellular ara-CTP levels increased significantly from day 1 to day 2 (p = 0.0002), but these increases did not differ significantly between arms (p=0.299); inhibition of DNA synthesis followed the same trend (data not shown). Despite the lack of significant differences in pharmacokinetic or pharmacodynamic parameters, patients in Arm B were significantly more likely to achieve remission after upfront therapy (p=0.026 and 0.068 for all randomized and non-DS de novo patients, respectively) and induction I (p=0.008 and 0.077 for all randomized and non-DS de novo patients, respectively) than their counterparts in arm A (Table 2). Remarkably, 44 of 46 (96%) patients in arm B achieved CR after only 1 course each of upfront therapy and DAV, compared to 38 of 50 (76%) in Arm A. Although the 2 arms did not differ significantly with respect to MRD, EFS, or OS, there was a trend toward better survival among patients in arm B than arm A (5-yr OS 60.9% ±7.2% vs. 40.0% ±6.8%, p = 0.069). It should be noted that potential outcome differences between the two arms may be confounded by differences in the timing of chemotherapy administration between arms. Although the median blast percentages at day 15 did not differ between arms A and B (5% for both arms), patients treated in arm A had shorter intervals between the initiation of the first course of therapy and the start of DAV #1 (median days, 18 for Arm A vs. 25 for Arm B, p = 0.008). Thus, it is possible that the reduced efficacy of arm A is partially compensated by more intense therapy for that group.
Overall treatment results
Table 3 shows CR rates, causes of failure and death, EFS, and OS for the 102 patients enrolled and the 78 non-DS patients with de novo AML. For all patients, 5-yr EFS and OS estimates were 44.1% ± 5.4 % and 50.0% ± 5.5% (Fig. 2), whereas corresponding estimates for patients without DS, secondary AML, or MDS were 48.7% ± 6.3% and 53.8% ± 6.3%.
Table 3.
Overall results and causes of failure
| All patients (n=102) | Non-DS de novo patients (n=78) | |
|---|---|---|
|
| ||
| Did not achieve CR | 12 (12%) | 4 (5%) |
| Refractory disease | 10 | 3 |
| Toxic death | 2 | 1 |
|
| ||
| Achieved CR | 90 (88%) | 74 (95%) |
| Died in first CR | 15 (15%) | 11 (14%) |
| Chemotherapy | 4 | 4 |
| Allogeneic HSCT | 11 | 7 |
| Autologous HSCT | 0 | 0 |
| Alive in first CR | 46 (45%) | 38 (49%) |
| Total alive | 51 (50%) | 42 (54%) |
| Relapsed | 25 (25%) | 23 (29%) |
| Chemotherapy | 14 | 13 |
| Allogeneic HSCT | 5 | 4 |
| Autologous HSCT | 6 | 6 |
| Protocol violation | 2 | 1 |
| Lineage switch | 1 | 0 |
| Increasing MRD | 1 | 1 |
|
| ||
| Causes of death | 51 (50%) | 36 (46%) |
| Leukemia | 24 | 15 |
| Infection | 16 | 11 |
| Chemotherapy | 8 | 7 |
| HSCT | 8 | 4 |
| HSCT complications | 5 | 4 |
| Other | 6 | 6 |
|
| ||
| 5-yr EFS ± 2SE | ||
| Chemotherapy | 45.9% ± 7.0% (n=61) | 51.1% ± 8.2% (n=47) |
| Allogeneic HSCT | 46.9% ± 9.1% (n=32) | 52.2% ±10.9% (n=23) |
| Autologous HSCT | 22.2% ± 11.3% (n=9) | 25.0% ± 12.5% (n=8) |
|
| ||
| 5-yr OS ± 2SE | ||
| Chemotherapy | 52.5% ± 7.4% (n=61) | 55.3% ± 8.3% (n=47) |
| Allogeneic HSCT | 46.9% ± 9.1% (n=32) | 52.2% ±10.9% (n=23) |
| Autologous HSCT | 44.4% ± 14.8% (n=9) | 50.0% ±15.8% (n=8) |
Figure 2.
Panel A. Event-free survival (EFS) and overall survival (OS) for the 102 patients enrolled in AML97.
Panel B. EFS for patients enrolled in Arm A before (Arm A Pre) and after (Arm A Post) treatment amendments, and for patients enrolled in Arm B before (Arm B Pre) and after (Arm B Post) treatment amendments. The curves for Arm B Pre and Arm A Post overlap.
Of the 102 patients, 12 did not achieve CR: 10 had refractory leukemia and 2 died of disseminated Candida infections during induction therapy. Of the 10 patients with refractory leukemia, 3 had treatment-related AML, 2 had MDS-related AML, 2 had mixed-lineage leukemia, and 3 had de novo AML (1 with megakaryoblastic leukemia, 1 with t(6;9), and 1 with monosomy 7). Of the 90 patients who achieved CR, 25 developed bone marrow or combined relapses (14 after chemotherapy, 5 after allogeneic HSCT, and 6 after autologous HSCT); there were no isolated CNS relapses. Of the 25 patients who relapsed, only 3 are alive. In addition, 15 patients (5 who received chemotherapy and 10 who received allogeneic HSCT) died in remission: 11 from infection (6 Aspergillus, 3 Candida, 1 S. maltophilia, 1 Parainfluenza) and 4 from other complications related to allogeneic HSCT. The cumulative incidence of grade 3-4 toxicity was 84% ± 4% and of grade 3-4 infection was 61% ± 5%, with no significant differences in toxicities between treatment arms. The rates of grade 3-4 infection ranged from 26% to 38% after each course of chemotherapy.
We studied the associations between EFS and OS rates and potential prognostic factors: sex, race, cytogenetics, CNS involvement, MRD, FAB, age, and leukocyte count at diagnosis (Table 4). MRD negativity after the cladribine—cytarabine upfront therapy was significantly associated with a higher EFS rate (p=0.029, Table 4), whereas MRD negativity after DAV #1 was significantly associated with higher OS (p=0.024) and EFS rates (p=0.003). Patients who were MRD-negative after DAV #1 had a 5-yr EFS estimate of 59.3% ± 9.5% compared with only 23.5% ± 9.2% for patients with detectable disease. Younger patients (<10 years old) had significantly higher OS (p=0.003) and EFS (p=0.002) rates than older patients. OS and EFS were not significantly affected by sex, race, cytogenetics, CNS involvement, FAB or leukocyte count, although there is a slight trend for M5 patients to have better OS (66.7% ± 10.7% vs. 46.4% ± 5.4%, p = 0.184) and EFS (61.1% ± 11.0% vs. 40.5% ± 5.4%, p = 0.157) than other subtypes among all randomized patients. However, EFS and OS estimates for the subgroups shown in Table 4 should be interpreted with caution because of low patient numbers. Additionally, favorable cytogenetics, de novo status, initial leukocyte count, age, and the presence of minimal residual disease were found to be prognostically relevant factors for event-free survival in a multi-predictor Cox regression analysis (Table 5). Except for initial leukocyte count, the same factors were important for overall survival (Table 5). When MRD was excluded as a predictor in order to include patients without MRD data in the analysis, the impact of the remaining factors was found to be qualatively similar as in Table 5 (data not shown).
Table 4.
Analysis of prognostic factors
| Feature | N | 5-yr OS ±SE (%) | p | 5-yr EFS ±SE (%) | p |
|---|---|---|---|---|---|
|
| |||||
| Sex | 0.274 | 0.321 | |||
| Female | 52 | 55.8 ± 6.8 | 50.0 ± 6.8 | ||
| Male | 50 | 44.0 ± 7.0 | 38.0 ± 6.9 | ||
|
| |||||
| Race | 0.277 | 0.272 | |||
| Black | 19 | 36.8 ± 10.4 | 31.6 ± 9.9 | ||
| Other | 19 | 68.4 ± 10.3 | 63.2 ± 10.6 | ||
| White | 64 | 48.4 ± 6.2 | 42.2 ± 6.2 | ||
|
| |||||
| Cytogenetics | 0.811 | 0.528 | |||
| inv(16) | 7 | 71.4 ± 15.6 | 71.4 ± 15.6 | ||
| t(8;21) | 11 | 36.4 ± 13.0 | 36.4 ± 13.0 | ||
| t(9;11) | 14 | 57.1 ± 12.5 | 57.1 ± 12.5 | ||
| Other 11q23 | 12 | 50.0 ± 13.4 | 41.7 ± 13.0 | ||
| Normal | 18 | 55.6 ± 11.7 | 33.3 ± 11.1 | ||
| Other | 39 | 46.2 ± 7.8 | 43.6 ± 7.7 | ||
|
| |||||
| CNS status | 0.367 | 0.338 | |||
| CNS-negative | 69 | 51.7 ± 5.4 | 46.0 ± 5.3 | ||
| CNS-positive | 12 | 40.0 ± 11.7 | 33.3 ± 11.1 | ||
|
| |||||
| FAB | 0.882 | 0.837 | |||
| M0/other | 7 | 57.1 ± 16.7 | 42.9 ± 16.2 | ||
| M1 | 18 | 44.4 ± 11.7 | 38.9 ± 11.5 | ||
| M2 | 22 | 45.5 ± 10.1 | 36.4 ± 9.7 | ||
| M4/M4Eo | 20 | 45.0 ± 10.6 | 40.0 ±10.3 | ||
| M5 | 18 | 66.7 ± 10.7 | 61.1 ± 11.0 | ||
| M7 | 16 | 50.0 ± 11.8 | 50.0 ± 11.8 | ||
|
| |||||
| MRD after window | 0.058 | 0.029 | |||
| < 0.1% | 21 | 66.7 ± 10.3 | 66.7 ± 10.3 | ||
| ≥ 0.1% | 20 | 40.0 ± 10.3 | 35.0 ± 10.0 | ||
|
| |||||
| MRD after DAV #1 | 0.024 | 0.003 | |||
| < 0.1% | 27 | 63.0 ± 9.3 | 59.3 ± 9.5 | ||
| ≥ 0.1% | 17 | 35.3 ± 10.7 | 23.5 ± 9.2 | ||
|
| |||||
| MRD after DAV #2 | 0.055 | 0.064 | |||
| < 0.1% | 26 | 69.2 ± 9.1 | 65.4 ± 9.3 | ||
| ≥ 0.1% | 10 | 40.0 ± 13.9 | 40.0 ± 13.9 | ||
|
| |||||
| Age >10 | 0.003 | 0.002 | |||
| Yes | 47 | 34.0 ± 6.9 | 27.7 ± 6.5 | ||
| No | 55 | 63.6 ± 6.4 | 58.2 ± 6.5 | ||
|
| |||||
| Age (continuous) | 0.003 | 0.002 | |||
|
| |||||
| Leukocyte count >50 | 0.316 | 0.228 | |||
| Yes | 24 | 37.5 ± 9.4 | 33.3 ± 9.1 | ||
| No | 78 | 53.8 ± 5.6 | 47.4 ± 5.7 | ||
|
| |||||
| Leukocyte count (continuous) | 0.282 | 0.110 | |||
Table 5.
Cox Regression Analysis
| Event-Free Survival | Overall Survival | |||||||
|---|---|---|---|---|---|---|---|---|
| Factor | HR | 95% CI | p | HR | 95% CI | p | ||
| Favorable cytogenetics | 0.322 | 0.097 | 1.072 | 0.065 | 0.433 | 0.143 | 1.314 | 0.139 |
| de novo AML | 0.013 | 0.001 | 0.120 | < 0.001 | 0.249 | 0.043 | 1.449 | 0.122 |
| Leukocyte count | 1.014 | 1.005 | 1.023 | 0.004 | 1.002 | 0.992 | 1.011 | 0.744 |
| Age | 1.114 | 1.008 | 1.231 | 0.034 | 1.115 | 1.015 | 1.225 | 0.023 |
| MRD+ | 3.817 | 1.321 | 11.033 | 0.013 | 2.662 | 0.942 | 7.519 | 0.065 |
HR, hazard ratio
For favorable cytogenetics, de novo AML, and MRD, reported hazard ratios indicate rate of event or death among favorable cytogenetics relative to other patients, de novo patients relative to other patients, and MRD+ patients relative to MRD- patients. For leukocyte count, hazard ratio indicates relative change in event or death rate associated with an increase of 1,000 units in initial leukocyte count. For age, hazard ratio indicates relative change in event or death rate associated with a one-year increase in age.
Effects of amendments
The protocol underwent 2 major revisions that potentially affected clinical endpoints: autologous HSCT was replaced with 2 courses of chemotherapy and 1 dose of IT cytarabine before window therapy was added. Overall, there were no statistically significant differences in EFS (5-yr estimates, 39.1% ±7.0 % vs. 48.2% ± 6.7%, p=0.450) or in OS (5-yr estimates, 43.5% ± 7.1% vs. 55.4% ± 6.6%, p=0.249) between those treated before or after the amendments. Within each arm, there were no significant differences in the pre- and post-amendment cohorts with respect to sex, race, cytogenetics, CNS involvement, age, initial leukocyte count, or FAB subtype. However, pre-amendment arm A patients were less frequently de novo than the other three therapy-defined groups (p = 0.039). Among patients treated in arm A, those treated after the amendments had a slightly better EFS (5-yr estimates, 50.0% ± 10% vs. 27.3% ± 8.8%, p=0.091, Fig. 2). In contrast, there were no differences in outcome among patients in arm B (Fig. 2). In addition, pre-amendment arm A patients had a higher rate of relapse or induction failure than other patients (p = 0.038). The 3-year estimates of cumulative incidence of relapse or induction failure for the 4 groups are as follows: Arm A pre-amendment, 50.0% ± 11.2%; Arm A post-amendment, 28.6% ± 8.8%; Arm B pre-amendment, 25% ± 9.1%; Arm B post-amendment, 31.2% ± 10.3%.
Multiple-variable logistic and Cox proportional hazards regression models were used to explain the disparity of outcomes between pre- and post-amendment patients in arm A. Although the difference did not reach statistical significance, pre-amendment patients in arm A were less likely to achieve remission by the end of window therapy than post-amendment patients (p=0.099), even when adjusting for initial leukocyte count, age, and karyotype. Also, for patients in arm A, treatment after replacing autologous HSCT with chemotherapy was significantly associated with better EFS (p = 0.015), after adjusting for age, initial leukocyte count, and cytogenetics.
DISCUSSION
Results of this study demonstrate that cladribine administration can augment cytarabine pharmacokinetics and pharmacodynamics, and the combination of cladribine and cytarabine can induce remission in over half of all patients. However, the CR rates of the present study cannot be directly compared with CR rates of other trials because of differences in the definitions of CR. Whereas most clinical trials define CR as less than 5% blasts in the marrow with a platelet count greater than 100 × 109/L and an ANC greater than 1.0 × 109/L, we defined CR as less than 5% blasts with a platelet count greater than 30 × 109/L and an ANC greater than 0.3 × 109/L. Nevertheless, despite an over-representation of t(9;11) in Arm A (cladribine with short daily infusions of cytarabine), the combination of cladribine and continuous-infusion cytarabine (Arm B) was more effective at inducing remission and appeared to contribute to a good overall outcome. Although our overall results were not better than those of other trials, the cladribine—cytarabine combination might be particularly valuable for specific subgroups of patients, such as those with monoblastic leukemia. In contrast, the outcome for patients with t(8;21) was relatively poor (5-yr OS, 36.4% ± 13.0%). This may reflect a lack of sensitivity of this subgroup of patients to the cladribine-cytarabine combination, the absence of anthracyclines during the first course of therapy, the relatively low cumulative doses of anthracyclines or cytarabine, or the higher percentage of t(8;21) patients who received autologous transplant (27% vs. 9.4%).
Replacing autologous HSCT with chemotherapy and adding early IT therapy appeared to favorably impact outcome, especially among patients who received cladribine with 2-hour daily cytarabine infusions (arm A). Because these therapeutic changes were made in close proximity (January 1999 and February 1999), we cannot separate the effects of these interventions. However, the high relapse rate in patients after autologous HSCT suggests that the greatest effect was because consolidation chemotherapy was used. Nevertheless, the additional early IT therapy may have helped improve the CR rate in patients in arm A after protocol amendments. Effects of both changes were most pronounced among patients in arm A, probably because that regimen seems inferior to arm B.
Overall, the results of AML97 compare favorably with recently reported trials for childhood AML conducted in the United States. In the CCG-2961 trial, EFS and OS estimates were 42% and 52%,(2) whereas corresponding estimates from the Pediatric Oncology Group 9421 trial were 36% and 54%.(16) Contemporary European clinical trials for pediatric AML include the AML-BFM 98 trial (EFS, 49%; OS, 62%)(1) and trials reported by investigators from the Medical Research Council (EFS, 48%; OS, 56%),(17, 18) and the Nordic Society for Paediatric Haematology and Oncology (EFS, 49%; OS, 64%).(19) In our trial, which enrolled patients up to 22 years old with de novo AML, treatment-related AML, and RAEB-T, the 5-yr EFS and OS estimates were 44.1% and 50.0%. However, for patients with de novo AML, which represent a patient group similar to that treated on other cooperative group trials, the estimates are 48.7% and 53.8%; EFS and OS estimates after treatment amendments are even better (57% and 62%).
Cumulative doses of anthracyclines (430 mg/m2) and cytarabine (35 g/m2) for our patients who did not receive HSCT are similar to those for other trials. However, patients in AML97 had excellent CR rates with relatively modest doses (180 mg/m2) of daunomycin during induction therapy. Also, outstanding CNS control (no isolated CNS relapses) was achieved in AML97 without using cranial irradiation. Although the follow up is too short to describe late effects in detail, we have not observed any cardiac toxicity or secondary malignancies in AML97 survivors. However, we did observe unacceptably high rates of toxic deaths, especially among patients who underwent HSCT. As in some previous AML trials,(20-22) our patients had significant infection-related toxicity: 11 patients died of disseminated fungal infections (2 during remission induction and 9 who had achieved CR). To reduce this toxicity, we are currently testing prophylactic voriconazole in patients with AML.(23)
In future studies, we will further explore the cytarabine-cladribine combination as consolidation therapy in selected patients. Because it is unlikely that further refinements in conventional chemotherapy will improve outcomes for AML in a major way, we must focus instead on better understanding the biology of the disease, refining risk stratification, improving supportive care, and developing alternative treatment approaches, including targeted therapy and cellular therapies.
Acknowledgement
The authors thank Vani Shanker for expert editorial review. The authors declare no competing financial interests.
This work was supported in part by Cancer Center Support (CORE) grant P30 CA-21765 from the National Institutes of Health, by a Center of Excellence Grant from the State of Tennessee, and by the American Lebanese Syrian Associated Charities (ALSAC). C-H Pui is an American Cancer Society Professor.
REFERENCES
- 1.Creutzig U, Zimmermann M, Lehrnbecher T, Graf N, Hermann J, Niemeyer CM, et al. Less toxicity by optimizing chemotherapy, but not by addition of granulocyte colony-stimulating factor in children and adolescents with acute myeloid leukemia: results of AML-BFM 98. J Clin Oncol. 2006;24:4499–4506. doi: 10.1200/JCO.2006.06.5037. [DOI] [PubMed] [Google Scholar]
- 2.Lange BJ, Smith FO, Feusner J, Barnard DR, Dinndorf P, Feig S, et al. Outcomes in CCG-2961, a children’s oncology group phase 3 trial for untreated pediatric acute myeloid leukemia: a report from the children’s oncology group. Blood. 2008;111:1044–1053. doi: 10.1182/blood-2007-04-084293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaspers GJ, Creutzig U. Pediatric acute myeloid leukemia: international progress and future directions. Leukemia. 2005;19:2025–2029. doi: 10.1038/sj.leu.2403958. [DOI] [PubMed] [Google Scholar]
- 4.Carrera CJ, Terai C, Lotz M, Curd JG, Piro LD, Beutler E, et al. Potent toxicity of 2-chlorodeoxyadenosine toward human monocytes in vitro and in vivo. A novel approach to immunosuppressive therapy. J Clin Invest. 1990;86:1480–1488. doi: 10.1172/JCI114865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robertson LE, Chubb S, Meyn RE, Story M, Ford R, Hittelman WN, et al. Induction of apoptotic cell death in chronic lymphocytic leukemia by 2- chloro-2′-deoxyadenosine and 9-beta-D-arabinosyl-2-fluoroadenine. Blood. 1993;81:143–150. [PubMed] [Google Scholar]
- 6.Santana VM, Mirro J, Jr., Harwood FC, Cherrie J, Schell M, Kalwinsky D, et al. A phase I clinical trial of 2-chlorodeoxyadenosine in pediatric patients with acute leukemia. J Clin Oncol. 1991;9:416–422. doi: 10.1200/JCO.1991.9.3.416. [DOI] [PubMed] [Google Scholar]
- 7.Santana VM, Mirro J, Jr., Kearns C, Schell MJ, Crom W, Blakley RL. 2-Chlorodeoxyadenosine produces a high rate of complete hematologic remission in relapsed acute myeloid leukemia. J Clin Oncol. 1992;10:364–370. doi: 10.1200/JCO.1992.10.3.364. [DOI] [PubMed] [Google Scholar]
- 8.Santana VM, Hurwitz CA, Blakley RL, Crom WR, Luo X, Roberts WM, et al. Complete hematologic remissions induced by 2-chlorodeoxyadenosine in children with newly diagnosed acute myeloid leukemia. Blood. 1994;84:1237–1242. [PubMed] [Google Scholar]
- 9.Krance RA, Hurwitz CA, Head DR, Raimondi SC, Behm FG, Crews KR, et al. Experience with 2-chlorodeoxyadenosine in previously untreated children with newly diagnosed acute myeloid leukemia and myelodysplastic diseases. J Clin Oncol. 2001;19:2804–2811. doi: 10.1200/JCO.2001.19.11.2804. [DOI] [PubMed] [Google Scholar]
- 10.Gandhi V, Estey E, Keating MJ, Chucrallah A, Plunkett W. Chlorodeoxyadenosine and arabinosylcytosine in patients with acute myelogenous leukemia: pharmacokinetic, pharmacodynamic, and molecular interactions. Blood. 1996;87:256–264. [PubMed] [Google Scholar]
- 11.Kornblau SM, Gandhi V, Andreeff HM, Beran M, Kantarjian HM, Koller CA, et al. Clinical and laboratory studies of 2-chlorodeoxyadenosine +/- cytosine arabinoside for relapsed or refractory acute myelogenous leukemia in adults. Leukemia. 1996;10:1563–1569. [PubMed] [Google Scholar]
- 12.Robak T, Wrzesien-Kus A, Lech-Maranda E, Kowal M, Dmoszynska A. Combination regimen of cladribine (2-chlorodeoxyadenosine), cytarabine and G-CSF (CLAG) as induction therapy for patients with relapsed or refractory acute myeloid leukemia. Leuk Lymphoma. 2000;39:121–129. doi: 10.3109/10428190009053545. [DOI] [PubMed] [Google Scholar]
- 13.Rubnitz JE, Razzouk BI, Srivastava DK, Pui CH, Ribeiro RC, Santana VM. Phase II trial of cladribine and cytarabine in relapsed or refractory myeloid malignancies. Leuk Res. 2004;28:349–352. doi: 10.1016/j.leukres.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 14.Crews KR, Gandhi V, Srivastava DK, Razzouk BI, Tong X, Behm FG, et al. Interim comparison of a continuous infusion versus a short daily infusion of cytarabine given in combination with cladribine for pediatric acute myeloid leukemia. J Clin Oncol. 2002;20:4217–4224. doi: 10.1200/JCO.2002.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Coustan-Smith E, Ribeiro RC, Rubnitz JE, Razzouk BI, Pui CH, Pounds S, et al. Clinical significance of residual disease during treatment in childhood acute myeloid leukaemia. Br J Haematol. 2003;123:243–252. doi: 10.1046/j.1365-2141.2003.04610.x. [DOI] [PubMed] [Google Scholar]
- 16.Becton D, Dahl GV, Ravindranath Y, Chang MN, Behm FG, Raimondi SC, et al. Randomized use of cyclosporin A (CsA) to modulate P-glycoprotein in children with AML in remission: Pediatric Oncology Group Study 9421. Blood. 2006;107:1315–1324. doi: 10.1182/blood-2004-08-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevens RF, Hann IM, Wheatley K, Gray RG. Marked improvements in outcome with chemotherapy alone in paediatric acute myeloid leukemia: results of the United Kingdom Medical Research Council’s 10th AML trial. MRC Childhood Leukaemia Working Party. Br J Haematol. 1998;101:130–140. doi: 10.1046/j.1365-2141.1998.00677.x. [DOI] [PubMed] [Google Scholar]
- 18.Gibson BE, Wheatley K, Hann IM, Stevens RF, Webb D, Hills RK, et al. Treatment strategy and long-term results in paediatric patients treated in consecutive UK AML trials. Leukemia. 2005;19:2130–2138. doi: 10.1038/sj.leu.2403924. [DOI] [PubMed] [Google Scholar]
- 19.Lie SO, Abrahamsson J, Clausen N, Forestier E, Hasle H, Hovi L, et al. Treatment stratification based on initial in vivo response in acute myeloid leukaemia in children without Down’s syndrome: results of NOPHO-AML trials. Br J Haematol. 2003;122:217–225. doi: 10.1046/j.1365-2141.2003.04418.x. [DOI] [PubMed] [Google Scholar]
- 20.Sung L, Lange BJ, Gerbing RB, Alonzo TA, Feusner J. Microbiologically documented infections and infection-related mortality in children with acute myeloid leukemia. Blood. 2007;110:3532–3539. doi: 10.1182/blood-2007-05-091942. [DOI] [PubMed] [Google Scholar]
- 21.Lehrnbecher T, Varwig D, Kaiser J, Reinhardt D, Klingebiel T, Creutzig U. Infectious complications in pediatric acute myeloid leukemia: analysis of the prospective multi-institutional clinical trial AML-BFM 93. Leukemia. 2004;18:72–77. doi: 10.1038/sj.leu.2403188. [DOI] [PubMed] [Google Scholar]
- 22.Creutzig U, Zimmermann M, Reinhardt D, Dworzak M, Stary J, Lehrnbecher T. Early deaths and treatment-related mortality in children undergoing therapy for acute myeloid leukemia: analysis of the multicenter clinical trials AML-BFM 93 and AML-BFM 98. J Clin Oncol. 2004;22:4384–4393. doi: 10.1200/JCO.2004.01.191. [DOI] [PubMed] [Google Scholar]
- 23.Kurt B, Flynn P, Shenep JL, Pounds S, Lensing S, Ribeiro RC, et al. Prophylactic antibiotics reduce morbidity due to septicemia during intensive treatment for pediatric acute myeloid leukemia. Cancer. 2008;113:376–382. doi: 10.1002/cncr.23563. [DOI] [PubMed] [Google Scholar]