Abstract
We reported that complement (C) becomes activated and cleaved in bone marrow (BM) during preconditioning for hematopoietic transplantation and the third C component (C3) cleavage fragments C3a and desArgC3a increase responsiveness of hematopoietic stem/progenitor cells (HSPCs) to stromal derived factor-1 (SDF-1). We also demonstrated that this homing promoting effect is not C3a receptor (C3aR) dependent. Herein, we report our new observation that transplantation of C3aR-/- HSPCs into lethally irradiated recipients results in: 1) ∼5-7 day delay in recovery of platelets and leukocytes; 2) decrease in formation of day 12 colony-forming units-spleen (CFU-S); and 3) decrease in the number of donor-derived CFU-granulocyte-macrophage (GM) progenitors detectable in the BM cavities at day 16 after transplantation. In agreement with the murine data, blockage of C3aR on human umbilical cord blood CD34+ cells by C3aR antagonist SB290157 impairs their engraftment in non-obese diabetic/severe combined immunodeficient (NOD/SCID) mice. However, HSPCs from C3aR-/- mice stimulated by C3a still better responded to SDF-1 gradient, after exposure to C3a, they secrete less matrix metalloprotease-9 (MMP-9) and show impaired adhesion to stroma cells. We conclude that C3a, in addition to enhancing responsiveness of HSPCs to SDF-1 gradient in a C3aR independent manner, may also directly modulate HSPC homing by augmenting C3aR-mediated secretion of MMP-9 and cell adhesion.
Keywords: C3, C3aR, CXCR4, SDF-1, stem cells
Introduction
The collective term “homing” refers to the early stages of bone marrow (BM) seeding by hematopoietic stem/progenitor cells (HSPCs) after transplantation, which precedes engraftment, proliferation, and differentiation of these cells. In general, homing, engraftment, and expansion of HSPCs after transplantation are closely related processes, although all the factors involved have not been identified (1, 2). However, it is well demonstrated that α-chemokine stromal-derived factor-1 (SDF-1) - chemokine receptor CXCR4 axis and vascular cell adhesion molecule 1 (VCAM-1) - very late antigen-4 (VLA-4) interactions play important roles in these processes. While SDF-1 secreted by BM stroma chemoattracts intravenously infused CXCR4+ HSPCs, VCAM-1 expressed on BM endothelium interacts with and tethers VLA-4 (α4/β1 integrin)+ HSPCs (3-11).
It is well known that conditioning for transplantation by chemo/radiotherapy destroys old hematopoiesis and by emptying hematopoietic niches, makes space available for transplanted cells and highly upregulates expression of SDF-1 in the BM microenvironment, which creates a proper chemoattracting gradient for HSPCs (12-14). In addition, SDF-1 activates adhesion molecules on HSPCs (e.g., VLA-4), which promotes their adhesion and upregulates secretion of metaloproteinases (e.g., matrix metalloprotease-9 [MMP-9]) by these cells. All these processes orchestrate transendothelial migration of HSPCs into the BM microenvironment (3, 5, 6, 9-11, 15-17).
We have recently reported that myeloablative conditioning for transplants by radio/chemotherapy activates complement (C) cascade in BM as well (18-19). The protein components of C including the third C component (C3) are activated through proteolysis in a cascade-like fashion leading to the generation of “liquid phase” activation peptides with potent pro-inflammatory properties termed anaphylatoxins (C3a and desArgC3a) as well as activated/cleaved “solid phase” proteins that bind to the surface of C-activating cells (e.g., iC3b). C3 cleavage fragments bind to specific C3 receptors that are expressed on several types of cells including HSPCs (18-22) . While C3a binds to G-protein coupled, seven transmembrane-spanning C3a receptor (C3aR) and C5L2, C3adesArg binds to C5L2 only. The receptor for iC3b is CR3 and is the αMβ2-integrin, which is also known as CD11b/CD18 or Mac-1 (22-27).
We previously reported that C3 liquid phase cleavage fragments (C3a and desArgC3a) enhance responsiveness of HSPCs to SDF-1 gradient. The molecular explanation of this phenomenon is based on our observation that both C3a and desArgC3a increase incorporation of CXCR4 into membrane lipid rafts, thus facilitating its more effective assembly with down stream signaling molecules (18, 22, 23, 28). In addition, C3a, but not desArgC3a, enhances secretion of MMP-9 in HSPCs and increases adhesion of these cells to VCAM-1. As a result, HSPCs primed before transplantation by C3a but not desArgC3a home and engraft better (23). On the other hand, iC3b deposited on cells in the BM microenvironment tethers HSPCs via CR3. To support this, we found that C3 deficient mice engraft poorly with HSPCs (18, 22, 29).
Because C3aR-/- cells show normal priming effect after C3a stimulation (18, 22, 29), we hypothesized that this phenomenon does not involve C3aR. However, to better address whether C3aR is also directly involved in engraftment,we employed HSPCs from C3aR-/- mice and compared their homing and engraftment characteristics to wildtype (WT) counterparts. Our data provides the first evidence that C3a increases homing responses of HSPCs by direct interaction with C3aR and that another C3a binding receptor (C5L2 receptor) cannot compensate for C3aR deficiency. We conclude that C3a plays an important but underappreciated role in engraftment of HSPCs by increasing non-C3aR-dependent responsiveness of HSPCs to SDF-1 gradient and by C3aR-mediated increase in MMP-9 secretion and cell adhesion.
Materials and Methods
Murine BM-derived cells
Animal studies were approved by the Institutional Animal Care and Use Committee of the University of Louisville. Murine BM mononuclear cells (BMMNCs) were flushed from the femora of BALB/c C3aR+/+ (The Jackson Laboratory, Bar Harbor, ME) and BALB/c C3aR-/- (kind gift from Dr. Rick A Wetzel, University of Texas, Houston). The protocols used were approved by the appropriate institutional ethics review boards. MNCs were depleted of adherent cells (A-) and then enriched for light-density MNCs by Ficoll-Paque centrifugation. Sca-1+ cells were isolated using paramagnetic mini-beads (Miltenyi Biotec, Auburn, CA). The purity of isolated BM Sca-1+ cells was >95%, as determined by fluorescein-activated cell sorting (FACS). For some of transplant experiments, Sca-1+kit+lin- (SKL) cells were purified by employing MoFlo sorter as described (18).
Human umbilical cord blood (UCB)—derived cells
Human light-density UCB cells were obtained after informed consent; the protocols used were approved by the appropriate institutional ethics review boards. Light-density cells were depleted of adherent cells and T lymphocytes (A-T- MNCs) and enriched for CD34+ cells by immunoaffinity selection with MiniMACS paramagnetic beads (Miltenyi Biotec). The purity of isolated BM CD34+ cells was >95%, as determined by FACS analysis using a FACscan (Becton Dickinson, San Jose, CA). Human BM CD34+ transplants were performed on non-obese diabetic/severe combined immunodeficient (NOD/SCID) inbred mice (The Jackson Laboratory).
Adhesion Assay
WT BM stroma was grown to confluence and WT or C3aR-/- Sca-1+ cells were non-stimulated or stimulated by C3a (1μg/ml) or desArgC3a (1μg/ml) and added to the cultures for either 1 or 3 hours. Non-adhered cells were washed off. Subsequently, the entire cultures were trypsinized and plated in methylcellulose cultures and stimulated to grow colony-forming units-granulocyte-macrophage (CFU-GM) colonies as described (18). The number of colonies was scored under an inverted microscope.
Zymography
To evaluate MMP-9 secretion by Sca-1+, 2 × 106 cells/mL were incubated (for 24 h at 37°C in 5% CO2) in the absence or presence of C3a or desArg C3a (1 μg/ml), and cell-were isolated for RT-PCR analysis of mRNA expression for MMP-2 and MMP-9 and cell-conditioned media were analyzed immediately by zymography as previously described by us (23). The intensity of the bands was quantified using the NIH ScionImage for Windows software (Scion Corp., Frederick, MD).
Short term homing assays
Mice were irradiated with a lethal dose of γ-irradiation (900 cGy). After 24 hours, the mice were transplanted (by tail vein injection) with 2×105 BM Sca-1+ cells. At 6 or 24 hours post transplant, BM was harvested from the femurs and plated in serum-free methylcellulose cultures and stimulated to grow CFU-GM colonies by stimulation with granulocyte macrophage-colony stimulating factor (GM-CSF) (25ng/mL) + Interleukin (IL)-3 (10 ng/ml).
BM transplantation
For transplant experiments, mice were irradiated with a lethal dose of γ-irradiation (900 cGy). After 24 hours, mice were transplanted with 105 BM Sca-1+ cells or 104 Sca-1+kit+lin- (SKL) cells by tail vein injection. Anesthetized transplanted mice were bled at various intervals from the retro-orbital plexus to obtain samples for leukocytes and platelets.
Evaluation of engraftment
CFU-spleen (S) assay
For the CFU-S assays, mice were transplanted with 5 × 104 Sca-1+ marrow cells. At day 12, spleens were removed, fixed in Telesyniczky’s solution, and CFU-S colonies were counted on the surface of the spleen as described (18).
Number of CFU-GM/femur
Femora of transplanted mice were flushed with phosphate-buffered saline (PBS) at day 16 post-transplant. Subsequently, Sca-1+ cells were purified and plated in serum-free methylcellulose cultures and stimulated to grow CFU-GM colonies by granulocyte-colony stimulating factor (G-CSF; 25 ng/ml) + IL-3 (10 ng/ml).
Statistical analysis
Arithmetic means and standard deviations were calculated using Instat 1.14 software (Graphpad, San Diego, CA). Statistical significance was defined as p<0.05. Data were analyzed using Student’s t-test for unpaired samples.
Results
Delayed engraftment after transplantation of C3aR-/- HSPCs
We reported that C3aR-/- mice or WT mice in which C3aR is blocked by small molecular antagonist SB 290157 are easy mobilizers in response to G-CSF (30, 31). This could indicate that C3aR plays an important role in retention of HSPCs in the BM microenvironment.
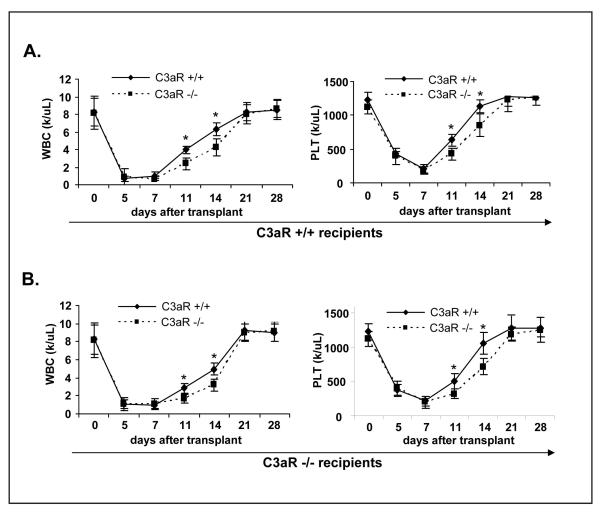
To better address this question, we performed hematopoietic transplantation experiments using HSPCs from C3aR-/- and WT mice to both WT and C3aR-/- animals. As shown in Figure 1 panel A, the kinetics of white blood cell (WBC) and platelet counts revealed delayed engraftment of C3aR-/- HSPCs. Next, to exclude a potential contribution of C3aR deficiency on cells in the hematopoietic environment, we performed transplants into C3aR-/- recipients (Fig. 1 panel B) and noticed that C3aR-/- HSPCs also show delayed engraftment as compared to WT HSPCs. Thus, regardless of the genetic background of the recipient animal, C3aR-/- HSPCs do not engraft as well as their WT counterparts.
Figure 1. HSPCs from C3aR-/- mice show defective engraftment.
This is demonstrated by a delayed recovery of platelets and leukocytes. Panel A. Lethally irradiated WT mice were transplanted with SKL cells (104/mouse) isolated from C3aR-/- and WT animals. Recovery of platelet and leukocyte counts was evaluated at days 0, 5, 7, 11, 16, and 28. Panel B. Lethally irradiated C3aR-/- mice were transplanted with SKL cells isolated from C3aR-/- and WT animals. Recovery of platelet and leukocyte counts was evaluated at days 0, 5, 7, 11, 16, and 28. The data shown represent the combined results from three independent experiments performed with 10 mice/group. *p<0.0001. All mice in irradiation control (non-transplanted) died before day 14 (not shown).
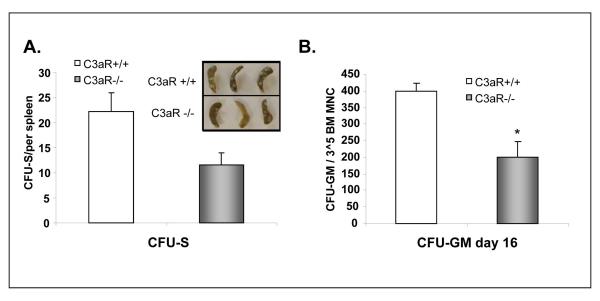
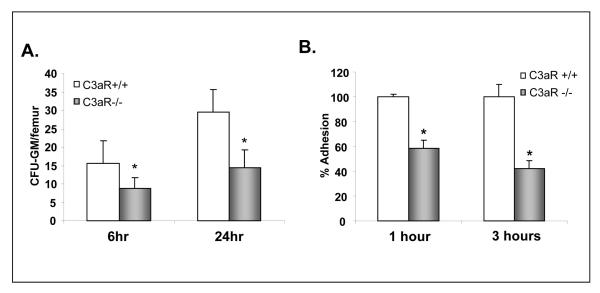
Our WBC and platelet counts recovery kinetic data were subsequently confirmed by evaluating the number of: a) day 11 CFU-S (Fig. 2 panel A) and b) cells able to grow CFU-GM colonies that were recovered at day 16 from the femora of transplanted mice (Fig. 2 panel B) or in WT mice transplanted either with C3aR-/- or WT HSPCs. Next, similar results were obtained after transplantation of HSPCs from C3aR-/- or WT mice to C3aR-/- deficient animals (not shown). Thus, our data revealed an engraftment defect of C3aR-/- HSPCs and show that this defect does not depend on C3aR expression in the BM microenvironment. Therefore, these data clearly demonstrate that C3a modulates engraftment and that some of its effects are mediated by its direct interaction with C3aR on HSPCs.
Figure 2. HSPCs from C3aR-/- mice show decrease in day 11 CFU-S formation and content of day 16 CFU-GM/femur.
Panel A. Lethally irradiated WT mice were transplanted with Sca-1+ cells isolated from C3aR-/- and WT animals. CFU-S colonies were counted 11 days after transplantation. Inset. Examples of spleens with CFU-S colonies. The data shown represent the combined results from three independent experiments performed with 10 mice/group. *p<0.0001. All mice in irradiation control (non-transplanted) did not have any endogenous CFU-S colonies and died before day 14. Panel B. Lethally irradiated WT mice were transplanted with Sca-1+ cells isolated from C3aR-/- and WT animals. BM from femora of transplanted mice were isolated and the contents of CFU-GM and femurs was evaluated in secondary in vitro cultures. The data shown represent the combined results from three independent experiments performed with 6 mice/group. *p<0.003. All mice in irradiation control (non-transplanted) died before day 14.
C3aR-/- cells show defective homing to BM
Homing is the first step of BM seeding by HSPCs after transplantation, which precedes their engraftment, proliferation, and differentiation. Because we demonstrated that the C3a-C3aR axis is not involved in proliferation of HSPCs (23), we asked if delayed recovery of hematopoiesis in mice transplanted with C3aR-/- cells could be explained by their defective homing and lodgment to the BM microenvironment.
To address this issue, we employed short-homing assay to evaluate lodging of HSPCs to BM. Accordingly, WT mice were lethally irradiated and, 24 hours later, they were transplanted with C3aR-/- or WT HSPCs. Subsequently, animals were sacrificed 6 or 24 hours after transplantation, the femora were removed, and BM cells were isolated and plated in methylocelluose cultures to grow CFU-GM colonies (Fig. 2 panel A). By employing this assay, we found that C3aR-/- HSPCs show defective homing and lodging to the BM microenvironment. This defective lodging could be the result of defective transendothelial migration of HSPCs to cells in the BM microenvironment.
To further address this question, we performed in vitro adhesion studies with sorted Sca-1+ cells. Accordingly, Sca-1+ cells isolated from the BM of C3aR-/- or WT mice were non-exposed, or exposed for 30 minutes to C3a or desArgC3a and subsequently plated over BM-derived irradiated stroma feeder layers established in well plates. Subsequently, at 1 and 3 hours later, plates were gently shaken and non-adherent cells were discarded. Finally, cells remaining in the wells were trypsinized and plated in methylcellulose to grow CFU-GM colonies. We noticed that C3a, but not desArgC3a, increased adhesion in WT but not C3aR-/- HSPCs (Table 1). Based on this, the C3a-C3aR axis seems to be involved in increasing adhesion of HSPCs. Because stimulation by desArgC3a was ineffective to compensate the C3aR deficiency in C3aR-/- cells that express C3a and desArgC3a binding C5L2 receptor, we excluded the potential involvement of C5L2 in this phenomenon.
Table 1. The C3a-C3aR axis enhances adhesion of BM-derived Sca-1+ cells to BM stroma.
The number of CFU-GM from Sca-1+ cells from WT mice that adhered to WT strom a stimulated for 30 m in by C3a was arbitrarily assumed to be 100% in 1 hour and 3 hour adhesion assays. Data are pooled from quadruplicate samples from three independent experiments (n=12).
| Source of Sca-1+ cells [adhesion time] |
Non-stimulated | Stimulated by C3a (1μg/ml, 30 min) |
Stimulated by desArgC3a (1μg/ml, 30 min) |
|---|---|---|---|
| WT [1 hr] | 58+/-11* | 100+/-21 | 67+/-15* |
| C3aR-/- [1 hr] | 62+/-19* | 64+/-17* | 62+/-17* |
| WT [3 hrs] | 49+/-11* | 100+/-8 | 46+/-13* |
| C3aR-/- [3 hrs] | 53+/-16* | 49+/-20* | 51+/-7* |
p<0.00001
C3a-C3aR axis regulates secretion of MMP9 by HSPCs
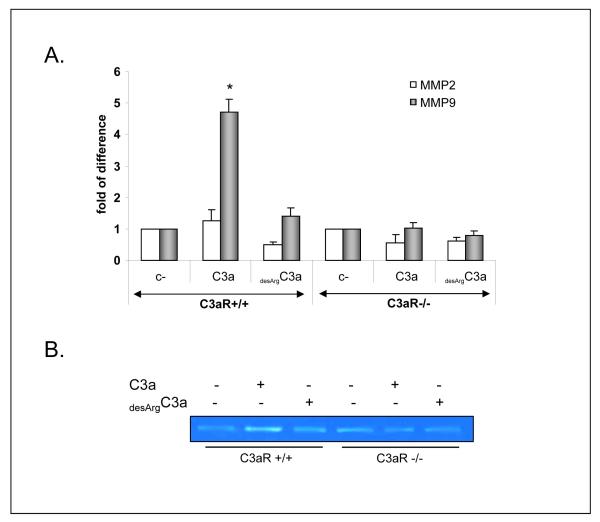
Following adhesion to endothelium, HSPCs have to migrate through the endothelial basement membrane in a process that involves secretion of proteolytic enzymes. MMP-9 was shown to play a pivotal role here. In our previous studies, we demonstrated that C3a stimulates secretion of MMP-9 by HSPCs (23). To address the importance of C3aR in this phenomenon, we exposed BM-derived Sca-1+ cells isolated from C3aR-/- and WT to C3a or desArgC3a and evaluated MMP-9 expression at the mRNA (Fig. 4 panel A) and protein (Fig. 4 panel B) levels. We noticed that C3a, but not desArgC3a, upregulates expression of MMP-9 in WT but not C3aR-/- cells. Again, because stimulation by desArgC3a was ineffective to compensate the C3aR deficiency in C3aR-/- cells that express C3a and desArgC3a binding C5L2 receptor, we excluded the potential involvement of C5L2 in this phenomenon.
Figure 4. C3a-C3aR axis enhances expression of MMP-9 by HSPC.
Sca-1+ BM cells from C3aR-/- or WT mice were non-stimulated or stimulated overnight by C3a (1 μg/ml) or desArgC3a (1 μg/ml) and mRNA was isolated for reverse transcriptase-polymerase chain reaction (RT-PCR) analysis of MMP-9 and MMP-2 expression (Panel A). Supernatants were collected for MMP-9 analysis by zymography (Panel B). The data shown represent the combined results from three independent experiments. *p<0.0001 for real-time quantitative (RQ)-PCR. One representative zymography study is shown.
C3aR also plays a role in engraftment of human HSPCs
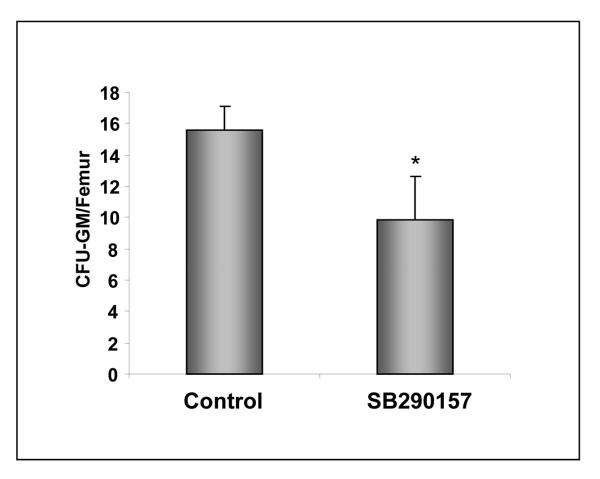
Finally, to address the role of C3aR in homing of human HSPCs, we performed xenotransplants in NOD/SCID mice (Fig. 5). Human UCB purified human CD34+ cells were pre-incubated or not exposed to C3aR antagonist SB 290157 and transplanted into lethally irradiated NOD/SCID mice 24 hours after an irradiation that eliminated murine endogenous HSPCs. Subsequently, 24 hours after transplantation of human UCBs, we recovered cells from the femora of transplanted mice and plated them in methylcellulose cultures to growth colonies in the presence of human hematopoietic factors. We noticed that the number of human CFU-GM progenitors recovered from murine femora was significantly lower in animals transplanted with UCB CD34+ cells in which C3aR was blocked by SB 290157 (Fig. 5), which also implicates a role of C3aR in homing of human HSPCs. Of note, in control experiments, no CFU-GM colonies were grown from BM cells recovered from lethally irradiated NOD/SCID mice that did not receive human UCB cells (not shown).
Figure 5. Blocking of C3aR on human UCB CD34+ cells impairs homing in NOD/SCID mice model.
Human UCB CD34+ cells were exposed or not exposed to C3aR antagonist SB 290157 and transplanted into lethally irradiated NOD/SCID mice. At 24 hours later, BM from femora of transplanted mice was isolated and the contents of human CFU-GM and femurs were evaluated in secondary in vitro cultures. The data shown represent the combined results from three independent experiments performed with 6 mice/group each. *p<0.001. No colonies grew from lethally irradiated and control animals not transplanted with UCB cells (n=5).
Discussion
The α-chemokine SDF-1 is upregulated in the BM microenvironment after conditioning for transplantation by radio-chemotherapy, which is a crucial chemoattractant for intravenously infused (1, 2, 4, 12, 13). However, the biological activity of the SDF-1-CXCR4 axis is modulated by several factors. For example, data from our laboratory demonstrated that during conditioning for transplantation, C cascade is activated. While C3 liquid phase cleavage fragments (C3a and desArgC3a) prime and enhance responsiveness of HSPCs to SDF-1 gradient (22, 23, 28, 29), solid phase iC3b tethers HSPCs to BM stroma (18).
In agreement with this, we have previously shown that HSPCs poorly engraft after transplantation into C3-/- mice (18). We have explained this defective engraftment in C3-/- mice as a lack of generated C3a and desArgC3a, which enhance responsiveness of HSPCs to SDF-1 gradient (18, 28). To our surprise, however, we noticed that C3 cleavage fragments increase responsiveness of HSPCs to SDF-1 gradient in vitro and that this effect does not seem to be C3aR dependent. In support of this, we demonstrated that cells from WT and C3aR-/- mice respond equally to priming effect to both C3a and desArgC3a (18).
On the other hand, we have also previously shown that C3aR-/- mice as well as WT animals exposed to C3aR antagonist SB290157 are easy G-CSF mobilizers (30, 31). This latter observation prompted us to investigate whether C3a-C3aR may play a role in the retention of HSPCs in BM, which could be a potentially novel role of C3a independent of its priming effect on HSPCs.
Here, we show for the first time that transplantation of murine C3aR-/- HSPCs results in delayed engraftment and hematopoietic recovery of lethally irradiated mice. Because C3aR-/- animals engraft normally with WT cells, this defect is intrinsic to HSPCs and does not depend on C3aR expressed on cells in the hematopoietic microenvironment. More importantly, our in vivo lodging experiments suggest that this defect is related to impaired homing of transplanted cells. Furthermore, our short-term lodging xenotransplants experiments in NOD/SCID mice revealed that this novel role for the C3aR-C3a axis also seems to be relevant for homing of human CD34+ cells. Thus, the C3a-C3aR axis plays an underappreciated role in homing of both human and murine HSPCs.
Based on these data, we postulate that activation of C cascade and release of C3a in BM after conditioning for transplantation not only primes responsiveness of HSPCs to SDF-1 gradient by a C3aR independent lipid raft formation-dependent mechanism (18, 22, 23, 29), but in addition, C3a directly engages C3aR on the surface of HSPCs and thus enhances homing of these cells to BM.
To better address the role of the C3aR-C3a axis in the early steps of homing and engraftment, we report here that murine HSPCs stimulated by C3a, but not desArgC3a, secrete more MMP-9 and adhere better to BM stroma. Thus, C3a-C3aR plays an important role in increasing secretion of MMP-9, which facilitates transmigration of HSPCs through the endothelial barrier as well as by increasing the interaction of HSPCs with cells in the hematopoietic microenvironment. Furthermore, because desArgC3a was not able to compensate for the C3aR deficiency, we excluded that another C3aR, the C5L2 receptor, is not involved in these phenomena.
In our previous work, we have also shown that another C3 cleavage fragment, iC3b, which is deposited during activation of C on the surface of damaged stroma cells in BM, increases tethering of HSPCs via the CR3 (CD11b/CD18) receptor. This dual role of C3 cleavage fragments, i.e., liquid phase C3a and solid phase iC3B, may explain why the engraftment defect in C3-/- mice was somehow slightly more pronounced than that observed after transplantation of WT cells into C3aR-/- mice. It is obvious that C3aR-/- mice still express and activate C3 and thus iC3b is normally deposited on BM stroma cells, providing a proper tethering for transplanted HSPCs via CR3 (CD11b/CD18) receptor (18).
Our data also explain our previous priming studies in vivo (22, 23, 29) showing that stimulation of HSPCs before transplantation with C3a, but not desArgC3a, accelerated engraftment of HSPCs into lethally irradiated WT mice. Because desArgC3a does not activate C3aR, the short exposure of HSPCs before transplantation to C3a accelerates engraftment by activating the homing function of C3aR. To support this and as we have demonstrated in this work, C3a, but not desArgC3a, increased MMP-9 secretion and enhanced adhesion of HSPCs to BM stroma cells. By employing C3aR-/- mice in this study, we clearly demonstrate that this effect is related to C3aR and another C3a binding receptor, C5L2, which also binds desArgC3a, cannot compensate for the C3aR deficiency. However, further research is required to identify if other proteases are released from HSPCs in addition to MMP-9 and to identify which adhesion molecules are directly affected by the C3a-C3aR axis.
In conclusion, we show for the first time that the C3a-C3aR axis plays an underappreciated role in homing of HSPCs to BM. By employing C3aR-/- mice, we excluded the involvement of the C5L2 receptor. Our data show that defective homing and engraftment of C3aR-/- HSPCs could be explained by the C3a-C3aR axis modulating secretion of MMP-9 by HSPCs as well as their adhesion. Thus, C3a plays a dual role in homing and engraftment (Figure 6). As reported previously, the first is by enhanced responsiveness of HSPCs to SDF-1 gradient through a C3aR independent mechanism (18, 22, 28, 29). Secondly and as shown here by engaging C3aR, it modulates several functions pertinent to transendothelial migration and homing (e.g., secretion of proteases and adhesion).
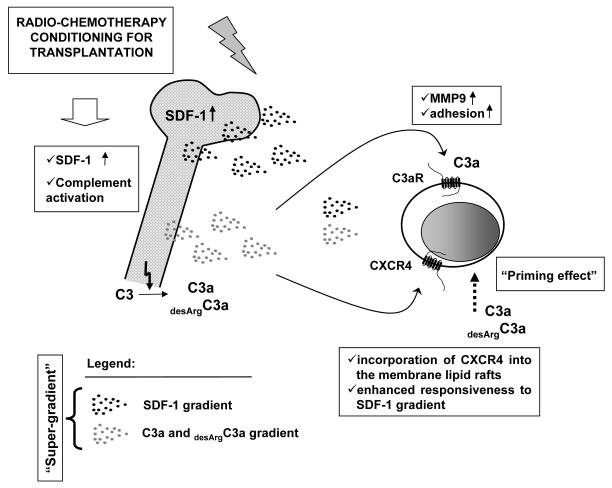
Figure 6. SDF-1 and C3 cleavage fragments create “super-gradient” for HSPC.
Conditioning for transplantation by radio-chemotherapy upregulates in BM i) expression of SDF-1 (12) and ii) activates complement cascade (18, 22, 23, 28, 29). While SDF-1 directly chemoattracts HSPCs by interacting with CXCR4 receptor, our work revealed that the homing function of the SDF-1-CXCR4 axis is modulated by C3 cleavage fragments (C3a and desArgC3a) — both in C3aR-dependent and C3aR-independent manner. In a first mechanism, C3a (but not desArgC3a) activates C3aR and increases adhesiveness of HSPCs as well as secretion of MMP-9 by these cells. In a second one (C3aR independent) both C3a and desArgC3a enhance/prime responsiveness of HSPCs to SDF-1 gradient by increasing incorporation of CXCR4 into membrane lipid rafts. CXCR4 incorporated in lipid rafts is better connected with signal transduction proteins what explains why CXCR4+ HSPCs respond more robust to SDF-1. However, the identification of receptor/receptors? involved in this C3a and desArgC3s mediated “priming” effect requires further studies.
Figure 3. HSPCs from C3aR-/- mice show defective homing in vivo.
Panel A. Lethally irradiated WT mice were transplanted with Sca-1+ cells isolated from C3aR-/- or WT animals. At 6 and 24 hours later, BM from femora of transplanted mice were isolated and the contents of CFU-GM and femurs were evaluated in secondary in vitro cultures. The data shown represent the combined results from three independent experiments performed with 6 mice/group. *p<0.0237 for 6 hours and *p<0.0016 for 24 hours. No colonies grew from lethally irradiated and non-transplanted animals.
Acknowledgments
Supported by NIH grant R01 CA106281, NIH R01 DK074720, and Stella and Henry Endowment to MZR.
Literature
- 1.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106(6):1901–1910. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 2.Chute JP. Stem cell homing. Current Opinion in Hematology. 2006;13(6):399–406. doi: 10.1097/01.moh.0000245698.62511.3d. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch E, Iglesias A, Potocnik AJ, Hartmann U, Fassler R. Impaired migration but not differentiation of haematopoietic stem cells in the absence of beta1 integrins. Nature. 1996;380(6570):171–175. doi: 10.1038/380171a0. [DOI] [PubMed] [Google Scholar]
- 4.Papayannopoulou T. Bone marrow homing: the players, the playfield, and their evolving roles. Current Opinion in Hematology. 2003;10(3):214–219. doi: 10.1097/00062752-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Papayannopoulou T, Priestley GV, Nakamoto B, Zafiropoulos V, Scott LM. Molecular pathways in bone marrow homing: dominant role of alpha(4)beta(1) over beta(2)-integrins and selectins. Blood. 2001;98(8):2403–2411. doi: 10.1182/blood.v98.8.2403. [DOI] [PubMed] [Google Scholar]
- 6.Peled A, Kollet O, Ponomaryov T, Petit I, Franitza S, Grabovsky V, Slav MM, Nagler A, Lider O, Alon R, Zipori D, Lapidot T. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000;95(11):3289–3296. [PubMed] [Google Scholar]
- 7.Pelus LM, Fukuda S. Chemokine-mobilized adult stem cells; defining a better hematopoietic graft. Leukemia. 2008;22(3):466–473. doi: 10.1038/sj.leu.2405021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papayannopoulou T, Craddock C, Nakamoto B, Priestley GV, Wolf NS. The VLA4/VCAM-1 adhesion pathway defines contrasting mechanisms of lodgement of transplanted murine hemopoietic progenitors between bone marrow and spleen. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(21):9647–9651. doi: 10.1073/pnas.92.21.9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vermeulen M, Le Pesteur F, Gagnerault MC, Mary JY, Sainteny F, Lepault F. Role of adhesion molecules in the homing and mobilization of murine hematopoietic stem and progenitor cells. Blood. 1998;92(3):894–900. [PubMed] [Google Scholar]
- 10.Sanz-Rodriguez F, Hidalgo A, Teixido J. Chemokine stromal cell-derived factor-1alpha modulates VLA-4 integrin-mediated multiple myeloma cell adhesion to CS-1/fibronectin and VCAM-1. Blood. 2001;97(2):346–351. doi: 10.1182/blood.v97.2.346. [DOI] [PubMed] [Google Scholar]
- 11.Peled A, Grabovsky V, Habler L, Sandbank J, Arenzana-Seisdedos F, Petit I, Ben-Hur H, Lapidot T, Alon R. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34(+) cells on vascular endothelium under shear flow. The Journal of Clinical Investigation. 1999;104(9):1199–1211. doi: 10.1172/JCI7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ponomaryov T, Peled A, Petit I, Taichman RS, Habler L, Sandbank J, Arenzana-Seisdedos F, Magerus A, Caruz A, Fujii N, Nagler A, Lahav M, Szyper-Kravitz M, Zipori D, Lapidot T. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. The Journal of Clinical Investigation. 2000;106(11):1331–1339. doi: 10.1172/JCI10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss L, Bullorsky E, Ashkenazi YJ, Slavin S. Optimal time interval between myeloablative whole body irradiation and reconstitution with syngeneic bone marrow graft. Bone Marrow Transplantation. 1988;3(3):207–210. [PubMed] [Google Scholar]
- 14.Shirota T, Tavassoli M. Alterations of bone marrow sinus endothelium induced by ionizing irradiation: implications in the homing of intravenously transplanted marrow cells. Blood Cells. 1992;18(2):197–214. [PubMed] [Google Scholar]
- 15.Janowska-Wieczorek A, Marquez LA, Dobrowsky A, Ratajczak MZ, Cabuhat ML. Differential MMP and TIMP production by human marrow and peripheral blood CD34(+) cells in response to chemokines. Experimental Hematology. 2000;28(11):1274–1285. doi: 10.1016/s0301-472x(00)00532-4. [DOI] [PubMed] [Google Scholar]
- 16.Janowska-Wieczorek A, Matsuzaki A, L AM. The Hematopoietic Microenvironment: Matrix Metalloproteinases in the Hematopoietic Microenvironment. Hematology. 2000;4(6):515–527. [PubMed] [Google Scholar]
- 17.Redondo-Munoz J, Escobar-Diaz E, Samaniego R, Terol MJ, Garcia-Marco JA, Garcia-Pardo A. MMP-9 in B-cell chronic lymphocytic leukemia is up-regulated by alpha4beta1 integrin or CXCR4 engagement via distinct signaling pathways, localizes to podosomes, and is involved in cell invasion and migration. Blood. 2006;108(9):3143–3151. doi: 10.1182/blood-2006-03-007294. [DOI] [PubMed] [Google Scholar]
- 18.Ratajczak MZ, Reca R, Wysoczynski M, Kucia M, Baran JT, Allendorf DJ, Ratajczak J, Ross GD. Transplantation studies in C3-deficient animals reveal a novel role of the third complement component (C3) in engraftment of bone marrow cells. Leukemia. 2004;18(9):1482–1490. doi: 10.1038/sj.leu.2403446. [DOI] [PubMed] [Google Scholar]
- 19.Ross GD, Lambris JD, Cain JA, Newman SL. Generation of three different fragments of bound C3 with purified factor I or serum. I. Requirements for factor H vs CR1 cofactor activity. J Immunol. 1982;129(5):2051–2060. [PubMed] [Google Scholar]
- 20.Walport MJ. Complement. Second of two parts. The New England Journal of Medicine. 2001;344(15):1140–1144. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 21.Walport MJ. Complement. First of two parts. The New England Journal of Medicine. 2001;344(14):1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 22.Ratajczak MZ, Reca R, Wysoczynski M, Yan J, Ratajczak J. Modulation of the SDF-1-CXCR4 axis by the third complement component (C3)--implications for trafficking of CXCR4+ stem cells. Experimental Hematology. 2006;34(8):986–995. doi: 10.1016/j.exphem.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 23.Reca R, Mastellos D, Majka M, Marquez L, Ratajczak J, Franchini S, Glodek A, Honczarenko M, Spruce LA, Janowska-Wieczorek A, Lambris JD, Ratajczak MZ. Functional receptor for C3a anaphylatoxin is expressed by normal hematopoietic stem/progenitor cells, and C3a enhances their homing-related responses to SDF 1. Blood. 2003;101(10):3784–3793. doi: 10.1182/blood-2002-10-3233. [DOI] [PubMed] [Google Scholar]
- 24.Teixido J, Hemler ME, Greenberger JS, Anklesaria P. Role of beta 1 and beta 2 integrins in the adhesion of human CD34hi stem cells to bone marrow stroma. The Journal of Clinical Investigation. 1992;90(2):358–367. doi: 10.1172/JCI115870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xia Y, Borland G, Huang J, Mizukami IF, Petty HR, Todd RF, 3rd, Ross GD. Function of the lectin domain of Mac-1/complement receptor type 3 (CD11b/CD18) in regulating neutrophil adhesion. J Immunol. 2002;169(11):6417–6426. doi: 10.4049/jimmunol.169.11.6417. [DOI] [PubMed] [Google Scholar]
- 26.Honczarenko M, Lu B, Nicholson-Weller A, Gerard NP, Silberstein LE, Gerard C. C5L2 receptor is not involved in C3a / C3a-desArg-mediated enhancement of bone marrow hematopoietic cell migration to CXCL12. Leukemia. 2005;19(9):1682–1683. doi: 10.1038/sj.leu.2403862. author reply 1684-1685. [DOI] [PubMed] [Google Scholar]
- 27.Kalant D, Cain SA, Maslowska M, Sniderman AD, Cianflone K, Monk PN. The chemoattractant receptor-like protein C5L2 binds the C3a des-Arg77/acylation-stimulating protein. The Journal of Biological Chemistry. 2003;278(13):11123–11129. doi: 10.1074/jbc.M206169200. [DOI] [PubMed] [Google Scholar]
- 28.Honczarenko M, Ratajczak MZ, Nicholson-Weller A, Silberstein LE. Complement C3a enhances CXCL12 (SDF-1)-mediated chemotaxis of bone marrow hematopoietic cells independently of C3a receptor. J Immunol. 2005;175(6):3698–3706. doi: 10.4049/jimmunol.175.6.3698. [DOI] [PubMed] [Google Scholar]
- 29.Reca R, Wysoczynski M, Yan J, Lambris JD, Ratajczak MZ. The role of third complement component (C3) in homing of hematopoietic stem/progenitor cells into bone marrow. Advances in Experimental Medicine and Biology. 2006;586:35–51. doi: 10.1007/0-387-34134-X_3. [DOI] [PubMed] [Google Scholar]
- 30.Ratajczak J, Reca R, Kucia M, Majka M, Allendorf DJ, Baran JT, Janowska-Wieczorek A, Wetsel RA, Ross GD, Ratajczak MZ. Mobilization studies in mice deficient in either C3 or C3a receptor (C3aR) reveal a novel role for complement in retention of hematopoietic stem/progenitor cells in bone marrow. Blood. 2004;103(6):2071–2078. doi: 10.1182/blood-2003-06-2099. [DOI] [PubMed] [Google Scholar]
- 31.Ratajczak MZ, Wysoczynski M, Reca R, Wan W, Zuba-Surma EK, Kucia M, Ratajczak J. A pivotal role of activation of complement cascade (CC) in mobilization of hematopoietic stem/progenitor cells (HSPC) Advances in Experimental Medicine and Biology. 2008;632:47–60. [PubMed] [Google Scholar]