Abstract
The intracochlear infusion of neurotrophic factors via a mini-osmotic pump has been shown to prevent deafness-induced spiral ganglion neuron (SGN) degeneration; however, the use of pumps may increase the incidence of infection within the cochlea, making this technique unsuitable for neurotrophin administration in a clinical setting. Cell- and gene-based therapies are potential therapeutic options. This study investigated whether Schwann cells which were genetically modified to over-express the neurotrophins brain-derived neurotrophic factor (BDNF) or neurotrophin 3 (Ntf3, formerly NT-3) could support SGN survival in an in vitro model of deafness. Co-culture of either BDNF over-expressing Schwann cells or Ntf3 over-expressing Schwann cells with SGNs from early postnatal rats significantly enhanced neuronal survival in comparison to both control Schwann cells and conventional recombinant neurotrophin proteins. Transplantation of neurotrophin over-expressing Schwann cells into the cochlea may provide an alternative means of delivering neurotrophic factors to the deaf cochlea for therapeutic purposes.
Keywords: auditory, ex vivo gene transfer, BDNF, Ntf3
Sensorineural hearing loss (SNHL) is a common cause of deafness, and normally results from damage to or loss of the sensory auditory hair cells, which are a key step in the transmission of sound signals to the brain. Cochlear implants are currently the only therapeutic intervention for patients with a severe-profound SNHL. These devices bypass the degenerated hair cells to directly electrically stimulate residual spiral ganglion neurons (SGNs) to provide the auditory cues required for speech perception. However, SGNs undergo progressive degeneration following a SNHL, and evidence from animal studies indicates that ongoing SGN degeneration has the potential to compromise the efficacy of the electrode-neural interface, including elevated thresholds (Hardie and Shepherd, 1999), prolonged refractory behaviour (Shepherd et al., 2004), bursting activity (Shepherd and Javel, 1997), and slower recovery of the neural membrane following action potential generation (Sly et al., 2007). It is therefore anticipated that maintenance of a robust SGN population may improve the efficacy of the electrode-neural interface and enhance cochlear implant performance.
Previous studies have shown that neurotrophic factors such as brain-derived neurotrophic factor (BDNF) and neurotrophin 3 (Ntf3), which are important for the normal development and maintenance of the auditory system (Pirvola et al., 1992; Ylikoski et al., 1993; Farinas et al., 1994; Pirvola et al., 1994; Wheeler et al., 1994; Ernfors et al., 1995; Farinas et al., 2001; Fritzsch et al., 2004; Fritzsch et al., 2005), can also support SGN survival and prevent degeneration in models of deafness (Ernfors et al., 1996; Miller et al., 1997; Gillespie et al., 2003; Gillespie et al., 2004; Richardson et al., 2005; Wise et al., 2005). These anatomical changes have also been shown to correspond with functional improvements of the deaf auditory system (Shinohara et al., 2002; Yamagata et al., 2004; Shepherd et al., 2005), meaning the surviving neurons retain the electrophysiological properties required to transfer sound signals to the brain. Unfortunately, however, the survival effects are lost if delivery of the exogenous neurotrophin stops (Gillespie et al., 2003; Shepherd et al., submitted). Since the loss of neurotrophic support from the hair cells is considered a major contributing factor to the degeneration of SGNs in the deaf ear, these results are not surprising, and together these concepts suggest that ongoing neurotrophic factor support may be required for long-term or permanent maintenance of SGNs in deafness.
Furthermore, clinically safe and effective drug delivery methods are required. The finite delivery period of osmotic pumps mean that long-term neurotrophic factor treatment would require repeated replacement of the pumps, thereby increasing the risk of infection associated with such surgical procedures. While refillable pump-based systems have been shown to function adequately for up to eight months in rats (Praetorius et al., 2001), the long-term bioactivity of neurotrophic factors at body temperature has not been confirmed, and the regular refilling of these devices still poses a risk for the introduction of infection directly into the cochlea. While cerebrospinal fluid shunts and drug delivery pumps are routinely implanted in the central nervous system on a chronic basis, infection is a major cause of morbidity and mortality of these devices, with infection rates in the order of 8–10% for both (Albright et al., 2004; Ferguson et al., 2007; Kan and Kestle, 2007; Motta et al., 2007; Sciubba et al., 2007). Such outcomes would be totally unacceptable if translated to an intracochlear drug delivery system. Prevention of infection within the inner ear is extremely important, firstly, to minimise further damage to and loss of SGNs, and secondly, to avoid infection spreading via the cochlear aqueduct to the central nervous system.
Gene therapy is an alternative method for increasing neurotrophin expression within the cochlea, and in fact in situ transduction of the spiral ganglion with genes encoding BDNF, Ntf3 or glial-derived neurotrophic factor has been shown to support SGN survival (Staecker et al., 1998; Yagi et al., 2000; Bowers et al., 2002; Lalwani et al., 2002; Nakaizumi et al., 2004). However, long-term transgene expression would require the use of viral vectors, which also raises concerns regarding clinical applicability.
Ex vivo gene transfer, which involves the transduction of a host population of cells in vitro followed by transplantation of these cells in vivo, is an alternative for neurotrophin therapy within the inner ear. Importantly, previous studies have reported that cells genetically modified to over-produce neurotrophins can continue to express the transgene for at least 12 months (Winn et al., 1996; Tuszynski et al., 1998), providing further support for this technique as a potential therapy for prevention of SGN degeneration in deafness.
This study therefore aimed to investigate the combination of cell- and gene-based techniques on SGN survival. Specifically, Schwann cells were genetically modified to over-express either BDNF or Ntf3 and the survival effects of these cells on SGNs was determined in vitro.
EXPERIMENTAL PROCEDURES
In order to test the survival-promoting effect of neurotrophin over-expressing Schwann cells on SGNs, Schwann cells transfected with either BDNF or Ntf3 were co-cultured with primary SGNs. All experiments were performed under the approval of the Animal Research and Ethics Committee of the Royal Victorian Eye and Ear Hospital, and in accordance with the guidelines of the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Expression vectors
Expression plasmids encoding for C-terminal enhanced green fluorescent protein (EGFP)-tagged rat prepro BDNF or human prepro Ntf3 were kindly provided by Dr Volkmar Lessmann, from the Johannes Gutenberg Universität, Mainz, Germany. The neurotrophin expression vectors had been constructed as previously described (Haubensak et al., 1998; Hartmann et al., 2001; Brigadski et al., 2005). Briefly, the complete sequence of either rat prepro BDNF cDNA or human prepro Ntf3 cDNA was introduced into the cytomegalovirus-promoter driven pEGFP-N1 expression vector (Clontech, Cambridge, UK), which also contains genes conferring resistance to kanamycin and neomycin. The original pEGFP-N1 vector was also provided as a control.
Transformation and plasmid collection
All constructs were introduced into TOP10 competent Escherichia coli bacterial cells (Invitrogen, Melbourne, VIC, Australia) at a concentration of 200ng plasmid per 50μl E. coli cells via heat shock, and cells were grown on kanamycin-containing Luria Broth agar plates. Plasmid DNA was purified from liquid cultures using the QIAprep Spin Miniprep Kit column-based method (QIAGEN, Melbourne, VIC, Australia) and stored at 20°C in Tris-EDTA (ethylene diaminetetra-acetic acid) buffer until required for transfection.
Schwann cell cultures
Schwann cell cultures were kindly provided by Dr Simon Murray and Professor Trevor Kilpatrick from the Howard Florey Institute, Melbourne, Australia. Briefly, Schwann cells were prepared from postnatal day (P) three rat sciatic nerve, and purified to >99.5% purity using the fibroblast inhibitor cytosine arabinoside followed by treatment with antiserum to the selective fibroblast antigen Thy-1, as previously described (Brockes et al., 1979). Schwann cells were grown on poly-lysine (25μg/ml; Sigma, Castle Hill, NSW, Australia) coated 75cm2 flasks (Greiner Bio-One [Interpath Services, West Heidelberg, VIC, Australia]) in 10ml Schwann cell media (SCM; Dulbecco’s modified Eagle’s medium [DMEM; Thermo Electron Corporation, Noble Park, VIC, Australia] containing 2mM L-glutamine [Thermo], 50U/ml penicillin/streptomycin [Thermo], 10% fetal calf serum [FCS; Thermo], 0.08% bovine pituitary extract [Sigma] and 2μM Forskolin [Sigma]) at 37°C, 10% CO2. The Schwann cells were maintained by sub-culturing when the cells were confluent (every 3–4 days) using 0.025% trypsin (Thermo) in 0.1M phosphate buffer with 0.018% EDTA (Merck Pty, Kilsyth, VIC, Australia).
Schwann cell transfection
On the day prior to transfection, Schwann cells were sub-cultured into two poly-lysine coated 6-well plates (Techno Plastic Products [Interpath Services, West Heidelberg, VIC, Australia]) at a concentration of 2×105 cells/well, ensuring cells would be in the log phase of differentiation on the day of transfection.
Four wells of Schwann cells were transfected with each of the three expression plasmids using the lipid-based transfection reagent Lipofectamine 2000 (LF2000; Invitrogen), according to manufacturer’s guidelines. Specifically, for each plasmid, 16μg DNA was gently diluted in 1mL Opti-MEM reduced serum media (Gibco [Invitrogen, Melbourne, VIC, Australia]), and 40μl of LF2000 was diluted with 960μl Opti-MEM and incubated at room temperature for 5 minutes. The diluted DNA and the diluted LF2000 were then combined in a 1:1 ratio and incubated at room temperature for 20–25 minutes.
Schwann cells were rinsed with phosphate buffered saline (PBS), and 1.5ml of fresh SCM was added to each well. The DNA/LF2000 solution (500μl) was added to each well of Schwann cells (four wells per plasmid), and mixed by gently rocking the plate. The plates were then incubated at 37°C, 10% CO2 overnight.
The following day, the presence of the EGFP reporter gene under direct fluorescence microscopy was used to confirm successful transfection. Schwann cells were then sub-cultured at 1:10 into 6-well plates, maintaining four wells per plasmid type. After a further 24 hours, the selective reagent Geneticin (G418 sulphate; Gibco) was added to each well at a concentration of 400μg/ml. Previous experimentation in our laboratory determined this as the optimal concentration for selection of Schwann cell transformants over a 10–14 day period (data not shown). Following two weeks of selective pressure, cells transfected with each plasmid were pooled and purified by fluorescence-activated cell sorting, which isolated the top 3–5% of each cell type. The three resultant cell populations, BDNF-Schwann cells (BDNF-SCs), Ntf3-Schwann cells (Ntf3-SCs) and control EGFP-Schwann cells (EGFP-SCs), were grown in 75cm2 flasks and selective conditions maintained with 200μg/ml Geneticin. Schwann cells were sub-cultured every 3–4 days at a 1:10 ratio, and conditioned media collected and stored at −80°C for analysis.
The concentration of neurotrophin secreted by the genetically modified cells was determined using enzyme-linked immunosorbent assays (ELISAs). Serial dilutions of conditioned media collected from the BDNF-SCs and EGFP-SCs was analysed for BDNF content (n=7), and media from Ntf3-SCs and EGFP-SCs was analysed for Ntf3 content (n=13), using an Emax Immunoassay System kit (Promega, Annandale, NSW, Australia) directed against the neurotrophin of interest, as per manufacturer’s instructions. Standard curves were also performed for each experiment, in accordance with manufacturer’s guidelines. The concentration of neurotrophin in the conditioned media was calculated from the respective standard curve.
Spiral ganglion neuron cultures
SGN cultures were prepared from P5–P6 albino Wistar rat pups, as previously described (Gillespie et al., 2001). Briefly, the rats were rendered unconscious on ice and rapidly decapitated. The bulla was dissected from each temporal bone, the bony otic capsule removed and the cochlea isolated, and the auditory nerve severed at the internal auditory meatus. The organ of Corti was then removed, and the central core of all cochleae, containing Rosenthal’s canal, were pooled and digested in a solution of calcium-magnesium-free Hank’s Buffered Salt Solution (Gibco) containing 0.025% trypsin (Merck) and 0.001% DNase (Roche, Castle Hill, NSW, Australia) for 30 minutes at 37°C. Trypsinisation was stopped with FCS and the solution was centrifuged at 2500 rpm for 5 minutes. The tissue was resuspended in HEPES buffered Eagle’s medium (Gibco) containing 0.001% DNase, mechanically digested through a series of needles, and spun as above. The resultant pellet was resuspended in DMEM-SATO (DMEM with 100μg/ml human transferrin [Sigma], 16μg/ml putrescine [Sigma], 10μg/ml insulin [Sigma], 0.4μg/ml thyroxine [Sigma], 0.3μg/ml thyronine [Sigma], 0.06μg/ml progesterone [Sigma], 0.04μg/ml selenium [Sigma], 2mM glutamine [Gibco] and 4.5g/L glucose [Gibco]; a modification of the medium described by (Bottenstein and Sato, 1979)). The dissociated SGN suspension was pre-plated in a 6-well tissue culture plate and incubated for 30 minutes at 37°C.
Spiral ganglion neuron-Schwann cell co-cultures
After the pre-plate incubation, SGNs were collected and plated onto poly-ornithine (500μg/mL; Sigma) and laminin (10μg/mL; Gibco) coated 8-well chamber slides (Nalge Nunc International, Rochester, NY, USA) at a density of approximately 20,000 cells/well, based upon previous in vitro studies (Gillespie et al., 2001). Schwann cells were prepared for the co-cultures by trypsinising as previously described and resuspending in SCM. BDNF-, Ntf3- or EGFP-SCs were added to the SGNs at 20,000 Schwann cells/well. This concentration was calculated by scaling down the number of Schwann cells normally seeded into a 75cm2 flask. Control SGN cultures were grown in DMEM-SATO and treated with recombinant human (rh) BDNF or rhNtf3 (50ng/mL; PeproTech, Rocky Hill, NJ, USA); control Schwann cell cultures were grown in SCM without additional exogenous neurotrophins.
All cultures were fixed after three days in vitro. The Schwann cell control cultures were fixed with 4% paraformaldehyde (30 minutes), rinsed in PBS, the chambers were removed and the slides mounted in DAPI mounting medium (Vector Laboratories, Burlingame, CA, USA). The SGN controls and SGN-Schwann cell co-cultures were fixed with 100% ice-cold methanol (30 minutes) for immunostaining.
Immunostaining
Immunostaining was performed using the avidin-biotin complex method of a standard Vectastain kit (Vector), as per manufacturer’s instructions. Following fixation, cultures were rinsed with PBS and immunostained by incubation with, in series: 2% FCS in PBS (30 minutes); rabbit anti-neurofilament 200 kDa (Chemicon, Boronia, VIC, Australia; 1:400; 30 minutes); 2% FCS in PBS (3 × 5 minutes); biotinylated anti-rabbit secondary antibody (1:200; 30 minutes); PBS (3 × 5 minutes); avidin-biotin (1:100; 30 minutes); PBS (3 × 5 minutes); diaminobenzidine chromogen substrate (Vector; 5 minutes); and distilled water (3 × 5 minutes). Following immunostaining, the chambers were removed and the slides were dehydrated in 100% alcohol (3 × 1 minute), cleared in Histoclear (3 × 1 minute), and cover-slipped with DePeX mounting medium (Merck).
Analysis and Statistics
SGNs were co-cultured with EGFP-SCs (n=20), BDNF-SCs (n=9), rhBDNF (n=16), Ntf3-SCs (n=13) or rhNtf3 (n=13), across 10 independent experiments. For each experiment, all surviving SGNs – identified by immunopositivity for neurofilament – were counted in each well of the control EGFP-SC co-cultures, and the average SGN survival (per well) elicited by the EGFP-SCs was standardised to 100%. The number of surviving SGNs in each of the other treatment conditions was then counted (per well) and expressed as a percentage of the control. This was repeated for each individual experiment, and the average percentage SGN survival for each treatment condition was then calculated.
Statistical analyses were performed using the SigmaStat software package. Significant differences were identified using one way analysis of variance, and a Bonferroni test was used for all pairwise multiple comparisons. A difference was considered to be statistically significant at p<0.05.
RESULTS
Schwann cells can be genetically modified to over-express the neurotrophins BDNF and Ntf3
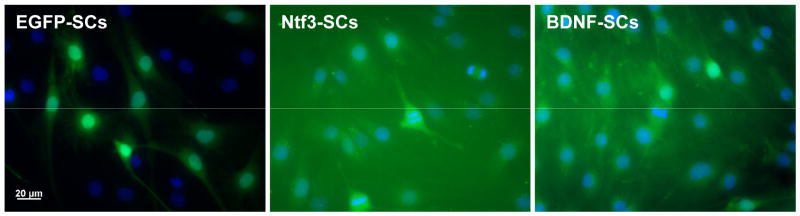
Successful transfection of the Schwann cells with each of the plasmids was initially confirmed 24 hours post-transfection by fluorescence microscopy. Transfected cells appeared green due to the presence of the EGFP reporter gene (Figure 1).
Figure 1.
Successful transformation of Schwann cells was confirmed by the presence of the EGFP reporter gene. ELISA analysis indicated that the BDNF-SCs produced 221±14 pg BDNF/day/106 cells, while the Ntf3-SCs produced 708±54 pg Ntf3/day/106 cells, significantly greater amounts of either neurotrophin than the control EGFP-SCs.
Transfection resulted in three Schwann cell lines: a) EGFP-SCs: Schwann cells that were genetically modified to express the fluorescent marker EGFP and serve as controls; b) BDNF-SCs: Schwann cells that were genetically modified to express EGFP-tagged BDNF; and c) Ntf3-SCs: Schwann cells that were genetically modified for EGFP-tagged Ntf3.
In addition, successful transfection was confirmed via ELISA analysis of conditioned media from each of the three cell types. Conditioned media was collected three days after sub-culturing when cells were confluent, at approximately 3×106 cells/flask. The control EGFP-SCs produced only a very small amount of BDNF (15.7±5 pg/day/106 cells [mean±SEM]), and no detectable amount of Ntf3. In contrast, the BDNF-SCs produced a significantly greater amount of BDNF (221±14 pg/day/106 cells) in comparison to the control Schwann cells (p<0.05). The Ntf3-SCs also produced significantly more Ntf3 than the control Schwann cells, producing 708±54 pg Ntf3/day/106 cells (p<0.001).
Neurotrophin over-expressing Schwann cells enhance SGN survival in vitro
SGNs were grown in co-culture with each of the three Schwann cell lines in order to assess the biological activity of the secreted neurotrophins and determine the survival-promoting effects of these cells in comparison to commercially available neurotrophins.
Co-culture of SGNs with the control EGFP-SCs resulted in the survival of 40±4 (mean±SEM) SGNs/well, which was standardised to 100% within each individual experiment.
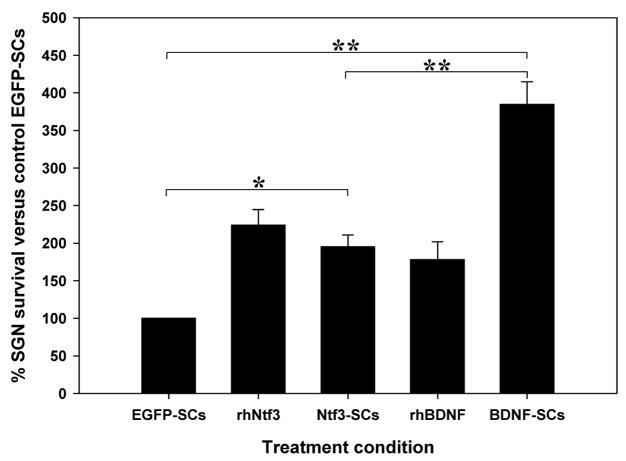
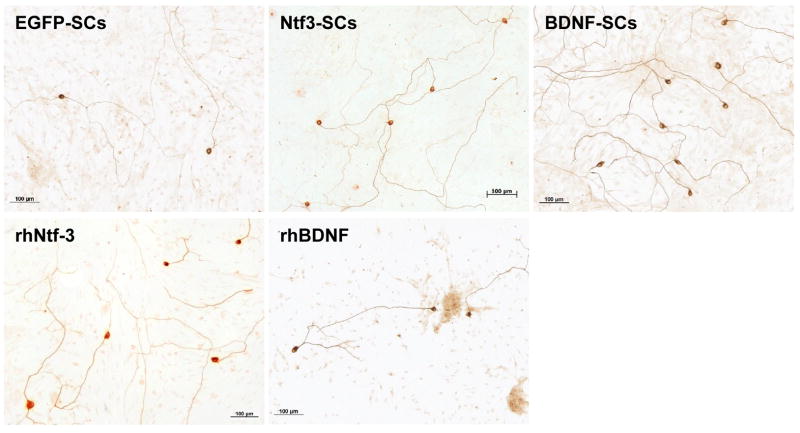
Co-culture with the neurotrophin over-expressing Schwann cells significantly enhanced SGN survival over that elicited by the control EGFP-SCs. In comparison to the survival effects of the EGFP-SCs, the Ntf3-SCs increased SGN survival by 2-fold to 195±15% (mean±SEM; p<0.01). The BDNF-SCs further enhanced SGN survival, leading to a survival rate of 384±30% (mean±SEM; p<0.001), a 4-fold increase in survival over the control EGFP-SCs, and a 2-fold increase over the Ntf3-SCs. In addition, while there was no significant difference in the survival effects of the Ntf3-SCs versus either rhBDNF or rhNtf3, the BDNF-SCs significantly improved SGN survival as compared to both the rhBDNF (178±23%; mean±SEM) and rhNtf3 (223±20%; mean±SEM) treatments (p<0.001), (Figures 2 and 3).
Figure 2.
Co-culture with either the BDNF-Schwann cells or the Ntf3-Schwann cells led to a significant increase in auditory neuron survival in comparison to that elicited by both the respective neurotrophin controls (rhBDNF and rhNtf3), and the control Schwann cells. * p<0.01; ** p<0.001.
Figure 3.
Survival of postnatal day six rat SGNs when grown in co-culture with the various Schwann cell lines, or in the presence of recombinant human neurotrophin proteins. SGN survival was greatest in the BDNF-Schwann cell co-cultures (p<0.001).
DISCUSSION
Schwann cells were successfully genetically modified using a lipid-based technique to over-express the neurotrophins BDNF and Ntf3. Both BDNF-SCs (221±14 pg/day/106 cells) and Ntf3-SCs (708±54 pg/day/106 cells) produced significantly greater amounts of the respective neurotrophin as compared to the control Schwann cells, which were modified to express the reporter gene EGFP only.
In addition, the neurotrophin over-expressing Schwann cells significantly enhanced SGN survival in vitro when compared to the control EGFP-SCs, with Ntf3-SCs increasing the number of surviving SGNs by 2-fold, and BDNF-SCs supporting the survival of four times as many SGNs.
These results indicate that Schwann cells could successfully process and release the EGFP-tagged neurotrophins, and that these neurotrophins retained biological activities, as has been previously reported (Haubensak et al., 1998; Hartmann et al., 2001; Brigadski et al., 2005).
BDNF-SCs support SGN survival in vitro better than Ntf3-SCs
While both types of neurotrophin over-expressing Schwann cells significantly enhanced SGN survival in comparison to the control Schwann cells, the BDNF-SCs elicited the most potent survival effects. In fact, the BDNF-SCs supported the survival of twice as many SGNs as any of the other neurotrophin treatments, including the Ntf3-SCs, despite the fact that Ntf3-SCs produced a significantly greater amount of Ntf3 than the BDNF-SCs did of BDNF. In addition, the BDNF-SCs enhanced SGN survival 2-fold over that elicited by the recombinant human neurotrophins, even though the concentration of these proteins (50ng/ml) was significantly greater than the amount of BDNF produced by the BDNF-SCs over the course of the experiments.
There are a number of arguments that could be used to explain these effects. Firstly, BDNF and Ntf3 are differentially expressed within the cochlea during development. Specifically, BDNF mRNA expression in SGNs is greatest in embryonic and early postnatal stages of development, while Ntf3 is only present in low levels during the early postnatal period (Ylikoski et al., 1993; Wheeler et al., 1994), suggesting that BDNF is more important for supporting the survival of immature SGNs. Also, the survival of early postnatal SGNs (P2–P10) has been shown to be greater in response to BDNF than Ntf3 (Mou et al., 1998). Therefore, since our SGN cultures were prepared from early postnatal (P6) rats, the SGNs may have been more responsive to the BDNF-SCs than the Ntf3-SCs, leading to the differences observed in terms of SGN survival. In comparison, the expression of Ntf3 seems essential for the maintenance of a robust SGN population in the mature adult cochlea (Ylikoski et al., 1993; Stankovic et al., 2004; Wissink et al., 2006). As such, it will be interesting to determine the response of SGNs to these neurotrophin over-expressing Schwann cells in adult animal models of deafness; in particular, whether mature SGNs are more responsive to Ntf3-SCs. Such experiments will also be imperative in ascertaining the most appropriate approach for clinically translational studies.
Secondly, Schwann cells have an intimate relationship with neurons and nerve fibres, with a number of important functions. For example, Schwann cells inherently produce small amounts of a variety of neurotrophic factors, including the neurotrophins BDNF, Ntf3, neurotrophin-4/5 and nerve growth factor, and the cytokines ciliary-derived neurotrophic factor (CNTF) and leukaemia inhibitory factor (LIF), to promote or ensure neuronal survival (Reynolds and Woolf, 1993; Frostick et al., 1998; Terenghi, 1999). It has also been reported that the survival effects of BDNF and Ntf3 on SGNs can be enhanced by combined application of CNTF or LIF. For example, CNTF can act in an additive fashion with each of BDNF and Ntf3 to improve SGN survival and neurite length (Staecker et al., 1995; Hartnick et al., 1996), while LIF can enhance the effects of both BDNF and Ntf3 in SGN cultures (Marzella et al., 1999; Gillespie et al., 2001), with BDNF and LIF acting synergistically to be the most potent stimulator of SGN survival in vitro (Marzella et al., 1999). This therefore suggests that the BDNF expressed by the BDNF-SCs may act in combination with other neurotrophic factors produced intrinsically by the Schwann cells, to synergistically boost the survival-promoting effects on SGNs. In contrast, the Ntf3 produced from the Ntf3-SCs may only act in an additive fashion with other inherently produced neurotrophic factors.
Furthermore, Schwann cells can influence the differentiation and growth state of neurons and control axon guidance by providing an environment that is favourable for growth (Reynolds and Woolf, 1993). Specifically, in addition to the production of neurotrophic factors, Schwann cells also produce and secrete extracellular matrix proteins such as laminin and fibronectin, and express cell adhesion molecules on their surface (Reynolds and Woolf, 1993; Frostick et al., 1998). Despite the fact that, during development, inner ear sensory neurons can differentiate in the absence of Schwann cells (Morris et al., 2006), nerve survival and growth in response to injury is facilitated and enhanced by the expression of these trophic and tropic molecules by Schwann cells. Furthermore, Schwann cells within the deafened cochlea can survive, at least for a limited period of time, despite SGN degeneration (Leake and Hradek, 1988; Hurley et al., 2007), and it has been suggested that Schwann cells can support their own survival by autocrine circuits involving neurotrophic signals (Mirsky and Jessen, 1999). The ability of Schwann cells to respond to neurotrophic factors therefore suggests that Schwann cells have the capacity to stimulate and enhance reparative responses within the damaged cochlea.
Importantly, Schwann cells have previously been suggested as a possible source of neurotrophic support for SGNs (Hansen et al., 2001; Andrew et al., 2007), and in vitro experiments have reported improved SGN survival in cultures containing non-neuronal tissue, with the enhanced effects attributed to the direct cell-cell contact between Schwann cells and SGNs, and the Schwann cells serving as a substrate for neuronal attachment and growth (Whitlon et al., 2006). Therefore, in the current study, the microenvironment produced by the Schwann cells is also likely to have played a role in the enhanced survival effects elicited by the neurotrophin over-expressing Schwann cells in comparison to the recombinant neurotrophin proteins.
Ex vivo gene transfer for clinically relevant SGN rescue
One of the current limitations to research attempting to prevent deafness-induced SGN degeneration is the method by which to deliver trophic support in a clinically relevant manner. Ex vivo gene transfer, which involves the transplantation of cells that have been genetically modified to express exogenous therapeutic gene products, may serve this purpose. Numerous studies have in fact attempted ex vivo gene transfer as a potential therapeutic option to deliver neurotrophic factors, in a variety of systems, and using a variety of cell types. For example, Schwann cells genetically modified to over-express CNTF enhance axonal regeneration from rat retinal ganglion cells following optic nerve transection (Hu et al., 2005). In addition, both Schwann cells and fibroblasts over-expressing either nerve growth factor or BDNF can elicit robust axonal outgrowth following transplantation into the chronically injured rat spinal cord (Tuszynski et al., 1996; Grill et al., 1997b; Menei et al., 1998; Weidner et al., 1999; Tobias et al., 2005). Furthermore, cellularly delivered Ntf3 induces significant growth of corticospinal axons and also improves sensorimotor functional deficits after spinal cord injury in the rat (Grill et al., 1997a; Blits et al., 2000). Importantly, a recent study has reported that a cochlear implant electrode array coated with BDNF-secreting fibroblasts could reduce auditory nerve degeneration in the deaf guinea pig (Rejali et al., 2007).
Several criteria are essential for ex vivo gene transfer to be considered clinically viable. Such a technique would require that the cell type used (i) is readily attainable, (ii) can survive grafting for extended periods of time, (iii) is a suitable target for genetic modification, and (iv) pose no ethical controversy (Hendriks et al., 2004).
Based on these specifications, Schwann cells can be considered a suitable candidate cell type for the ex vivo delivery of neurotrophic factors. Firstly, Schwann cells can be obtained by peripheral nerve biopsy, allowing for autologous transplantation and thereby eliminating concerns of immunological rejection and inflammatory reactions, as well as eliminating ethical concerns. In addition, Schwann cells can be cultured in vitro such that sufficient numbers of cells can be obtained for implantation. Secondly, Schwann cells can survive transplantation into the guinea pig cochlea, and have been demonstrated to enhance SGN survival in comparison to deaf, untreated controls (Andrew et al., 2007), presumably as a result of the secretion of neurotrophic factors. Schwann cells have also been demonstrated to chronically survive grafting in other systems (Tuszynski et al., 1998), suggesting that there is also the potential for long-term survival within the fluid spaces of the cochlea. Finally, Schwann cells can be successfully genetically modified to over-express neurotrophins, and in fact, can maintain neurotrophin transgene expression for at least 12 months (Tuszynski et al., 1998). Most importantly, as demonstrated in the present study, neurotrophin over-expressing Schwann cells can enhance SGN survival over that achieved with both normal Schwann cells and traditional recombinant neurotrophin proteins.
However, ex vivo gene transfer within the cochlea may require combined and/or co-ordinated therapies, such as electrical stimulation via a cochlear implant, for ongoing, permanent rescue of SGNs from deafness-induced degeneration. While it has previously been shown that neurotrophin-induced SGN survival is not maintained beyond the treatment period (Gillespie et al., 2003), a recent study has demonstrated that the initial trophic advantage afforded by osmotic pump-based BDNF infusion can be maintained by chronic electrical stimulation (Shepherd et al., submitted). Interestingly, BDNF secretion can be triggered by electrical stimulation (Lessmann et al., 2003), which may have promising implications for combined neurotrophin and cochlear implant therapies. Future studies will need to assess the effects of cell-based neurotrophin delivery with electrical stimulation, in particular in terms of whether transient neurotrophin expression is sufficient or if stable production is required. The use of inducible promoters, in which transgene expression can be regulated and switched on and off, may also prove useful.
CONCLUSION
This study demonstrates that Schwann cells genetically modified to over-express the neurotrophins BDNF or Ntf3 can enhance SGN survival in vitro compared to both control Schwann cells and recombinant neurotrophin proteins. Transplantation of these neurotrophin over-expressing Schwann cells into the cochlea may provide a clinically relevant means of providing neurotrophic support and preventing SGN degeneration following a sensorineural hearing loss.
Acknowledgments
The authors would like to thank Dr Volkmar Lessmann from the Johannes Gutenberg Universität, Mainz, Germany, for generously providing the expression plasmids used for this study, and Dr Simon Murray and Professor Trevor Kilpatrick from the Howard Florey Institute, Melbourne, Australia, for providing the Schwann cells. We are also grateful to Dr Rachael Richardson and Dr Justin Tan for technical advice regarding the molecular biology aspects of the experiments. This work was supported by the NIDCD (N01-DC-3-1005), the Macquarie Bank Foundation, the State Government of Victoria, and the Bionic Ear Institute.
ABBREVIATIONS
- BDNF
brain-derived neurotrophic factor
- BDNF-SCs
Schwann cells over-expressing BDNF
- CNTF
ciliary-derived neurotrophic factor
- DMEM
Dulbecco’s modified Eagle’s medium
- EGFP
enhanced green fluorescent protein
- EGFP-SCs
Schwann cells expressing EGFP
- ELISA
enzyme-linked immunosorbent assay
- FCS
fetal calf serum LF2000, Lipofectamine 2000
- LIF
leukaemia inhibitory factor
- Ntf3
neurotrophin 3
- Ntf3-SCs
Schwann cells over-expressing Ntf3
- P
postnatal day
- PBS
phosphate buffered saline
- rh
recombinant human
- SCM
Schwann cell media
- SGN
spiral ganglion neuron
- SNHL
sensorineural hearing loss
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- Albright AL, Awaad Y, Muhonen M, Boydston WR, Gilmartin R, Krach LE, Turner M, Zidek KA, Wright E, Swift D, Bloom K. Performance and complications associated with the synchromed 10-ml infusion pump for intrathecal baclofen administration in children. J Neurosurg. 2004;101:64–68. doi: 10.3171/ped.2004.101.2.0064. [DOI] [PubMed] [Google Scholar]
- Andrew JK, Geaney MS, Wise A, Pettingill LN, Richardson RT, Coco A, Skinner SJ, Shepherd RK. Therapeutic Potential of Encapsulated Neuroprotective Cells in the Cochlea. Proceedings of the IBRO World Congress of Neuroscience; Melbourne, Australia. 2007. [Google Scholar]
- Blits B, Dijkhuizen PA, Boer GJ, Verhaagen J. Intercostal nerve implants transduced with an adenoviral vector encoding neurotrophin-3 promote regrowth of injured rat corticospinal tract fibers and improve hindlimb function. Exp Neurol. 2000;164:25–37. doi: 10.1006/exnr.2000.7413. [DOI] [PubMed] [Google Scholar]
- Bottenstein JE, Sato GH. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979;76:514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers WJ, Chen X, Guo H, Frisina DR, Federoff HJ, Frisina RD. Neurotrophin-3 transduction attenuates cisplatin spiral ganglion neuron ototoxicity in the cochlea. Mol Ther. 2002;6:12–18. doi: 10.1006/mthe.2002.0627. [DOI] [PubMed] [Google Scholar]
- Brigadski T, Hartmann M, Lessmann V. Differential vesicular targeting and time course of synaptic secretion of the mammalian neurotrophins. J Neurosci. 2005;25:7601–7614. doi: 10.1523/JNEUROSCI.1776-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockes JP, Fields KL, Raff MC. Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 1979;165:105–118. doi: 10.1016/0006-8993(79)90048-9. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Duan ML, ElShamy WM, Canlon B. Protection of auditory neurons from aminoglycoside toxicity by neurotrophin-3. Nat Med. 1996;2:463–467. doi: 10.1038/nm0496-463. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Van De Water T, Loring J, Jaenisch R. Complementary roles of BDNF and NT-3 in vestibular and auditory development. Neuron. 1995;14:1153–1164. doi: 10.1016/0896-6273(95)90263-5. [DOI] [PubMed] [Google Scholar]
- Farinas I, Jones KR, Backus C, Wang XY, Reichardt LF. Severe sensory and sympathetic deficits in mice lacking neurotrophin-3. Nature. 1994;369:658–661. doi: 10.1038/369658a0. [DOI] [PubMed] [Google Scholar]
- Farinas I, Jones KR, Tessarollo L, Vigers AJ, Huang E, Kirstein M, de Caprona DC, Coppola V, Backus C, Reichardt LF, Fritzsch B. Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. J Neurosci. 2001;21:6170–6180. doi: 10.1523/JNEUROSCI.21-16-06170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SD, Michael N, Frim DM. Observations regarding failure of cerebrospinal fluid shunts early after implantation. Neurosurg Focus. 2007;22:E7. doi: 10.3171/foc.2007.22.4.8. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Pauley S, Matei V, Katz DM, Xiang M, Tessarollo L. Mutant mice reveal the molecular and cellular basis for specific sensory connections to inner ear epithelia and primary nuclei of the brain. Hear Res. 2005;206:52–63. doi: 10.1016/j.heares.2004.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Tessarollo L, Coppola E, Reichardt LF. Neurotrophins in the ear: their roles in sensory neuron survival and fiber guidance. Prog Brain Res. 2004;146:265–278. doi: 10.1016/S0079-6123(03)46017-2. [DOI] [PubMed] [Google Scholar]
- Frostick SP, Yin Q, Kemp GJ. Schwann cells, neurotrophic factors, and peripheral nerve regeneration. Microsurgery. 1998;18:397–405. doi: 10.1002/(sici)1098-2752(1998)18:7<397::aid-micr2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Gillespie LN, Clark GM, Bartlett PF, Marzella PL. LIF is more potent than BDNF in promoting neurite outgrowth of mammalian auditory neurons in vitro. Neuroreport. 2001;12:275–279. doi: 10.1097/00001756-200102120-00019. [DOI] [PubMed] [Google Scholar]
- Gillespie LN, Clark GM, Bartlett PF, Marzella PL. BDNF-induced survival of auditory neurons in vivo: Cessation of treatment leads to an accelerated loss of survival effects. J Neurosci Res. 2003;71:785–790. doi: 10.1002/jnr.10542. [DOI] [PubMed] [Google Scholar]
- Gillespie LN, Clark GM, Marzella PL. Delayed neurotrophin treatment supports auditory neuron survival in deaf guinea pigs. Neuroreport. 2004;15:1121–1125. doi: 10.1097/00001756-200405190-00008. [DOI] [PubMed] [Google Scholar]
- Grill R, Murai K, Blesch A, Gage FH, Tuszynski MH. Cellular delivery of neurotrophin-3 promotes corticospinal axonal growth and partial functional recovery after spinal cord injury. J Neurosci. 1997a;17:5560–5572. doi: 10.1523/JNEUROSCI.17-14-05560.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill RJ, Blesch A, Tuszynski MH. Robust growth of chronically injured spinal cord axons induced by grafts of genetically modified NGF-secreting cells. Exp Neurol. 1997b;148:444–452. doi: 10.1006/exnr.1997.6704. [DOI] [PubMed] [Google Scholar]
- Hansen M, Zha X-M, Bok J, Green S. Multiple distinct signal pathways, including an autocrine neurotrophic mechanism, contribute to the survival-promoting effect of depolarisation on spiral ganglion neurons in vitro. Journal of Neuroscience. 2001;21:2256–2267. doi: 10.1523/JNEUROSCI.21-07-02256.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie NA, Shepherd RK. Sensorineural hearing loss during development: morphological and physiological response of the cochlea and auditory brainstem. Hear Res. 1999;128:147–165. doi: 10.1016/s0378-5955(98)00209-3. [DOI] [PubMed] [Google Scholar]
- Hartmann M, Heumann R, Lessmann V. Synaptic secretion of BDNF after high-frequency stimulation of glutamatergic synapses. Embo J. 2001;20:5887–5897. doi: 10.1093/emboj/20.21.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartnick CJ, Staecker H, Malgrange B, Lefebvre PP, Liu W, Moonen G, Van de Water TR. Neurotrophic effects of BDNF and CNTF, alone and in combination, on postnatal day 5 rat acoustic ganglion neurons. J Neurobiol. 1996;30:246–254. doi: 10.1002/(SICI)1097-4695(199606)30:2<246::AID-NEU6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Haubensak W, Narz F, Heumann R, Lessmann V. BDNF-GFP containing secretory granules are localized in the vicinity of synaptic junctions of cultured cortical neurons. J Cell Sci. 1998;111(Pt 11):1483–1493. doi: 10.1242/jcs.111.11.1483. [DOI] [PubMed] [Google Scholar]
- Hendriks WT, Ruitenberg MJ, Blits B, Boer GJ, Verhaagen J. Viral vector-mediated gene transfer of neurotrophins to promote regeneration of the injured spinal cord. Prog Brain Res. 2004;146:451–476. doi: 10.1016/S0079-6123(03)46029-9. [DOI] [PubMed] [Google Scholar]
- Hu Y, Leaver SG, Plant GW, Hendriks WT, Niclou SP, Verhaagen J, Harvey AR, Cui Q. Lentiviral-mediated transfer of CNTF to schwann cells within reconstructed peripheral nerve grafts enhances adult retinal ganglion cell survival and axonal regeneration. Mol Ther. 2005;11:906–915. doi: 10.1016/j.ymthe.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Hurley PA, Crook JM, Shepherd RK. Schwann cells revert to non-myelinating phenotypes in the deafened rat cochlea. Eur J Neurosci. 2007;26:1813–1821. doi: 10.1111/j.1460-9568.2007.05811.x. [DOI] [PubMed] [Google Scholar]
- Kan P, Kestle J. Lack of efficacy of antibiotic-impregnated shunt systems in preventing shunt infections in children. Childs Nerv Syst. 2007;23:773–777. doi: 10.1007/s00381-007-0296-7. [DOI] [PubMed] [Google Scholar]
- Lalwani AK, Han JJ, Castelein CM, Carvalho GJ, Mhatre AN. In vitro and in vivo assessment of the ability of adeno-associated virus-brain-derived neurotrophic factor to enhance spiral ganglion cell survival following ototoxic insult. Laryngoscope. 2002;112:1325–1334. doi: 10.1097/00005537-200208000-00001. [DOI] [PubMed] [Google Scholar]
- Leake PA, Hradek GT. Cochlear pathology of long term neomycin induced deafness in cats. Hear Res. 1988;33:11–33. doi: 10.1016/0378-5955(88)90018-4. [DOI] [PubMed] [Google Scholar]
- Lessmann V, Gottmann K, Malcangio M. Neurotrophin secretion: current facts and future prospects. Prog Neurobiol. 2003;69:341–374. doi: 10.1016/s0301-0082(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Marzella PL, Gillespie LN, Clark GM, Bartlett PF, Kilpatrick TJ. The neurotrophins act synergistically with LIF and members of the TGF- beta superfamily to promote the survival of spiral ganglia neurons in vitro. Hear Res. 1999;138:73–80. doi: 10.1016/s0378-5955(99)00152-5. [DOI] [PubMed] [Google Scholar]
- Menei P, Montero-Menei C, Whittemore SR, Bunge RP, Bunge MB. Schwann cells genetically modified to secrete human BDNF promote enhanced axonal regrowth across transected adult rat spinal cord. Eur J Neurosci. 1998;10:607–621. doi: 10.1046/j.1460-9568.1998.00071.x. [DOI] [PubMed] [Google Scholar]
- Miller JM, Chi DH, O’Keeffe LJ, Kruszka P, Raphael Y, Altschuler RA. Neurotrophins can enhance spiral ganglion cell survival after inner hair cell loss. Int J Dev Neurosci. 1997;15:631–643. doi: 10.1016/s0736-5748(96)00117-7. [DOI] [PubMed] [Google Scholar]
- Mirsky R, Jessen KR. The neurobiology of Schwann cells. Brain Pathol. 1999;9:293–311. doi: 10.1111/j.1750-3639.1999.tb00228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JK, Maklad A, Hansen LA, Feng F, Sorensen C, Lee KF, Macklin WB, Fritzsch B. A disorganized innervation of the inner ear persists in the absence of ErbB2. Brain Res. 2006;1091:186–199. doi: 10.1016/j.brainres.2006.02.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta F, Buonaguro V, Stignani C. The use of intrathecal baclofen pump implants in children and adolescents: safety and complications in 200 consecutive cases. J Neurosurg. 2007;107:32–35. doi: 10.3171/PED-07/07/032. [DOI] [PubMed] [Google Scholar]
- Mou K, Adamson CL, Davis RL. Time-dependence and cell-type specificity of synergistic neurotrophin actions on spiral ganglion neurons. J Comp Neurol. 1998;402:129–139. [PubMed] [Google Scholar]
- Nakaizumi T, Kawamoto K, Minoda R, Raphael Y. Adenovirus-mediated expression of brain-derived neurotrophic factor protects spiral ganglion neurons from ototoxic damage. Audiol Neurootol. 2004;9:135–143. doi: 10.1159/000077264. [DOI] [PubMed] [Google Scholar]
- Pirvola U, Arumae U, Moshnyakov M, Palgi J, Saarma M, Ylikoski J. Coordinated expression and function of neurotrophins and their receptors in the rat inner ear during target innervation. Hear Res. 1994;75:131–144. doi: 10.1016/0378-5955(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Pirvola U, Ylikoski J, Palgi J, Lehtonen E, Arumae U, Saarma M. Brain-derived neurotrophic factor and neurotrophin 3 mRNAs in the peripheral target fields of developing inner ear ganglia. Proc Natl Acad Sci U S A. 1992;89:9915–9919. doi: 10.1073/pnas.89.20.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praetorius M, Limberger A, Muller M, Lehner R, Schick B, Zenner HP, Plinkert P, Knipper M. A novel microperfusion system for the long-term local supply of drugs to the inner ear: implantation and function in the rat model. Audiol Neurootol. 2001;6:250–258. doi: 10.1159/000046130. [DOI] [PubMed] [Google Scholar]
- Rejali D, Lee VA, Abrashkin KA, Humayun N, Swiderski DL, Raphael Y. Cochlear implants and ex vivo BDNF gene therapy protect spiral ganglion neurons. Hear Res. 2007;228:180–187. doi: 10.1016/j.heares.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds ML, Woolf CJ. Reciprocal Schwann cell-axon interactions. Curr Opin Neurobiol. 1993;3:683–693. doi: 10.1016/0959-4388(93)90139-p. [DOI] [PubMed] [Google Scholar]
- Richardson RT, O’Leary S, Wise A, Hardman J, Clark G. A single dose of neurotrophin-3 to the cochlea surrounds spiral ganglion neurons and provides trophic support. Hear Res. 2005;204:37–47. doi: 10.1016/j.heares.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Sciubba DM, Lin LM, Woodworth GF, McGirt MJ, Carson B, Jallo GI. Factors contributing to the medical costs of cerebrospinal fluid shunt infection treatment in pediatric patients with standard shunt components compared with those in patients with antibiotic impregnated components. Neurosurg Focus. 2007;22:E9. doi: 10.3171/foc.2007.22.4.11. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Coco A, Epp S. Neurotrophins and electrical stimulation for protection and repair of spiral ganglion neurons following sensorineural hearing loss. Hearing Research. doi: 10.1016/j.heares.2007.12.005. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd RK, Coco A, Epp SB, Crook JM. Chronic depolarisation enhances the trophic effects of BDNF in rescuing auditory neurons following a sensorineural hearing loss. J Comp Neurol. 2005;486:145–158. doi: 10.1002/cne.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd RK, Javel E. Electrical stimulation of the auditory nerve. I. Correlation of physiological responses with cochlear status. Hear Res. 1997;108:112–144. doi: 10.1016/s0378-5955(97)00046-4. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Roberts LA, Paolini AG. Long-term sensorineural hearing loss induces functional changes in the rat auditory nerve. Eur J Neurosci. 2004;20:3131–3140. doi: 10.1111/j.1460-9568.2004.03809.x. [DOI] [PubMed] [Google Scholar]
- Shinohara T, Bredberg G, Ulfendahl M, Pyykko I, Olivius NP, Kaksonen R, Lindstrom B, Altschuler R, Miller JM. Neurotrophic factor intervention restores auditory function in deafened animals. Proc Natl Acad Sci U S A. 2002;99:1657–1660. doi: 10.1073/pnas.032677999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sly DJ, Heffer LF, White MW, Shepherd RK, Birch MG, Minter RL, Nelson NE, Wise AK, O’Leary SJ. Deafness alters auditory nerve fibre responses to cochlear implant stimulation. Eur J Neurosci. 2007;26:510–522. doi: 10.1111/j.1460-9568.2007.05678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staecker H, Gabaizadeh R, Federoff H, Van De Water TR. Brain-derived neurotrophic factor gene therapy prevents spiral ganglion degeneration after hair cell loss. Otolaryngol Head Neck Surg. 1998;119:7–13. doi: 10.1016/S0194-5998(98)70194-9. [DOI] [PubMed] [Google Scholar]
- Staecker H, Liu W, Hartnick C, Lefebvre P, Malgrange B, Moonen G, Van de Water TR. NT-3 combined with CNTF promotes survival of neurons in modiolus-spiral ganglion explants. Neuroreport. 1995;6:1533–1537. doi: 10.1097/00001756-199507310-00017. [DOI] [PubMed] [Google Scholar]
- Stankovic K, Rio C, Xia A, Sugawara M, Adams JC, Liberman MC, Corfas G. Survival of adult spiral ganglion neurons requires erbB receptor signaling in the inner ear. J Neurosci. 2004;24:8651–8661. doi: 10.1523/JNEUROSCI.0733-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terenghi G. Peripheral nerve regeneration and neurotrophic factors. J Anat. 1999;194:1–14. doi: 10.1046/j.1469-7580.1999.19410001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias CA, Han SS, Shumsky JS, Kim D, Tumolo M, Dhoot NO, Wheatley MA, Fischer I, Tessler A, Murray M. Alginate encapsulated BDNF-producing fibroblast grafts permit recovery of function after spinal cord injury in the absence of immune suppression. J Neurotrauma. 2005;22:138–156. doi: 10.1089/neu.2005.22.138. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH, Gabriel K, Gage FH, Suhr S, Meyer S, Rosetti A. Nerve growth factor delivery by gene transfer induces differential outgrowth of sensory, motor, and noradrenergic neurites after adult spinal cord injury. Exp Neurol. 1996;137:157–173. doi: 10.1006/exnr.1996.0016. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH, Weidner N, McCormack M, Miller I, Powell H, Conner J. Grafts of genetically modified Schwann cells to the spinal cord: survival, axon growth, and myelination. Cell Transplant. 1998;7:187–196. doi: 10.1177/096368979800700213. [DOI] [PubMed] [Google Scholar]
- Weidner N, Blesch A, Grill RJ, Tuszynski MH. Nerve growth factor-hypersecreting Schwann cell grafts augment and guide spinal cord axonal growth and remyelinate central nervous system axons in a phenotypically appropriate manner that correlates with expression of L1. J Comp Neurol. 1999;413:495–506. doi: 10.1002/(sici)1096-9861(19991101)413:4<495::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Wheeler EF, Bothwell M, Schecterson LC, von Bartheld CS. Expression of BDNF and NT-3 mRNA in hair cells of the organ of Corti: quantitative analysis in developing rats. Hear Res. 1994;73:46–56. doi: 10.1016/0378-5955(94)90281-x. [DOI] [PubMed] [Google Scholar]
- Whitlon DS, Ketels KV, Coulson MT, Williams T, Grover M, Edpao W, Richter CP. Survival and morphology of auditory neurons in dissociated cultures of newborn mouse spiral ganglion. Neuroscience. 2006;138:653–662. doi: 10.1016/j.neuroscience.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Winn SR, Lindner MD, Lee A, Haggett G, Francis JM, Emerich DF. Polymer-encapsulated genetically modified cells continue to secrete human nerve growth factor for over one year in rat ventricles: behavioral and anatomical consequences. Exp Neurol. 1996;140:126–138. doi: 10.1006/exnr.1996.0123. [DOI] [PubMed] [Google Scholar]
- Wise AK, Richardson R, Hardman J, Clark G, O’Leary S. Resprouting and survival of guinea pig cochlear neurons in response to the administration of the neurotrophins brain-derived neurotrophic factor and neurotrophin-3. J Comp Neurol. 2005;487:147–165. doi: 10.1002/cne.20563. [DOI] [PubMed] [Google Scholar]
- Wissink TF, Moes C, Beisel KW, Fritzsch B. Neurotrophins and hearing dysfunction: Comparing models to stop nerve fiber loss. Drug Discovery Today: Disease Models. 2006;3:391–396. [Google Scholar]
- Yagi M, Kanzaki S, Kawamoto K, Shin B, Shah PP, Magal E, Sheng J, Raphael Y. Spiral ganglion neurons are protected from degeneration by GDNF gene therapy. J Assoc Res Otolaryngol. 2000;1:315–325. doi: 10.1007/s101620010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata T, Miller JM, Ulfendahl M, Olivius NP, Altschuler RA, Pyykko I, Bredberg G. Delayed neurotrophic treatment preserves nerve survival and electrophysiological responsiveness in neomycin-deafened guinea pigs. J Neurosci Res. 2004;78:75–86. doi: 10.1002/jnr.20239. [DOI] [PubMed] [Google Scholar]
- Ylikoski J, Pirvola U, Moshnyakov M, Palgi J, Arumae U, Saarma M. Expression patterns of neurotrophin and their receptor mRNAs in the rat inner ear. Hear Res. 1993;65:69–78. doi: 10.1016/0378-5955(93)90202-c. [DOI] [PubMed] [Google Scholar]