Abstract
Previous studies have shown a link between inhaled particulate matter (PM) exposure in urban areas and susceptibility to cardiovascular diseases. Although an oxidative stress pathway is strongly implicated, the locus of generation of reactive oxygen species (ROS) and the mechanisms by which these radicals exert their effects remain to be characterized. To test the hypothesis that exposure to environmentally relevant inhaled concentrated ambient PM (CAPs) enhances atherosclerosis through induction of vascular ROS and reactive nitrogen species. High-fat chow fed apolipoprotein E–/– mice were exposed to CAPs of less than 2.5 μm (PM2.5) or filtered air (FA), for 6 h/day, 5 days/week, for 4 months in Manhattan, NY. Atherosclerotic lesions were analyzed by histomorphometricly. Vascular reactivity, superoxide generation, mRNA expression of NADPH (nicotinamide adenine dinucleotide phosphate, reduced) oxidase subunits, inducible nitric oxide synthase, endothelial nitric oxide synthase, and GTP cyclohydrolase I were also assessed. Manhattan PM2.5 CAPs were characterized by higher concentrations of organic and elemental carbon. Analysis of vascular responses revealed significantly decreased phenylephrine constriction in CAPs-exposed mice, which was restored by a soluble guanine cyclase inhibitor 1H-[1,2,4]oxadiazole[4,3-a]quinoxalin-1-one. Vascular relaxation to A23187, but not to acetylcholine, was attenuated in CAPs mice. Aortic expression of NADPH oxidase subunits (p47phox and rac1) and iNOS were markedly increased, paralleled by increases in superoxide generation and extensive protein nitration in the aorta. The composite plaque area of thoracic aorta was significantly increased with pronounced macrophage infiltration and lipid deposition in the CAPs mice. CAPs exposure in Manhattan alters vasomotor tone and enhances atherosclerosis through NADPH oxidase dependent pathways.
Keywords: air pollution, PM2.5, CAPs, ApoE–/– mice, atherosclerosis, reactive oxygen species, reactive nitrogen species
Epidemiological studies have linked ambient particulate matter (PM) air pollution with cardiovascular mortality and morbidity (Mills et al., 2007; Peters et al., 2004; Pope et al., 2004; Rossi et al., 1999). However, the mechanisms whereby PM air pollution exerts adverse effects on the vasculature remains elusive. The cardiovascular effect of airborne PM is most closely associated with particles with an aerodynamic size of less than 2.5 μm (PM2.5) which are primarily derived from stationary and traffic-related combustion sources (Brook et al., 2004; Schwarze et al., 2007). The biologic effects of PM2.5 although extra-ordinarily complex are further complicated by substantial spatial and temporal heterogeneity in PM composition and concentration that may yield disparate effects depending on the location and time of the exposure (Bhatnagar, 2006; Schwarze et al., 2007). Using a versatile aerosol concentration enrichment system (VACES), we previously showed that in the ApoE−/− mouse model, long term exposure (6 months) to concentrated PM2.5 (CAPs) at Tuxedo, New York, induces vascular oxidant stress, a small but definitive change in vasomotor tone, inflammation, and atherosclerosis (Sun et al., 2005). These experiments were performed in a rural area in a large New York state park that is approximately 40 miles from Manhattan. In the present study, we attempted to address the issue of whether regional differences in CAPs composition may contribute to differential vascular response and to address in detail the mechanism and source of vascular nitric oxide (NO) and superoxide (·O2−) generation. Both radicals can be generated in inflammatory states including exposure to airborne PM, and simultaneously form peroxynitrite (ONOO−), a powerful oxidant that itself induces tissue injury and alteration in intracellular signaling cascades through the diffusion limited reaction of NO and ·O2− (Huie and Padmaja, 1993; Ischiropoulos et al., 1992). Large amounts of NO and ·O2−, are generated in the inflammatory response to CAPs in various types of cells that may directly interact with inhaled PM (Bai et al., 2001; Becker et al., 2002; Gurgueira et al., 2002; Uppu et al., 2007).
Accordingly, we performed studies to assess the effects of CAPs using a VACES system in the Upper East Side of Manhattan and investigated the sources of reactive oxygen species in the vasculature and the impact of this exposure on experimental atherosclerosis.
MATERIALS AND METHODS
Animal model.
Six-week-old apolipoprotein E−/− (ApoE−/−) male mice (Taconic Europe, Denmark) were enrolled and housed in an Association for Assessment and Accreditation of Laboratory Animal Care–accredited animal housing facility. They were fed high-fat chow (Adjusted Calories Diet, TD 88137, Harlan, Indianapolis, IN) for 10 weeks before exposure to CAPs or filtered air (FA). Assignments to CAPs versus FA were randomized and the exposure was performed for a total duration of 4 months. The Committees on Use and Care of Animals from New York University and Mount Sinai School of Medicine approved all experimental procedures.
Protocol for exposure to CAPs.
The mouse exposure, the monitoring of the exposure atmosphere and ambient aerosol particles, and the exposure concentration calculation were performed as previously described (Chen and Hwang, 2005; Sun et al., 2005). Briefly, six ApoE−/− mice were exposed to CAPs, which is composed of the northeastern regional background in Manhattan at Mount Sinai School of Medicine (New York, NY), at nominal 10× ambient concentrations for 6 h per day, 5 days per week, for a total of 4 months from May to September, 2007. Another six ApoE−/− mice served as controls and were exposed to an identical protocol with the exception of a high-efficiency particulate air filter positioned in the inlet valve position to remove all of the CAPs in the FA stream. The exposure chamber was located on the fourth floor at an elevation of approximately 100 feet.
Vascular physiology studies.
Mice were killed by injection of lethal doses of pentobarbital. The ascending aortas were removed and the 2-mm thoracic aortic rings were suspended in individual organ chambers filled with physiological salt solution buffer (sodium chloride, 130 mEq/l; potassium chloride, 4.7 mEq/l; calcium dichloride, 1.6 mEq/l; magnesium sulfate, 1.17 mEq/l; potassium diphosphate, 1.18 mEq/l; sodium bicarbonate, 14.9 mEq/l; ethylenediaminetetraacetic acid, 0.026 mEq/l; and glucose, 99.1 mg/dl [5.5 mmol/l]; pH, 7.4), aerated continuously with 5% carbon dioxide in oxygen at 37°C, as previously described (Sun et al., 2005). Briefly, for vasoconstrictor responses, vessels were allowed to equilibrate for at least 1 hour at a resting tension of 700 mg before being subjected to graded doses of phenylephrine (PE; 10−9 to 10−5 mEq/l). Responses were then expressed as a percentage of the peak response to 120 mEq/l of potassium chloride. The vessels were then washed thoroughly and allowed to equilibrate for 1 h before beginning experiments with acetylcholine. After a stable contraction plateau was reached with PE, which was about 50% of peak tension generated with maximal dose potassium chloride, the rings were exposed to graded doses of the endothelium-dependent agonist acetylcholine (10−9 to 10−5 mEq/l) or A23187. To investigate a role for the cGMP-soluble guanylate cyclase (sGC) pathway, vessel segments were incubated in 1H-[1,2,4]oxadiazole[4,3-a]quinoxalin-1-one (ODQ; 10μM) for 10 min and subsequently subjected contraction by PE.
Immunohistochemistry and morphometry.
Segments of thoracic aorta were frozen in liquid nitrogen and embedded in Optimal Cutting Temperature compound (Tissue-Tek, Sakura Finetek USA, Inc., Torrance, CA). Antibodies against CD68, inducible nitric oxide synthase (iNOS), and endothelial nitric oxide synthase (eNOS) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). A polyclonal anti-nitrotyrosine antibody was obtained from Upstate Cell Signaling Solutions (Lake Placid, NY). Immunohistochemical staining was performed by using the primary antibodies (1:200 concentration) and a detection system (Immunoperoxidase Secondary Detection System; Chemicon International, Temecula, CA), and quantified with software (National Institutes of Health [NIH] Image) after digitization of the images with a camera system (Zeiss Axioskop with Spot I digital camera, Jena, Germany). Data are expressed as the percentage of the total analyzed area. For estimation of atherosclerotic plaque size, four successive sections were collected on the same slide, and at least 10 sections from three consecutive slides per area per mouse were examined. Each image was digitized with a digital camera and analyzed under a research microscope (Zeiss Axioskop with Spot I digital camera, Jena, Germany) with NIH Image software version 1.61 (Wayne Rasband, NIH, http://rsb.info.nih.gov/nih-image). Plaque areas were adjusted for the cross-sectional vessel cavity area and expressed as a percentage value. All analyses were performed blindly without knowledge of the origin of the samples.
Quantitative RT-PCR analysis.
Total RNA was isolated with TRIzol reagent (Invitrogen, Carlsbad, CA). Four microgram of total RNA was reverse transcribed by random hexamers and ThermoScript RT-PCR System (Invitrogen). Quantitative real-time PCR was performed with the Stratagene Mx3005 using SYBER Green PCR Master Mix (Applied Biosystems, Carlsbad, CA). The primers were previously reported (Das et al., 2005), and their sequences were as follows: eNOS (sense, 5′-CAC CAG GAA GAA GAC CTT TAA GGA-3′; antisense, 5′-CAC ACG CTT CGC CAT CAC-3′), iNOS (sense, 5′-CAG GAG GAG AGA GAT CCG ATT TAG-3′; antisense, 5′-TGA CCC GTG AAG CCA TGA C-3′), glyceraldehyde 3-phosphate dehydrogenase (sense, 5′-TAC AAG TTC AAG AGC GCC TCA CCA-3′; antisense, 5′-CCA TGC ACA TGT GTG TCG CTT CCA A-3′), GAPDH (sense, 5′-TGA ACG GGA AGC TCA CTG G-3′; antisense, 5′-TCC ACC ACC CTG TTG CTG TA-3′), p47Phox (sense, 5′-ACC TGT CGG AGA AGG TGG T-3′; antisense, 5′-TAG GTC TGA AGG ATG ATG GG-3′), p22Phox (sense, 5′-TGC GGG ACG CTT CAC GCA GTG G-3′; antisense, 5′-GGT TGG TAG GTG GCT GCT TGA TGG-3′), gp91Phox (sense, 5′-ACT CCT TGG GTC AGC ACT GG-3′; antisense, 5′-GTT CCT GTC CAG TTG TCT TCG-3′), and RAC1 (sense, 5′-TGG GAC ACA GCT GGA CAA GAA GAT-3′; antisense, 5′-TCA GGA TACC ACT TTG CAC GGA CA-3′). The relative expression level was obtained as previously described (Ying et al., 2006).
Superoxide anion measurement by chemiluminescence.
Mouse aorta were placed in chilled, modified Kreps-Hepes Buffer (pH = 7.4; initially gassed with 95% O2, and 5% CO2), cleaned of excessive adventitial tissue and cut into 2- to 3-mm segments. The vessel segments were placed in a modified Kreps-Hepes Buffer and equilibrated for 30 min at 37°C. Scintillation vials containing 300 μl of Krebs-Hepes buffer with 5μM Lucigenin were placed into an EG&G Berthold luminometer (Berthold Australia Pty Ltd, Australia). After a dark adaptation, background counts were recorded and an aortic segment was added to the vial. Scintillation counts were then recorded every 2 min at 30-s intervals. After baseline measurements for 5 min, nicotinamide adenine dinucleotide (30μM) was added to each vial and measurements made over 10 min. Values were reported after subtracting respective background counts. In some experiments, the aortic segments were pre-incubated with NOS blocker L-nitro-arginine methyl ester (L-NAME; 1mM).
Statistical analyses.
Data are expressed as mean ± SD unless otherwise indicated. The half-maximal dose (either dilation or constriction) value for each experiment involving physiologic assessment of vascular tone was obtained by logarithmic transformation. Comparisons of vascular function variables were made by analysis of variance with p values; p < 0.05 considered significant. Post hoc Bonferroni correction was used for multiple testing. For immunohistochemical, RT-PCR and plaque comparisons, paired t-tests were employed. All statistical analyses were performed by using GraphPad Prism software version 3.02 (GraphPad Software, Inc., San Diego, CA).
RESULTS
Characteristics of Exposure
The exposure characteristics in Manhattan are summarized in Table 1. The elemental composition, as well as concentration of organic and elemental carbon (EC and OC), are presented in Table 2. CAPs collected at Sterling Forest, New York during the same period was used as a control reference against which the particles derived from Manhattan were compared. Although the total CAPs during the periods of exposure in the two locations was not significantly different, there was a trend towards high concentrations in Manhattan. CAPs collected in the upper east side of Manhattan, contained substantially more EC and OC. The body weight of CAPs and FA mice was not significantly different (39.07 ± 4.82 and 42.98 ± 3.06, respectively) at the end of CAPs exposure.
TABLE 1.
Characteristics of Exposure Protocol
| Groups | FA, CAPs |
| Exposure time (dates) | 05/29/07–09/28/07 |
| Ambient PM2.5 (μg/m3) | 23 ± 75.9 |
| FA chamber PM2.5 (μg/m3) | 21.4 ± 17.5 |
| PM chamber PM2.5 (μg/m3) | 138.4 ± 83.7 |
| PM chamber particle enrichment factor | 6.8 ± 1.1 |
Note. ApoE–/– mice were exposed on the fourth floor of a laboratory located at 10 East 101st Street, between Fifth and Madison Avenues, New York, NY. Values shown are mean ± SD.
TABLE 2.
Elemental Data of CAPs
| Sterling forest |
Manhattan |
|||
| Mean | SD | Mean | SD | |
| PM2.5 | 11,358 | 8301 | 17,326 | 9642 |
| Na | 39 | 44 | 85 | 67 |
| Mg | 12 | 15 | 20 | 16 |
| Al | 42 | 57 | 44 | 50 |
| Si | 67 | 126 | 134 | 166 |
| S | 1512 | 1432 | 1583 | 1317 |
| Cl | −2 | 11 | 37 | 273 |
| K | 36 | 27 | 50 | 49 |
| Ca | 20 | 19 | 60 | 36 |
| Ti | 4 | 18 | 4 | 4 |
| V | 3 | 3 | 10 | 6 |
| Mn | 1 | 1 | 7 | 11 |
| Fe | 39 | 40 | 194 | 131 |
| Ni | 4 | 14 | 24 | 14 |
| Cu | 1 | 2 | 6 | 13 |
| Zn | 9 | 7 | 44 | 97 |
| Se | 1 | 1 | 3 | 2 |
| Br | 3 | 9 | 8 | 37 |
| Sr | 1 | 1 | 3 | 3 |
| Ba | 0 | 3 | 9 | 5 |
| Pb | 2 | 5 | 9 | 36 |
| OC | 1700 | 856 | 3597* | 1260 |
| EC | 174 | 139 | 1226* | 580 |
Note. PM2.5 fine particulate matter was collected and elemental composition of PM2.5 was analyzed as previously described. Results are presented as ng/m3. Manhattan is the location where mice were exposed to CAPs, and Sterling Forest is a control location where previous CAPs inhalation studies were performed by members of the study team. The sources of CAPs are clearly different from New York City. *p < 0.05, one-way ANOVA.
CAPs Exposure Induced Alterations in Vascular Tone
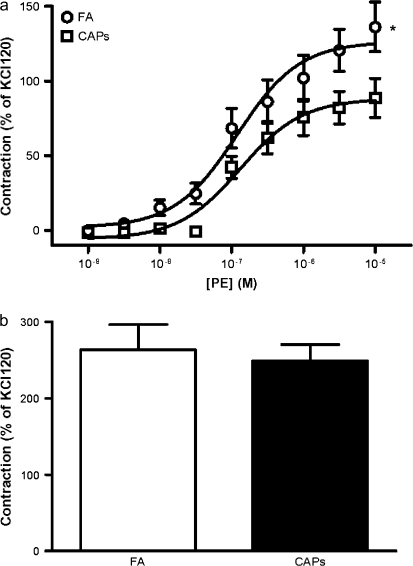
To test the vascular effects of CAPs on vascular function, intact aortic rings from CAPs and FA mice were prepared and mounted on the myograph chamber. Contraction was induced with a nonspecific depolarizing agent KCl and in response to the alpha adrergic agonist PE. Constrictive responses to 120mM KCl were comparable between FA mice and CAPs mice (1.01 ± 0.27 g and 0.99 ± 0.33 g, respectively, p > 0.05). Figure 1a depicts a significant decrease in maximum contraction induced by PE in CAPs mice (126.4 ± 7.6% and 88.3 ± 5.6% of contraction by 120mM KCl in the FA and CAPs groups, respectively). The sensitivity (EC50) to PE was however not significantly different in these two groups (95% CI: 0.06–0.25 and 0.07–0.26μM, FA and CAPs, respectively). To investigate the role of NO/sGC signaling in the reduced vascular responsiveness to PE, we repeated maximum response to PE in the presence of the sGC inhibitor ODQ. Pretreatment with ODQ completely restored the decrease in constriction noted in response to CAPs indicating a role for abnormal NO/sGC signaling in PM2.5 exposed mice (Fig. 1b).
FIG. 1.
Exposure to CAPs attenuates PE induced contraction by enhancing basal NO production. (a) Aortic rings were mounted on myography, and PE was added in an accumulative manner. Results were expressed as percentage of contraction by 120mM KCl. n = 6. *p < 0.05, ANOVA. (b) Aortic rings were contracted by 10μM PE in the presence of 10μM ODQ or vehicle. n = 6.
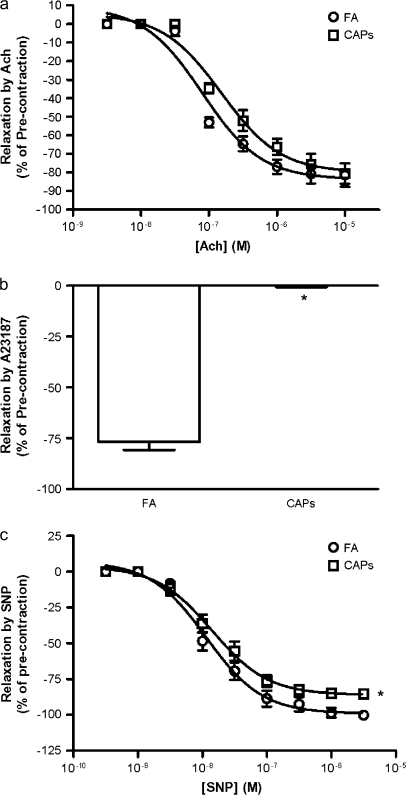
Figure 2a depicts responses to acetylcholine in FA and CAPs-exposed mice. Although there were no significant differences in response to acetylcholine, CAPs exposure completely abolished the relaxation induced by calcium ionophore A23187 (Fig. 2b). CAPs exposure slightly but significantly decreased maximum relaxation induced by sodium nitroprusside (SNP) (Fig. 2c; 86.02 ± 2.07 vs. 92.6 ± 3.78 in the CAPs and FA groups, respectively).
FIG. 2.
Exposure to CAPs alters NO signaling. (a) Aortic rings were precontracted by 0.1μM PE, and acetylcholine was added in an accumulative manner. n = 6. (b) Aortic rings were precontracted by 0.1μM PE, and relaxed by 1μM A23187. n = 6. *p < 0.05, Students t-test. (c) Aortic rings were precontracted by 0.1μM PE, and SNP was added in an accumulative manner. n = 6. *p < 0.05, one-way ANOVA.
iNOS Expression was Increased by CAPs Exposure
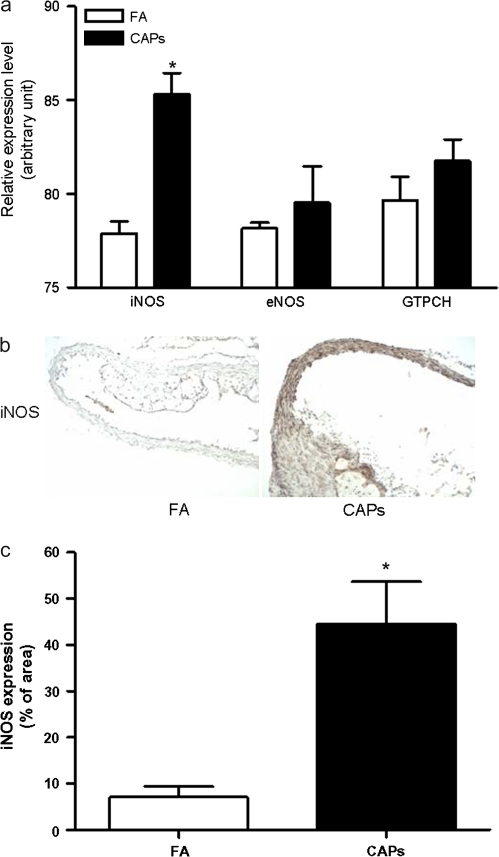
In light of previous data demonstrating that ONOO− may serve as a key oxidant mediating impairment in smooth muscle response owing to facile nitration of specific molecules (Uppu et al., 2007), we investigated the involvement of this pathway in CAPs-exposed animals. We initially investigated expression levels of iNOS, eNOS, and the rate limiting enzyme for tetrahydrobiopterin (BH4) synthesis (GTP cyclohydrolase I [GTPCH]). BH4 is an essential cofactor for NOS. Although CAPs exposure did not significantly change eNOS or GTPCH mRNA levels, iNOS mRNA expression was significantly increased (Fig. 3a). iNOS protein expression in aorta was evaluated by immunohistochemistry which demonstrated marked increase in iNOS expression in the aortas of CAPs mice. Notably, the distribution of iNOS in aorta was also different between FA and CAPs mice. Although in FA mice, iNOS was mainly expressed in the plaque, in CAPs mice, it was expressed in both the plaque and extensively in the media (Figs. 3b and 3c).
FIG. 3.
Exposure to CAPs increases vascular iNOS expression. (a) Total RNA was extracted from aorta, and the mRNA expression of iNOS, eNOS, and GTPCH were measured by real-time PCR. n = 6. *p < 0.05, Students t-test adjusted by Bonferroni correction. (b and c) Aorta were frozen and sectioned. The expression of iNOS protein was measured by immunohistochemistry analysis. A representative picture (b) and the summary of results (c) are presented. n = 6. *p < 0.05, Student's t-test adjusted by Bonferroni correction.
CAPs Exposure increased Vascular ·O2− Production
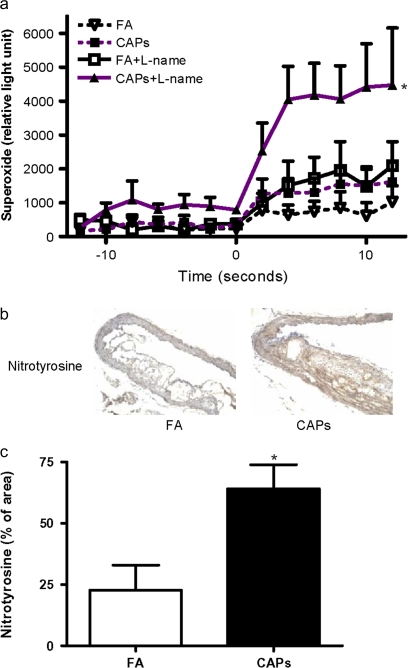
Aortic ·O2− levels in CAPs mice were significantly increased compared with FA exposed mice (Fig. 4a). Pretreatment of the aortic segments with the NOS inhibitor L-NAME significantly increased ·O2− production in both FA and CAPs mice, suggesting that there is a basal release of NO in these segments. L-NAME treated segments from CAPs mice released significantly higher levels of ·O2− compared with FA exposed mice (1782 ± 379 and 5578 ± 1943 counts/mg in the FA and CAPs groups, respectively). Similar to results for iNOS expression, immunohistochemical analysis with anti-nitrotyrosine antibody revealed extensive protein nitration in the aorta of CAPs mice. Moreover, the distribution pattern of nitrated proteins was very similar to that of iNOS (Figs. 4b and 4c). These results suggest that CAPs-exposed animals demonstrate increases in stimulated ·O2− production in conjunction with large increases in NO production presumably from an iNOS source.
FIG. 4.
Exposure to CAPs increases vascular superoxide production. (a) Aortic superoxide production was measured by lucigenin chemiluminescence assay in the presence or absence of 10μM L-NAME. Nicotinamide adenine dinucleotide was added at second 0. n = 6. *Compared with FA without L-NAME, p < 0.05; #compared with FA with L-NAME, p < 0.05; $compared with PM without L-NAME, p < 0.05; ANOVA. (b and c) The aortic protein nitration was measured by immunohistochemistry analysis with anti-nitrotyrosine. A representative picture (b) and the summary of results (c) are presented. n = 6. *p < 0.05, Student's t-test adjusted by Bonferroni correction.
CAPs Exposure Increased NADPH Oxidase Expression in Atherosclerosis
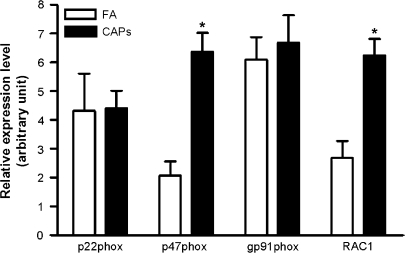
In light of the fact that we observed large increase in ·O2− production in the vasculature of CAPs treated animals we investigated the specific contribution of NADPH (nicotinamide adenine dinucleotide phosphate, reduced) oxidase, which is believed to be the most important source of vascular ·O2− production (Bedard and Krause, 2007). mRNA expression of key NADPH oxidase subunits and Rac1was measured by real-time RT-PCR. Figure 5 depicts mRNA expression of key NADPH oxidase subunits. Although Rac1 and p47phox was significantly increased in CAPs mice, expression of other NADPH oxidase subunits were not affected by CAPs exposure.
FIG. 5.
Exposure to CAPs increases vascular expression of p47phox and RAC1. Total RNA was extracted from aorta, and the mRNA expression of NADPH oxidase subunits and RAC1 were measured by real-time PCR. n = 6. *p < 0.05, Student's t-test adjusted by Bonferroni correction.
CAPs Exposure and Atherosclerosis
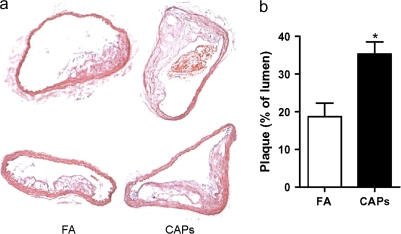
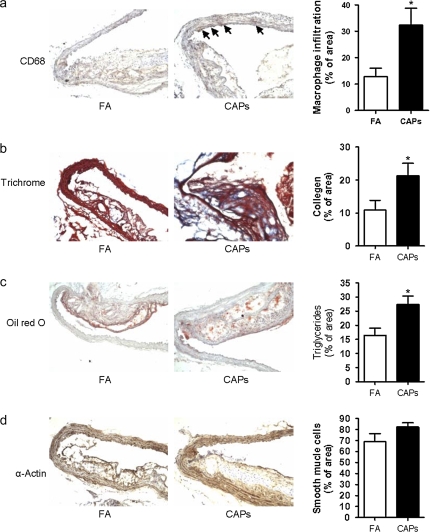
Atherosclerotic burden assessed by quantitative morphometry of the thoracic aorta revealed a significant increase in mice exposed to CAPs compared with FA (Figs. 6a and 6b). Analysis of atherosclerotic plaque composition including, macrophage, smooth muscle cells, lipid, and collagen content revealed higher levels of macrophage infiltration, collagen deposition, and lipid composition in aortas from CAPs mice (Figs. 7a-c) Smooth muscle actin levels were not different between CAPs and FA mice (Fig. 7d).
FIG. 6.
Exposure to CAPs increases atherosclerosis burden. ApoE–/– mice fed with high-fat chow were exposed to PM2.5 for 4 months. (a and b) Thoracic aorta from FA and CAPs mice were sectioned and analyzed after hematoxylin and eosin staining. (a) Two representative pictures of FA (right) and CAPs (left) mice were presented. (b) The summary of results. n = 6. *p < 0.05, Student's t-test.
FIG. 7.
Exposure to CAPs increases vascular macrophage infiltration and collagen accumulation. Aortic slides were stained with anti-CD68 (a), trichrome (b), oil red O (c), and α-actin (d). n = 6. *p < 0.05, Student's t-test adjusted by Bonferroni correction.
DISCUSSION
This paper has several important findings: (1) ambient CAPs exposure in Manhattan has potent effects on vascular responsiveness in experimental atherosclerosis; (2) CAPs stimulates vascular ·O2− generation through NADPH oxidase dependent mechanism; (3) PM2.5 is an important stimulus for iNOS expression in the vasculature; (4) CAPs increases atherosclerotic burden likely through pro-oxidant and inflammatory effects. Our findings extend previous observations from our group and others (Kunzli et al., 2005; Mills et al., 2007; Sun et al., 2005; Suwa et al., 2002) that CAPs is an important stimulus for atherosclerosis.
The biological effects of ambient particulate exposure are complex and vary according to the timing, duration, and location of exposure. The current experiments were performed in Manhattan, New York where motor vehicle exhaust is the primary source of CAPs (Lall, 2006). Consistent with previous study (Lall, 2006), our elemental data demonstrate that EC and OC are major constituents of CAPs at Manhattan, New York, whereas EC and OC are relatively lower in Sterling Forest (Tuedo, NY) where motor vehicle traffic is limited. Because epidemiological studies have shown that of the constituents of CAPs, EC, and OC are associated with mortality (Lippmann et al., 2000; Mar et al., 2000), the present study offers new insights into the mechanisms by which these constituents may exert health effects. In addition to EC and OC, CAPs from Manhattan also appeared to have higher constitutes of transition metals such as Zn, V, Fe, and Ni. Therefore, our results are also consistent with studies showing that these metals can play an important role in mediating cardiovascular effects of particulate air pollution (Campen et al., 2001; Dreher et al., 1997; Ghio and Cohen, 2005; Gottipolu et al., 2008; Huang et al., 2003; Lippmann et al., 2006)
Particulate air pollution has been shown to exert complex effects on vasomotor functions in vitro (Bagate et al., 2004; Cheng and Kang, 1999; Knaapen et al., 2001; Tzeng et al., 2003). The disparity in results from these studies may be related to the artificiality of in vitro exposure, and therefore provides a strong imperative to examine the in vivo effects of exposure to real world particles and to understand if varying timing, duration, and location of exposure has different effects on physiologic responses. Our results show that CAPs exposure attenuates PE induced contraction, and it is restored by ODQ, indicating that CAPs exposure increases NO production. These results were consistent with the information obtained by immunohistochemistry and RT-PCR that revealed the important role of CAPs in increasing vascular iNOS expression in the setting of atherosclerosis. Although NO produced by eNOS and nNOS exert anti-inflammatory effects and contribute to the maintenance of cardiovascular homeostasis, the large amount of NO produced by iNOS is pro-inflammatory and is implicated in dysfunctional physiologic responses partly through the diffusion limited reaction of ·O2− with NO to form ONOO-, which leads to the rapid inactivation of proteins through nitrosylation reactions (Huie and Padmaja, 1993; Ischiropoulos et al., 1992; Ortiz and Garvin, 2003). In contrast, our previous studies showed that 6-month CAPs exposure in a rural area in New York State approximately 40 miles from Manhattan did not decrease contraction by PE (Sun et al., 2005). Because the total dose of airborne CAPs were similar in these two studies (85 μg/m3 for 6 months vs. 138 μg/m3 for 4 months), the difference in effects should be due to the different durations of exposure as well as the varying composition of CAPs, and a head to head study in the future may be necessary to define the mechanism for the different effects of CAPs exposure.
Although vascular response to acetylcholine appears to be similar in FA and CAPs mice, a markedly decreased response to A23187 is observed in CAPs mice, indicating that CAPs exposure may also lead to important consequences in the NO/sGC pathway. A23187 induces NO releasing through Ca2+-dependent mechanisms. Notably, because activation of eNOS but not iNOS is Ca2+-dependent (Ortiz and Garvin, 2003; Sun et al., 2005), these results support prior findings including our own that eNOS function is abnormal in vasculature from CAPs mice (Mills et al., 2007; Sun et al., 2005; Tamagawa et al., 2008). These results are consistent with studies by Nukiewicz showing that pulmonary PM exposure siginificantly impaired A23187-induced arteriolar endothelium-dependent dilation (Nurkiewicz et al., 2004). The aortic segments from PM2.5 mice also reveal a slightly decreased response to NO donor SNP. This could be related to downstream abnormalities in cGMK dependent kinases as has been demonstrated previously (Warnholtz et al., 2002).
Our studies confirm the important effect of inhaled PM in driving ·O2− generation in the vasculature and provide yet another piece of evidence to support their complex and often variable physiologic effects. We demonstrate that increased vascular ·O2− is associated with increased activity of NADPH oxidase and heightened expression of the NADPH oxidase subunit p47phox and Rac1. p47phox is an important cytosolic subunit of NADPH oxidase that once phosphorylated, migrates to the membrane where it binds to the p22phox subunit resulting in ·O2− generation. Rac1 is a small GTP binding protein that is necessary for the full activation of NADPH oxidase (Bedard and Krause, 2007). The increased expression of p47phox and Rac1 in vasculature from CAPs mice supports the concept that NADPH oxidase may be a major source of vascular ·O2− induced by particulate air pollution. eNOS uncoupling is another important mechanism for vascular ·O2− generation (Forstermann and Munzel, 2006), In this investigation we however found no evidence of functional eNOS uncoupling in the aorta as evidenced by an increase rather than a decrease of ·O2− production with L-NAME incubation. Although we did not measure tetrahydrobiopterin levels in the aorta in this paper, we cannot rule out this possibility entirely. However, an increase in vascular ·O2− generation with L-NAME along with a trend towards an increase in expression of the rate-limiting enzyme for BH4 synthesis is supportive evidence that BH4 levels may be intact. However in conditions where oxidative stress is increased to levels even higher than that seen in atherosclerosis such as in states associated with Angiotensin II excess, CAPs may have an additive impact on BH4 depletion (Sun et al., 2008).
In conclusion, we demonstrate that 4-month exposure to particulate air pollution increases ·O2− and NO through NADPH oxidase and iNOS pathways that may contribute to vascular dysfunction and atherosclerosis noted with particulate air pollution. The compositional characteristics and duration of CAPs exposure may have disparate effects on vascular responses in vulnerable disease models. Together with other studies, these results support the hypothesis that exposure to low concentrations of particulate air pollution may aggravate atherosclerosis through promotion of remote vascular oxidant and inflammatory effects.
FUNDING
National Institutes of Health (NIH) (R01ES013406 and R01ES015146 to S.R. and K01ES016588 to Q.S.). The whole-body exposure was performed in facilities at Mount Sinai that were supported by NIH grants ES015146, ES00260, and ES015495 and a Health Effects Institute grant (4750-RFA05–1A/06–11 to L.C.C.).
References
- Bagate K, Meiring JJ, Cassee FR, Borm PJ. The effect of particulate matter on resistance and conductance vessels in the rat. Inhal. Toxicol. 2004;16:431–436. doi: 10.1080/08958370490439588. [DOI] [PubMed] [Google Scholar]
- Bai Y, Suzuki AK, Sagai M. The cytotoxic effects of diesel exhaust particles on human pulmonary artery endothelial cells in vitro: Role of active oxygen species. Free Radic. Biol. Med. 2001;30:555–562. doi: 10.1016/s0891-5849(00)00499-8. [DOI] [PubMed] [Google Scholar]
- Becker S, Soukup JM, Gallagher JE. Differential particulate air pollution induced oxidant stress in human granulocytes, monocytes and alveolar macrophages. Toxicol. In Vitro. 2002;16:209–218. doi: 10.1016/s0887-2333(02)00015-2. [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Bhatnagar A. Environmental cardiology: Studying mechanistic links between pollution and heart disease. Circ. Res. 2006;99:692–705. doi: 10.1161/01.RES.0000243586.99701.cf. [DOI] [PubMed] [Google Scholar]
- Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC, Jr, Tager I. Air pollution and cardiovascular disease: A statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- Campen MJ, Nolan JP, Schladweiler MC, Kodavanti UP, Evansky PA, Costa DL, Watkinson WP. Cardiovascular and thermoregulatory effects of inhaled PM-associated transition metals: a potential interaction between nickel and vanadium sulfate. Toxicol. Sci. 2001;64:243–252. doi: 10.1093/toxsci/64.2.243. [DOI] [PubMed] [Google Scholar]
- Chen LC, Hwang JS. Effects of subchronic exposures to concentrated ambient particles (CAPs) in mice. IV. Characterization of acute and chronic effects of ambient air fine particulate matter exposures on heart-rate variability. Inhal. Toxicol. 2005;17:209–216. doi: 10.1080/08958370590912789. [DOI] [PubMed] [Google Scholar]
- Cheng YW, Kang JJ. Inhibition of agonist-induced vasocontraction and impairment of endothelium-dependent vasorelaxation by extract of motorcycle exhaust particles in vitro. J. Toxicol. Environ. Health A. 1999;57:75–87. doi: 10.1080/009841099157791. [DOI] [PubMed] [Google Scholar]
- Das A, Xi L, Kukreja RC. Phosphodiesterase-5 inhibitor sildenafil preconditions adult cardiac myocytes against necrosis and apoptosis. Essential role of nitric oxide signaling. J. Biol. Chem. 2005;280:12944–12955. doi: 10.1074/jbc.M404706200. [DOI] [PubMed] [Google Scholar]
- Dreher KL, Jaskot RH, Lehmann JR, Richards JH, McGee JK, Ghio AJ, Costa DL. Soluble transition metals mediate residual oil fly ash induced acute lung injury. J. Toxicol. Environ. Health. 1997;50:285–305. [PubMed] [Google Scholar]
- Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- Ghio AJ, Cohen MD. Disruption of iron homeostasis as a mechanism of biologic effect by ambient air pollution particles. Inhal. Toxicol. 2005;17:709–716. doi: 10.1080/08958370500224482. [DOI] [PubMed] [Google Scholar]
- Gottipolu RR, Landa ER, Schladweiler MC, McGee JK, Ledbetter AD, Richards JH, Wallenborn GJ, Kodavanti UP. Cardiopulmonary responses of intratracheally instilled tire particles and constituent metal components. Inhal. Toxicol. 2008;20:473–484. doi: 10.1080/08958370701858427. [DOI] [PubMed] [Google Scholar]
- Gurgueira SA, Lawrence J, Coull B, Murthy GG, Gonzalez-Flecha B. Rapid increases in the steady-state concentration of reactive oxygen species in the lungs and heart after particulate air pollution inhalation. Environ. Health Perspect. 2002;110:749–755. doi: 10.1289/ehp.02110749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YC, Ghio AJ, Stonehuerner J, McGee J, Carter JD, Grambow SC, Devlin RB. The role of soluble components in ambient fine particles-induced changes in human lungs and blood. Inhal. Toxicol. 2003;15:327–342. doi: 10.1080/08958370304460. [DOI] [PubMed] [Google Scholar]
- Huie RE, Padmaja S. The reaction of no with superoxide. Free Radic. Res. Commun. 1993;18:195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H, Zhu L, Beckman JS. Peroxynitrite formation from macrophage-derived nitric oxide. Arch. Biochem. Biophys. 1992;298:446–451. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- Knaapen AM, den Hartog GJ, Bast A, Borm PJ. Ambient particulate matter induces relaxation of rat aortic rings in vitro. Hum. Exp. Toxicol. 2001;20:259–265. doi: 10.1191/096032701678227677. [DOI] [PubMed] [Google Scholar]
- Kunzli N, Jerrett M, Mack WJ, Beckerman B, LaBree L, Gilliland F, Thomas D, Peters J, Hodis HN. Ambient air pollution and atherosclerosis in Los Angeles. Environ. Health Perspect. 2005;113:201–206. doi: 10.1289/ehp.7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lall Ra T G D. Identifying and quantifying transported vs. local sources of New York City PM2.5 fine particulate matter air pollution. Atmospheric Environ. 2006;40:S333–S346. [Google Scholar]
- Lippmann M, Ito K, Hwang JS, Maciejczyk P, Chen LC. Cardiovascular effects of nickel in ambient air. Environ. Health Perspect. 2006;114:1662–1669. doi: 10.1289/ehp.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann M, Ito K, Nadas A, Burnett RT. Association of particulate matter components with daily mortality and morbidity in urban populations. Res. Rep. Health Eff. Inst. 2000:5–72. discussion 73–82. [PubMed] [Google Scholar]
- Mar TF, Norris GA, Koenig JQ, Larson TV. Associations between air pollution and mortality in Phoenix, 1995-1997. Environ. Health Perspect. 2000;108:347–353. doi: 10.1289/ehp.00108347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills NL, Tornqvist H, Robinson SD, Gonzalez MC, Soderberg S, Sandstrom T, Blomberg A, Newby DE, Donaldson K. Air pollution and atherothrombosis. Inhal. Toxicol. 2007;19(Suppl. 1):81–89. doi: 10.1080/08958370701495170. [DOI] [PubMed] [Google Scholar]
- Nurkiewicz TR, Porter DW, Barger M, Castranova V, Boegehold MA. Particulate matter exposure impairs systemic microvascular endothelium-dependent dilation. Environ. Health Perspect. 2004;112:1299–1306. doi: 10.1289/ehp.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz PA, Garvin JL. Cardiovascular and renal control in NOS-deficient mouse models. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;284:R628–638. doi: 10.1152/ajpregu.00401.2002. [DOI] [PubMed] [Google Scholar]
- Peters A, von Klot S, Heier M, Trentinaglia I, Hormann A, Wichmann HE, Lowel H. Exposure to traffic and the onset of myocardial infarction. N. Engl. J. Med. 2004;351:1721–1730. doi: 10.1056/NEJMoa040203. [DOI] [PubMed] [Google Scholar]
- Pope CA, 3rd, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution: Epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- Rossi G, Vigotti MA, Zanobetti A, Repetto F, Gianelle V, Schwartz J. Air pollution and cause-specific mortality in Milan, Italy, 1980-1989. Arch. Environ. Health. 1999;54:158–164. doi: 10.1080/00039899909602254. [DOI] [PubMed] [Google Scholar]
- Schwarze PE, Ovrevik J, Hetland RB, Becher R, Cassee FR, Lag M, Lovik M, Dybing E, Refsnes M. Importance of size and composition of particles for effects on cells in vitro. Inhal. Toxicol. 2007;19(Suppl. 1):17–22. doi: 10.1080/08958370701490445. [DOI] [PubMed] [Google Scholar]
- Sun Q, Wang A, Jin X, Natanzon A, Duquaine D, Brook RD, Aguinaldo JG, Fayad ZA, Fuster V, Lippmann M, et al. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA. 2005;294:3003–3010. doi: 10.1001/jama.294.23.3003. [DOI] [PubMed] [Google Scholar]
- Sun Q, Yue P, Ying Z, Cardounel AJ, Brook RD, Devlin R, Hwang JS, Zweier JL, Chen LC, Rajagopalan S. Air pollution exposure potentiates hypertension through reactive oxygen species-mediated activation of Rho/ROCK. Arterioscler Thromb. Vasc. Biol. 2008;28:1760–1766. doi: 10.1161/ATVBAHA.108.166967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwa T, Hogg JC, Quinlan KB, Ohgami A, Vincent R, van Eeden SF. Particulate air pollution induces progression of atherosclerosis. J. Am. Coll. Cardiol. 2002;39:935–942. doi: 10.1016/s0735-1097(02)01715-1. [DOI] [PubMed] [Google Scholar]
- Tamagawa E, Bai N, Morimoto K, Gray C, Mui T, Yatera K, Zhang X, Xing L, Li Y, Laher I, et al. Particulate matter exposure induces persistent lung inflammation and endothelial dysfunction. Am. J. Physiol. Lung Cell Mol. Physiol. 2008;295:L79–85. doi: 10.1152/ajplung.00048.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng HP, Yang RS, Ueng TH, Lin-Shiau SY, Liu SH. Motorcycle exhaust particulates enhance vasoconstriction in organ culture of rat aortas and involve reactive oxygen species. Toxicol. Sci. 2003;75:66–73. doi: 10.1093/toxsci/kfg164. [DOI] [PubMed] [Google Scholar]
- Uppu RM, Nossaman BD, Greco AJ, Fokin A, Murthy SN, Fonseca VA, Kadowitz PJ. Cardiovascular effects of peroxynitrite. Clin. Exp. Pharmacol. Physiol. 2007;34:933–937. doi: 10.1111/j.1440-1681.2007.04641.x. [DOI] [PubMed] [Google Scholar]
- Warnholtz A, Mollnau H, Heitzer T, Kontush A, Moller-Bertram T, Lavall D, Giaid A, Beisiegel U, Marklund SL, Walter U, et al. Adverse effects of nitroglycerin treatment on endothelial function, vascular nitrotyrosine levels and cGMP-dependent protein kinase activity in hyperlipidemic Watanabe rabbits. J. Am. Coll. Cardiol. 2002;40:1356–1363. doi: 10.1016/s0735-1097(02)02133-2. [DOI] [PubMed] [Google Scholar]
- Ying Z, Jin L, Palmer T, Webb RC. Angiotensin II up-regulates the leukemia-associated Rho guanine nucleotide exchange factor (RhoGEF), a regulator of G protein signaling domain-containing RhoGEF, in vascular smooth muscle cells. Mol. Pharmacol. 2006;69:932–940. doi: 10.1124/mol.105.017830. [DOI] [PubMed] [Google Scholar]