Abstract
The neurological sequelae of chronic Mn exposure include psychiatric, cognitive, and motor deficits, suggesting the potential involvement of multiple neurotransmitter systems and brain regions. Available evidence in rodents suggests that Mn causes dysregulation of glutamatergic and γ-aminobutyric acidergic (GABAergic) neurotransmitter systems. However, this has never been studied comprehensively in the nonhuman primate brain. Cynomolgus macaques were given weekly i.v. injections of 3.3–5.0 mg Mn/kg, 5.0–6.7 mg Mn/kg, or 8.3–10.0 mg Mn/kg for 7–59 weeks. Total glutamate, glycine, and GABA concentrations were measured by high performance liquid chromatography (HPLC) with fluorescence detection in 13 brain areas in Mn-treated and control monkeys. Neurotransmitter concentrations did not change with chronic Mn exposure. Quantitative autoradiography of the N-methyl-D-aspartate receptor, the GABAa receptor, and glutamate transporters was used to assess their regional distribution. Each of these neurotransmitter receptors remained almost universally unchanged with Mn treatment. Immunohistochemical analysis of glutamine synthetase (GS) demonstrated a selective Mn-induced decrease in the globus pallidus, which could potentially alter synaptic and/or astrocytic levels of glutamate. This study shows that in nonhuman primates with previous documentation of Mn-induced brain pathology, the glutamatergic and GABAergic systems appear to be mostly unaffected by chronic Mn exposure with the exception of reduced GS expression in the globus pallidus.
Keywords: manganese, neurotoxicity, nonhuman primates, glutamate, GABA, brain
Manganese (Mn) is a naturally occurring element that is an essential nutrient, and cofactor for certain metalloenzymes required for normal cellular homeostasis (Aschner and Aschner, 2005). Although Mn consumption is necessary in humans, excessive exposure is associated with adverse psychiatric, cognitive, and motor effects. Increased human exposures to Mn occur in a variety of settings. For example, excessive Mn exposure occurs in occupations such as ferroalloy smelting (Mergler et al., 1994), welding (Bowler et al., 2003), mining (Montes et al., 2008; Rodriguez-Agudelo et al., 2006), battery assembly (Bader et al., 1999), and the manufacture of glass and ceramics (Srivastava et al., 1991). Iatrogenic exposure to manganese may occur to individuals receiving total parenteral nutrition (Hardy et al., 2008; Iinuma et al., 2003). Medical conditions such as liver failure have also been shown to accumulate Mn in the brain (Brunberg et al., 1991). Furthermore, the use of methylcyclopentadienyl manganese tricarbonyl as a gasoline anti-knock agent has raised concerns over potential increased environmental Mn exposures to the general population (Gulson et al., 2006).
The neurological sequelae of exposure to high levels of Mn occur in stages. The early phase of Mn intoxication involves a psychiatric component, characterized in part by irritability, apathy, and psychosis, and is sometimes called Mn mania (Mergler and Baldwin, 1997). Deficits in measures of executive function, such as short-term memory and computational ability, are also early manifestations of Mn neurotoxicity (Lucchini et al., 1995). Characteristics of the late phase of Mn intoxication include dystonia, rigidity, and gait abnormalities (Lucchini et al., 1999).
Magnetic resonance imaging studies are in agreement that Mn accumulates to a large extent in the globus pallidus (Hauser et al., 1996; Josephs et al., 2005). The globus pallidus and related basal ganglia structures have therefore been a focus of the preponderance of the research efforts on Mn neurotoxicity. Several nonhuman primate studies show that Mn accumulates in other brain areas but not to the same extent as the globus pallidus (Bock et al., 2008; Dorman et al., 2006; Guilarte et al., 2006b). Examination of other brain regions may elucidate possible mechanisms by which Mn produces its early effects.
There is a persistent notion in the literature that a component of Mn neurotoxicity may be mediated by dysregulation of excitatory glutamatergic and inhibitory γ-aminobutyric acid (GABA) neurotransmission. Early evidence for this hypothesis stems from studies conducted two decades ago in which it was shown that chronic Mn administration to rodents produced an increase in striatal GABA concentrations (Gianutsos and Murray, 1982) and altered the levels of glutamic acid decarboxylase (Lai et al., 1981), the enzyme that converts glutamate to GABA. Since then, a series of rodent studies from several laboratories suggested that Mn exposure increases brain glutamate and GABA concentrations (Gwiazda et al., 2002; Lipe et al., 1999; Reaney et al., 2006) while other studies suggest opposite effects (Brouillet et al., 1993; Seth et al., 1981; Zwingmann et al., 2007). Other research suggests that chronic Mn exposure in rodents has no effect on amino acid neurotransmitter concentrations in the corpus striatum (Bonilla et al., 1994).
There is much more limited data available on the effect of chronic Mn exposure in nonhuman primates and its effects on the glutamatergic and GABAergic systems. For example, it was shown that over two years of repeated monthly injections of Mn does not alter GABAa receptor density (Eriksson et al., 1992). Other studies showed that nonhuman primates exposed to Mn via inhalation for 13 weeks did not have altered total glutamate or GABA tissue levels in the globus pallidus, caudate, and putamen (Struve et al., 2007). However, in several brain regions of the same animals, they noted increased mRNA expression and decreased protein levels of GLAST and GLT-1—the two main astrocytic glutamate transporters—and glutamine synthetase (GS), the enzyme that converts glutamate to glutamine (Erikson et al., 2007).
Given the contradictory data available from rodent studies, and the paucity of experiments conducted in nonhuman primates, a comprehensive study is warranted on the effect of chronic Mn exposure on the glutamatergic and GABAergic neurotransmitter systems and their receptors and transporters throughout the brain. Therefore, we used a model of chronic Mn exposure in nonhuman primates to evaluate the potential effects on these systems. The current study is part of a multidisciplinary effort to characterize the behavioral, neuroimaging, neuropathological and neurochemical consequences of chronic Mn exposure in nonhuman primates. This report provides data that indicates a Mn-induced deficit in GS levels in the globus pallidus that could potentially alter astrocytic or synaptic levels of glutamate.
MATERIALS AND METHODS
Mn administration and animal care.
Young adult male Cynomolgus macaques, 5–6 years of age at the start of the study, were used. All animal studies were reviewed and approved by the Johns Hopkins and the Thomas Jefferson University Animal Care and Use Committees. Treated animals received intravenous injections of manganese sulfate (3.3–5.0 mg Mn/kg, n = 4, 44–50 weeks; 5.0–6.7 mg Mn/kg, n = 5, 27–59 weeks; or 8.3–10 mg Mn/kg, n = 2, 15, and 38 weeks) into the saphenous vein under 1–3% isoflurane anesthesia approximately once or twice per week (see Table 1). Separate reports of neuroimaging data from this cohort of animals include two additional Mn-exposed animals, #6697 and #7782. However, post-mortem analysis could not be performed on these two animals due to lack of tissue availability. These animals, in addition to four naïve animals of the same age and two animals that went through behavioral and imaging protocols but did not receive Mn injections, were euthanized by ketamine injection (20–30 mg/kg) followed by an overdose of pentobarbital (100 mg/kg) and the brains were harvested. Brain tissue from the animals used in this work has been used in multiple studies. In some animals, therefore, prior sampling has limited the availability of tissue for some of the experiments in the current study.
TABLE 1.
Animal Exposure Parameters
| Animal ID | Dose level (MnSO4) | Dose level (Mn) | Dosing interval | Exposure duration (weeks) | Cumulative MnSO4 | Cumulative Mn |
| 75W | 10–15 mg/kg | 3.3–5.0 mg/kg | 1/week | 44 | 455 mg/kg | 151.7 mg/kg |
| 144T | 10–15 mg/kg | 3.3–5.0 mg/kg | 1/week | 50 | 515 mg/kg | 171.7 mg/kg |
| 107-705 | 10–15 mg/kg | 3.3–5.0 mg/kg | 1/week | 42 | 500 mg/kg | 166.7 mg/kg |
| 3154 | 10–15 mg/kg | 3.3–5.0 mg/kg | 1/week | 45 | 515 mg/kg | 173.8 mg/kg |
| 3114 | 15–20 mg/kg | 5.0–6.7 mg/kg | 1/week | 46 | 635 mg/kg | 206.4 mg/kg |
| 7782 | 15–20 mg/kg | 5.0–6.7 mg/kg | 2/weeka | 27 | 435 mg/kg | 141.4 mg/kg |
| 9093 | 15–20 mg/kg | 5.0–6.7 mg/kg | 2/weeka | 59 | 770 mg/kg | 250.8 mg/kg |
| 7469 | 15–20 mg/kg | 5.0–6.7 mg/kg | 2/weeka | 32 | 525 mg/kg | 170.7 mg/kg |
| 000-8001 | 15–20 mg/kg | 5.0–6.7 mg/kg | 2/weeka | 34 | 535 mg/kg | 173.9 mg/kg |
| 001-1099 | 15–20 mg/kg | 5.0–6.7 mg/kg | 2/weeka | 32 | 535 mg/kg | 173.9 mg/kg |
| 7839 | 25–30 mg/kg | 8.3–10.0 mg/kg | 2/weeka | 38 | 640 mg/kg | 218.3 mg/kg |
| 6697 | 25–30 mg/kg | 8.3–10.0 mg/kg | Bolus, 2/weeka | 7 | 206 mg/kg | 68.3 mg/kg |
| 7426 | 25–30 mg/kg | 8.3–10.0 mg/kg | Bolus, 2/weeka | 15 | 340 mg/kg | 113.3 mg/kg |
Note. Dosing schedule and cumulative doses for Mn-exposed animals used in this study.
For animals receiving a weekly dose as two injections, the total weekly dose was split and administered on two different occasions.
Brains were harvested and embedded in warm 3% low-melting point agarose. After the agarose hardened, the brains were sliced in a series of 0.4 cm coronal slabs using a commercial meat slicer. The left hemisphere was immediately frozen on dry ice, and stored at −80°C until use for metals and neurotransmitter analysis or quantitative autoradiography of neuroreceptors and transporters. The 0.4 cm slabs from the left hemisphere were sectioned at 20 μm and thaw-mounted onto slides. The slides were then stored at −80°C until use. The right hemisphere was post-fixed in paraformaldehyde and used for immunohistochemistry. Fifty-micron sections were prepared free-floating on a freezing microtome and stored in 50% glycerol at −20°C until use.
High-performance liquid chromatography–fluorescence detection of glutamate, glycine, and GABA in brain tissue.
Multiple brain areas were assayed for concentrations of glutamate, GABA, and glycine using an high-performance liquid chromatography (HPLC) (Shimadzu North America, Columbia, MD) system with fluorescence detection. Each sample was assayed according to a modification of a previously described protocol (Guilarte, 1989). A 50mM internal standard homoserine stock solution was prepared in 50% methanol. The homogenization solution was prepared by diluting this internal standard solution 1:20 in absolute methanol. Tissue from each brain area was processed independently, and the samples were assayed immediately following processing. Tissue samples were sonicated with 10, 1-s pulses in homogenization solution in a volume (in μl) 10 times that of the sample mass (in mg). The disrupted tissue was then centrifuged at 27,000 × g at 4°C for 5 min, and the resulting supernatant was filtered through Phenex 4 mm nonsterile 0.2-μm nylon syringe filters. The samples were then stored at 4°C until HPLC analysis on the same day. Derivatizing solution was prepared by mixing 65.5 mg O-phthaldialdehyde, 100 μl of β-mercaptoethanol, and 2.5 ml of methanol, and the volume was brought up to 25 ml with 0.1M sodium tetraborate, pH 9.1. The derivatizing solution was used within 5 days of preparation. The processed samples were diluted 1:1 in cold absolute methanol. Fifty microliters of the filtered diluent was added to 200 μl of derivatizing solution. The samples were vortexed and allowed to react for 2.5 min, and 20 μl was injected onto the HPLC. The HPLC system consisted of an isocratic pump (LC-20AT, Shimadzu; flow rate 0.75 ml/min), a SIL-9A injector (Shimadzu), a 5 μm C18 (4.6 × 150 mm) reverse-phase column, and a fluorescence detector (RF-535, Shimadzu; excitation 330 nm, emission 418 nm). The mobile phase consisted of 65% 0.1M sodium phosphate, pH 5.5, and 35% methanol. Amino acids in the biological samples were identified by comparison of their retention times to that of the individual standards. The ratio of the area under the curve for a given amino acid to that of the internal standard was determined. This ratio was compared with the ratio derived from injecting the standards alone, and the concentrations for individual amino acids in biological samples were calculated.
Quantitative autoradiography of GABAa receptors, N-methyl-D-aspartate receptors and glutamate transporters.
Slides were thawed and warmed on a slide warmer at 37°C for 30 min. For GABAa receptor autoradiography, brain sections were then equilibrated in 50mM Tris citrate buffer, pH 7.1, three times for five minutes each (Pan et al., 1983). Binding sites for muscimol were labeled in buffer containing 20nM [3H]-muscimol (36.6 Ci/mmol, Perkin Elmer, Waltham, MA), a ligand that binds to GABAa receptors, with or without 10mM of unlabeled GABA to correct for nonspecific binding, for 40 min at 4°C. Slides were then rinsed twice for one minute each in ice-cold buffer.
For N-methyl-D-aspartate (NMDA) receptor autoradiography, slides were preincubated for 30 min at room temperature in 50mM Tris acetate buffer, pH 7.4 (Jett and Guilarte, 1995). Slides were then dried for 30 min at 37°C. Slides were labeled in buffer containing 50μM glutamate and 20μM glycine in the presence of 2.5nM [3H]-MK-801 (27.5 Ci/mmol, Perkin Elmer), a selective ligand for the NMDA receptor, with or without 100μM of unlabeled MK-801 in adjacent sections to correct for nonspecific binding, for 1 h at room temperature. Slides were then rinsed twice for two minutes in ice-cold buffer, followed by a thirty minute rinse in ice-cold buffer.
For glutamate transporter autoradiography, slides were then preincubated for 20 min in ice-cold 50mM Tris-HCl buffer, pH 7.4, containing 300mM NaCl (Parsons and Rainbow, 1983). Slides were labeled in buffer containing 40nM [3H]-D-aspartate (23.9 Ci/mmol, Perkin Elmer), a ligand that binds to all glutamate transporter subtypes, with or without 200μM of unlabeled D-aspartate to correct for nonspecific binding, for 20 min at 4°C. Slides were then rinsed twice for five minutes in ice-cold buffer.
In all quantitative receptor autoradiography studies, slides were dipped in cold water and dried under a stream of cool air, then apposed to KODAK BioMax MR film, MR-1, along with [3H]-Microscales (Amersham, Arlington Heights, IL), for 4–8 weeks. Reference standards were included with each film to ensure the linearity of optical density and to allow quantitative analysis of the images. Images were captured and digitized using Inquiry (Loats Assoc., Westminster, MD) and the optical density was quantified (NIH Image Version 1.63; National Institute of Health, Bethesda, MD).
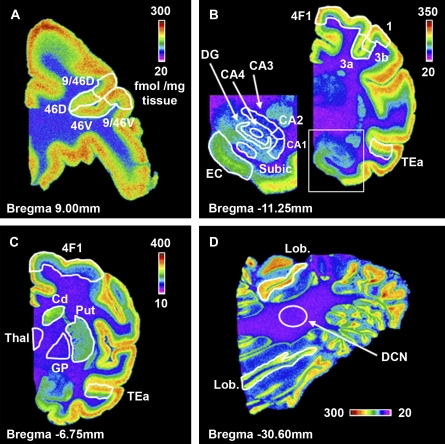
Autoradiography was conducted using slides of coronal brain sections that represent the following areas: frontal cortex (Bregma 9.00 mm); caudate/putamen and globus pallidus (Bregma −6.75 mm); hippocampus (Bregma −11.25 mm); and, cerebellum (Bregma −30.60 mm). A rhesus monkey brain atlas was used to define distinct brain areas (Paxinos et al. 2000). Imaging software was use to delineate and measure binding intensity in all areas. Areas 9/46D, 46D, 46V, and 9/46V (see Fig. 1A) reflect regions of the dorsolateral frontal cortex. Area 4F1 (Figs. 1B, 1C) is part of the motor cortex. This region was analyzed at the level of the globus pallidus and hippocampus in order to determine if an observed trend was dependent on the level at which it was analyzed. Area TEa (Figs. 1B, 1C), part of the temporal cortex, was similarly analyzed at multiple levels. Cd, Put, Thal, and GP (Fig. 1C) are abbreviations for the caudate, putamen, thalamus, and globus pallidus, respectively. Areas 3a, 3b, and 1 (Fig. 1B) are subregions of the somatosensory cortex. The inset in Figure 1B shows the CA fields, the dentate gyrus, subiculum, and entorhinal cortex. Figure 1D depicts representative [3H]-muscimol binding in the cerebellum. Specific binding in the deep cerebellar nucleus (DCN) and the lobules was measured. Lobular intensity above and below the DCN was averaged to yield a single measure of specific binding in the cerebellar lobules.
FIG. 1.
Representative pseudocolor coronal images depicting brain regions analyzed following [3H]-muscimol autoradiography. Representative images of the frontal cortex (A), hippocampus (B), globus pallidus (C), and cerebellum (D). Specific subregions at each level of the brain analyzed were identified in order to make appropriate comparisons among animals. 9/46D, 46D, 46V, and 9/46V = areas of the frontal cortex; 4F1 = area of the motor cortex; 3a, 3b and 1 = areas of the somatosensory cortex; TEa = area of the parietal cortex; dentate gyrus (DG), subiculum (subic), entorhinal cortex (EC), CA fields (CA1–4), thalamus (Thal), globus pallidus (GP), caudate (Cd), putamen (Put), deep cerebellar nucleus (DCN), and cerebellar lobules (Lob). Pseudocolor scale bars indicate specific binding (fmol/mg tissue). (B) The boxed area is enlarged and regions identified on the left side.
Immunohistochemistry of GS.
Tissue sections were pretreated with 3% H2O2 and 10% methanol in Tris-buffered saline (TBS) for 20 min. Sections were then incubated with 5% normal horse serum and 0.2% Triton X-100 in TBS for 1 h. Further, sections were incubated with a mouse monoclonal antibody reactive to human GS (BD Biosciences, San Jose, CA, 1:5000, 48 h at 4°C) followed by incubation with the corresponding secondary biotinylated IgG (1:250, Vector, Burlingame, CA, 1.5 h, at room temperature) and the avidin-biotin-peroxidase complex solution (1:170, 1 h at room temperature). The reaction product was visualized with 0.25 mg/ml 3,3′-diaminobenzidine and 0.03% H2O2. Sections were mounted on slides, stained with cresyl violet, dehydrated in graded ethanols and coverslipped using dibutyl phthalate xylene mounting media.
Metals analysis in brain tissue.
Concentrations of Mn were measured in tissue from the frontal cortex, frontal white matter, globus pallidus, caudate, and putamen from control and Mn-exposed animals as previously described (Guilarte et al., 2006a).
Statistics.
Comparison of paired groups of control versus Mn-exposed tissue was conducted using a Student's t-test, with p < 0.05 used as a threshold for statistical significance. Alternatively, to correct for multiple comparisons of means, a threshold of p < 0.025 was also considered for statistical significance in the Student's t-test. Linear regression analysis with p < 0.05 was used to determine statistical significance of the correlation between GS and Mn levels in the globus pallidus.
RESULTS
Analysis of Brain Manganese Concentrations
Brain Mn concentrations were determined by High-Resolution Inductively Coupled Plasma Mass Spectrometry in the frontal cortex, frontal white matter, globus pallidus, caudate, and putamen of control (n = 6) and Mn-exposed (n = 11) animals. Significant accumulation of Mn occurred in all regions analyzed, with the greatest degree of accumulation present in the globus pallidus (Table 2).
TABLE 2.
Brain Manganese Concentrations
| Control | Mn exposed | |
| Frontal cortex | 0.20 ± 0.02 | 0.41 ± 0.03* |
| Frontal white matter | 0.17 ± 0.01 | 0.82 ± 0.11* |
| Caudate | 0.36 ± 0.02 | 1.37 ± 0.18* |
| Putamen | 0.41 ± 0.04 | 1.97 ± 0.25* |
| Globus pallidus | 0.67 ± 0.12 | 3.30 ± 0.38* |
Note. Mn concentrations in the nonhuman primate brain. Each value represents the mean ± SEM (μg Mn/g tissue) of n = 6 controls and n = 11 (frontal cortex, frontal white matter), n = 10 (putamen) and n = 9 (caudate, globus pallidus) Mn-exposed animals. In some cases, tissue was not available in a brain region for a particular animal due to use for other analyses. *p < 0.05 relative to control value.
HPLC Detection of Amino Acid Neurotransmitters
Glutamate, glycine, and GABA concentrations were measured in 13 brain areas including white and gray matter, regions of the basal ganglia, hippocampus, thalamus, and cerebellum. Table 3 shows the average glutamate, GABA, and glycine concentrations for control and Mn-exposed animals. Statistical analysis revealed no significant differences between control and Mn-exposed monkeys in any of the brain regions analyzed.
TABLE 3.
Brain Glutamate, GABA, and Glycine Concentrations
| Glutamate |
GABA |
Glycine |
||||
| Brain region | Control | Mn exposed | Control | Mn exposed | Control | Mn exposed |
| Frontal cortex | 8.52 ± 0.43 | 9.09 ± 0.21 | 1.96 ± 0.13 | 1.93 ± 0.09 | 1.36 ± 0.11 | 1.40 ± 0.05 |
| Frontal white matter | 6.96 ± 0.52 | 6.92 ± 0.24 | ND | ND | 1.31 ± 0.06 | 1.52 ± 0.16 |
| Caudate | 9.32 ± 0.34 | 10.09 ± 1.20 | 3.01 ± 0.26 | 3.17 ± 0.37 | 1.58 ± 0.08 | 1.79 ± 0.18 |
| Putamen | 9.43 ± 0.42 | 8.81 ± 0.56 | 3.48 ± 0.38 | 3.53 ± 0.59 | 2.10 ± 0.43 | 1.53 ± 0.16 |
| Globus pallidus | 2.99 ± 0.20 | 3.56 ± 0.49 | 8.28 ± 0.91 | 7.17 ± 0.55 | 1.55 ± 0.20 | 1.76 ± 0.14 |
| Hippocampus | 10.36 ± 0.45 | 10.24 ± 0.57 | 3.25 ± 0.26 | 2.70 ± 0.30 | 1.41 ± 0.09 | 1.42 ± 0.09 |
| Thalamus | 6.84 ± 0.70 | 7.63 ± 0.31 | 1.83 ± 0.26 | 1.71 ± 0.18 | 0.98 ± 0.09 | 1.12 ± 0.05 |
| Motor cortex | 7.45 ± 0.38 | 7.49 ± 1.03 | 1.76 ± 0.08 | 1.70 ± 0.28 | 1.21 ± 0.09 | 1.16 ± 0.13 |
| Somatosensory cortex | 7.70 ± 0.48 | 7.30 ± 0.43 | 2.27 ± 0.16 | 1.94 ± 0.11 | 1.27 ± 0.06 | 1.11 ± 0.09 |
| Parietal cortex | 7.04 ± 0.58 | 8.30 ± 0.30 | 1.74 ± 0.16 | 1.91 ± 0.10 | 1.07 ± 0.10 | 1.28 ± 0.08 |
| Visual cortex | 6.83 ± 0.52 | 8.04 ± 0.48 | 2.23 ± 0.19 | 2.06 ± 0.17 | 1.15 ± 0.10 | 1.24 ± 0.09 |
| Simple lobule | 8.42 ± 0.28 | 7.37 ± 0.41 | 2.27 ± 0.15 | 1.84 ± 0.14 | 1.46 ± 0.07 | 1.23 ± 0.10 |
| Deep cerebellar nuclei | 3.05 ± 0.06 | 3.62 ± 0.38 | 4.61 ± 0.21 | 4.00 ± 0.30 | 1.77 ± 0.05 | 1.91 ± 0.15 |
Note. Glutamate, GABA, and glycine concentrations in various regions of the nonhuman primate brain. Values are mean ± SEM (nmol/mg tissue) of n = 6 controls and n = 11 Mn-exposed animals, with the exception of the parietal and motor cortex which represents n = 10 Mn-exposed animals. ND = GABA not detected in this region.
[3H]-Muscimol Autoradiography
The regional distribution of GABAa receptors was determined by using the specific ligand [3H]-muscimol. A representative image of total [3H]-muscimol binding in each brain region analyzed is shown in Figure 1. Table 4 shows the average levels of [3H]-muscimol binding in control and Mn-exposed animals. [3H]-muscimol binds prominently throughout the cortex, with a greater degree of binding present in the superficial cortical layers. Superficial and deep cortical layers were measured separately and as a whole. However, because the trends for each were not different than the trends for the layers combined (data not shown), the cortical data presented represents the superficial and deep layers combined. At Bregma −6.75 mm, a statistically significant (p < 0.05) 19% increase in specific binding in area TEa of the temporal cortex was measured in Mn-exposed animals. However, at Bregma −11.25 mm this difference was not observed in this same area of the temporal cortex. Furthermore, if a lower threshold of p < 0.025 is used to determine statistical significance to correct for multiple means comparisons, then there is no statistically significant difference between control and Mn-exposed animals in any of the brain regions analyzed. Binding of [3H]-muscimol in the thalamus, globus pallidus and DCN was too low to accurately measure.
TABLE 4.
Brain GABAa Receptor, NMDA Receptor, and Glutamate Transporter Levels
| [3H]-Muscimol (GABAaR) |
[3H]-MK-801 (NMDAR) |
[3H]-D-Asp (Glu Transp.) |
||||
| Control | Mn exposed | Control | Mn exposed | Control | Mn exposed | |
| Frontal cortex | ||||||
| Area 9/46D | 169.2 ± 9.2 | 186.9 ± 13.8 | 79.3 ± 3.4 | 79.0 ± 2.7 | ** | ** |
| Area 46D | 149.9 ± 9.0 | 175.8 ± 15.1 | 78.9 ± 3.5 | 78.1 ± 2.1 | ** | ** |
| Area 46V | 163.4 ± 10.1 | 174.8 ± 14.8 | 80.0 ± 4.0 | 78.2 ± 2.4 | ** | ** |
| Area 9/46V | 187.5 ± 10.1 | 196.9 ± 15.3 | 82.6 ± 3.6 | 74.9 ± 2.8 | ** | ** |
| Basal ganglia | ||||||
| Caudate | 107.0 ± 10.2 | 132.7 ± 6.1 | 23.2 ± 4.1 | 27.6 ± 5.8 | 102.1 ± 7.7 | 95.1 ± 8.5 |
| Putamen | 78.8 ± 8.8 | 99.0 ± 4.4 | 16.2 ± 3.9 | 19.2 ± 3.5 | 80.7 ± 8.0 | 77.2 ± 9.2 |
| Globus Pallidus | N/A | N/A | N/A | N/A | N/A | N/A |
| Area 4F1 | 136.4 ± 8.4 | 143.8 ± 6.8 | 49.9 ± 4.7 | 45.6 ± 5.8 | 97.4 ± 11.9 | 85.8 ± 6.3 |
| Area Tea | 173.1 ± 10.2 | 205.9 ± 7.4* | 71.8 ± 3.0 | 77.1 ± 7.2 | 92.9 ± 14.3 | 120.0 ± 9.2 |
| Thalamus | N/A | N/A | N/A | N/A | N/A | N/A |
| Hippocampus | ||||||
| CA1 | 63.9 ± 6.2 | 58.2 ± 4.8 | 90.1 ± 2.8 | 90.0 ± 4.5 | 174.6 ± 19.9 | 192.4 ± 18.2 |
| CA3 | 26.5 ± 1.5 | 28.2 ± 5.0 | 76.7 ± 3.7 | 83.5 ± 7.5 | 217.1 ± 18.7 | 213.2 ± 21.2 |
| CA4 | 41.2 ± 2.5 | 41.0 ± 5.1 | 83.5 ± 7.5 | 79.5 ± 7.4 | 280.7 ± 16.0 | 303.8 ± 37.3 |
| Dentate gyrus | 79.6 ± 6.5 | 77.7 ± 5.2 | 115.6 ± 5.3 | 121.0 ± 3.5 | 324.3 ± 21.8 | 307.7 ± 24.6 |
| Subiculum | 50.9 ± 5.0 | 45.6 ± 3.7 | 63.5 ± 5.4 | 57.4 ± 3.7 | 179.1 ± 17.1 | 181.1 ± 15.7 |
| Entorhinal cortex | 96.1 ± 7.3 | 95.3 ± 7.1 | 68.9 ± 2.1 | 67.7 ± 2.7 | 174.8 ± 18.1 | 187.9 ± 14.9 |
| Area 4F1 | 143.6 ± 17.5 | 127.0 ± 9.5 | 64.2 ± 6.2 | 63.3 ± 3.9 | 157.9 ± 15.4 | 156.5 ± 10.7 |
| Area TEa | 171.2 ± 5.1 | 155.5 ± 7.9 | 78.4 ± 2.4 | 73.3 ± 3.9 | 127.2 ± 20.3 | 161.9 ± 10.6 |
| Area 3a | 141.8 ± 10.3 | 132.0 ± 10.6 | 59.1 ± 4.2 | 56.2 ± 3.4 | 143.1 ± 24.2 | 149.6 ± 13.8 |
| Area 3b | 150.4 ± 10.5 | 146.2 ± 9.2 | 70.7 ± 2.9 | 60.5 ± 3.6* | 138.7 ± 21.3 | 154.0 ± 12.5 |
| Area 1 | 177.0 ± 6.3 | 163.8 ± 10.3 | 71.9 ± 3.2 | 65.8 ± 3.6 | 139.2 ± 17.5 | 163.2 ± 17.9 |
| Cerebellum | ||||||
| DCN | N/A | N/A | N/A | N/A | 81.5 ± 6.22 | 98.4 ± 5.1 |
| Lobules | 427.2 ± 38.0 | 493.1 ± 22.8 | N/A | N/A | 387.8 ± 19.4 | 398.9 ± 20.6 |
Note. Quantitative receptor autoradiography of GABAa receptors ([3H]-muscimol), NMDA receptors ([3H]-MK-801), and glutamate transporters ([3H]-D-aspartate). N/A = measurement not available due to inability to obtain specific binding in this brain region. *0.025 < p < 0.05 for [3H]-muscimol binding in area TEa and for [3H]-MK-801 binding in area 3b relative to control value. **Data are unavailable for [3H]-D-aspartate in the frontal cortex due to high nonspecific binding in multiple experiments.
[3H]-MK-801 Autoradiography
The distribution of NMDA receptors was visualized using quantitative autoradiography with the selective ligand [3H]-MK-801. Table 4 shows the average levels of specific [3H]-MK-801 binding in control and Mn-exposed animals in the brain regions analyzed. Only area 3b of the somatosensory cortex (Bregma −11.25 mm) of Mn-exposed animals was statistically significantly different (p < 0.05) from controls. In this area, [3H]-MK-801 specific binding was decreased by 14% in Mn-exposed animals. Similar to the statistical analysis for [3H]-muscimol, if a lower statistical threshold of p < 0.025 is used for comparisons of [3H]-MK-801 binding, then there are no statistically significant differences between control and Mn-exposed animals. Binding of [3H]-MK-801 in the globus pallidus, thalamus, and cerebellum was too low to measure.
[3H]-D-Aspartate Autoradiography
Regional levels of glutamate transporters were visualized using quantitative autoradiography with the glutamate-transporter specific ligand [3H]-D-aspartate. Under conditions of high sodium concentration, D-aspartate is taken up into astrocytes and neurons by all glutamate transporters (Danbolt 2001). Therefore, this method is not specific for individual glutamate transporter subtypes. Binding of [3H]-D-aspartate was quantified at the level of the basal ganglia, hippocampus, and the cerebellum. Table 4 shows the specific binding of [3H]-D-aspartate in multiple brain areas. No statistically significant differences were observed between control and Mn-exposed animals in the brain regions measured. Negligible uptake of [3H]-D-aspartate was noted in the thalamus and globus pallidus.
GS Immunohistochemistry
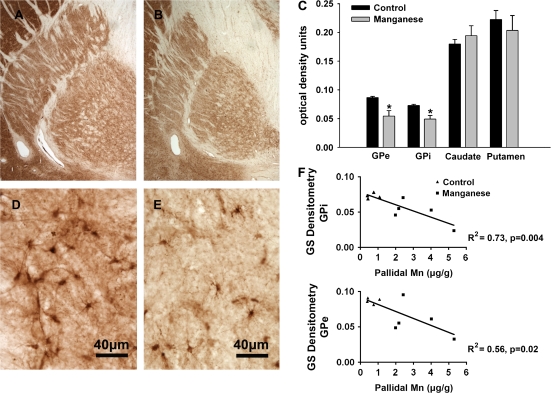
Immunohistochemistry was performed to assess the relative levels of GS in the globus pallidus, caudate and putamen in control and Mn-exposed animals. Semiquantitative densitometry of GS-stained sections revealed comparable levels of expression of GS in the caudate and putamen in control and Mn-exposed animals (Fig. 2). However, in both the exterior and interior globus pallidus, GS staining was consistently reduced (37%, GPe, p = 0.01; 33%, GPi, p = 0.03) in the Mn-exposed animals relative to the control animals. These data represent tissue from animals in which matching sections of globus pallidus were available (four control and six Mn-exposed animals). Nevertheless, the results indicate that chronic Mn exposure affects GS protein levels. When the GS immunohistochemistry was plotted against Mn concentrations in the globus pallidus, there were highly significant negative correlations in both the internal and external globus pallidus. That is, GS levels decreased as the Mn content in the GP increased (Fig. 2).
FIG. 2.
Immunohistochemistry for GS in a control animal (A and D) and a Mn-exposed animal (B and E). (C) Semi-quantitative densitometry of GS in exterior globus pallidus (GPe), interior globus pallidus (GPi), caudate, and putamen in control (n = 4) and Mn-exposed (n = 6) animals. Staining intensity was comparable among all animals in the caudate and putamen. However, in the globus pallidus, GS staining was consistently reduced (37%, GPe, p = 0.01; 33%, GPi, p = 0.03) in the Mn-exposed animals. (F) GS levels in the globus pallidus decreased with increasing concentrations of Mn in this brain region. Note: pallidal Mn concentration was not obtained in one experimental animal due to lack of tissue. *p < 0.05 relative to control value.
DISCUSSION
In the present study, we examine markers for the glutamatergic and GABAergic systems in the brain of Mn-exposed nonhuman primates. Our findings indicate that these systems, at the levels of Mn exposure used, are minimally affected with the exception of the enzyme GS that is decreased in the globus pallidus of Mn-exposed animals. It is important to note that previous studies in this same group of animals have shown significant reductions of in vivo dopamine release in the striatum measured by positron emission tomography that were associated with movement abnormalities (Guilarte et al., 2006a, 2008a). Further, we have also documented decreased N-acetyl aspartate/creatine ratios in the parietal cortex suggestive of neuronal dysfunction or degeneration (Guilarte et al., 2006b) and a significant degree of neuropathology in the frontal cortex (Guilarte et al., 2008b). Lastly, behavioral assessments of these animals have shown deficits in cognitive function (Schneider et al., 2006) and working memory (Schneider et al., 2009), with subtle effects on fine motor control (Schneider et al., 2006). The findings within indicate these behavioral effects of chronic Mn exposure are not likely to be attributable to modulation of glutamate, GABA, and glycine neurotransmitter levels.
Several studies conducted in rodents have documented Mn-induced alterations in the brain concentrations of glutamate and GABA. However, little information is available from higher species that addresses this association. Struve et al. (2007) reported unchanged total tissue glutamate and GABA concentrations in the basal ganglia following subchronic Mn inhalation. The current study provides confirmation that chronic Mn exposure does not alter the concentration of glutamate, GABA, and glycine neurotransmitters. Defining the scope of Mn-induced biological effects is imperative to the clinical management of excessive Mn exposure in humans.
Although we have measured total levels of glutamate, GABA, and glycine in this study, our analysis does not allow the determination of the compartmentalization of these neurotransmitters at the synaptic level. In future studies, in vivo microdialysis would allow the differentiation of distinct pools of glutamate, GABA, and glycine at the synapse. In addition, the data suggest that chronic Mn exposure does not significantly alter the distribution of NMDA subtype of glutamate receptors or GABAa receptors.
Evidence from in vitro and rodent studies has suggested the possibility that Mn interferes with glutamate uptake by reducing the expression of glutamate transporters, particularly GLAST and GLT-1. Erikson et al. (2007) demonstrated that nonhuman primates chronically exposed to Mn by inhalation exhibited decreased levels of GLAST and GLT-1 protein in the olfactory cortex, frontal cortex, caudate, cerebellum, and globus pallidus (Erikson et al., 2007). In addition, they also observed a decrease in GS protein levels. In this study, [3H]-D-aspartate autoradiography indicates that Mn-exposure does not decrease glutamate transporter levels. However, it is important to note that autoradiography of glutamate transporters using [3H]-D-aspartate is useful in identifying all glutamate transporters, not individual transporters, as [3H]-D-aspartate is taken up in neurons and astrocytes by all glutamate transporters (Danbolt, 2001). Due to limited tissue availability, we were unable to analyze these parameters in parallel by Western blot analysis.
Immunohistochemical analysis of GS did show a consistent decrease in the globus pallidus among the available Mn-exposed animals. GS binds four molecules of Mn per octamer, and up to 80% of brain Mn can normally be associated with GS (Prohaska, 1987). This astrocytic enzyme consumes ATP in the conversion of glutamate and ammonia into glutamine. Glutamine is transported to presynaptic neurons and used as a precursor for glutamate and GABA synthesis. A direct effect of Mn on GS levels may partially explain the decrease in GS in the globus pallidus but not the caudate or putamen, given that Mn accumulated to the greatest extent in the globus pallidus of these animals. GS is sensitive to oxidative stress and reactive oxygen species can cause structural changes in GS (Butterfield et al., 1997). Furthermore, oxygen radicals inactivate GS in vitro and in vivo (Schor, 1988). Increased astrocytic ammonia, which would result from a decrease in GS levels, has been associated with protein oxidation, proteasomal activity, and an increase in astrocytic reactive oxygen species (Widmer et al., 2007). Reduced GS activity may therefore reflect an increase in oxidative stress within astrocytes. In addition, an elevation of intracellular ammonia is known to cause astrocytic swelling (Norenberg et al., 2005). Interestingly, a 24-h exposure of cultured astrocytes to Mn also causes cellular swelling (Rama Rao et al., 2007). This suggests a potential linkage between reduced GS levels observed in our Mn-exposed animals and a Mn-induced pathological astrocytic change. Further studies are required to establish the mechanism by which Mn can produce decreased GS expression.
Irrespective of the cause of reduced GS levels, the effect of this reduction must be considered. GS is involved in the glutamate-glutamine cycle, which is responsible for the recycling of glutamate at the level of the synapse. Reductions in GS may cause dysregulation of this recycling pathway and by mass action a reduction in the conversion of glutamate into glutamine that could lead to an increase in astrocytic or synaptic glutamate and ammonia concentrations. This could produce altered astrocytic function and disrupt the tight control of glutamate concentration in the synapse. Increased ammonia in astrocytes causes a decrease in glutamate uptake and an increase in glutamate release (Rose, 2006), which appears to be mediated through the reversal of astrocytic glutamate transporters (Rose, 2006) and calcium-dependent vesicular release from astrocytes (Rose et al., 2005). This further underscores the need to perform in vivo microdialysis to determine whether Mn alters the distribution of glutamate at the synaptic level.
FUNDING
National Institute of Environmental Health Sciences grants (ES010975) to T.R.G.; and training grant (T32 ES07141) to N.C.B.
References
- Aschner JL, Aschner M. Nutritional aspects of manganese homeostasis. Mol. Aspects Med. 2005;26:353–362. doi: 10.1016/j.mam.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader M, Dietz MC, Ihrig A, Triebig G. Biomonitoring of manganese in blood, urine and axillary hair following low-dose exposure during the manufacture of dry cell batteries. Int. Arch. Occup. Environ. Health. 1999;72:521–527. doi: 10.1007/s004200050410. [DOI] [PubMed] [Google Scholar]
- Bock NA, Paiva FF, Nascimento GC, Newman JD, Silva AC. Cerebrospinal fluid to brain transport of manganese in a non-human primate revealed by MRI. Brain Res. 2008;1198:160–170. doi: 10.1016/j.brainres.2007.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla E, Arrieta A, Castro F, Davila JO, Quiroz I. Manganese toxicity: Free amino acids in the striatum and olfactory bulb of the mouse. Invest. Clin. 1994;35:175–181. [PubMed] [Google Scholar]
- Bowler RM, Gysens S, Diamond E, Booty A, Hartney C, Roels HA. Neuropsychological sequelae of exposure to welding fumes in a group of occupationally exposed men. Int. J. Hyg. Environ. Health. 2003;206:517–529. doi: 10.1078/1438-4639-00249. [DOI] [PubMed] [Google Scholar]
- Brouillet EP, Shinobu L, McGarvey U, Hochberg F, Beal MF. Manganese injection into the rat striatum produces excitotoxic lesions by impairing energy metabolism. Exp. Neurol. 1993;120:89–94. doi: 10.1006/exnr.1993.1042. [DOI] [PubMed] [Google Scholar]
- Brunberg JA, Kanal E, Hirsch W, Van Thiel DH. Chronic acquired hepatic failure: MR imaging of the brain at 1.5 T. Am. J. Neuroradiol. 1991;12:909–914. [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Hensley K, Cole P, Subramaniam R, Aksenov M, Aksenova M, Bummer PM, Haley BE, Carney JM. Oxidatively induced structural alteration of glutamine synthetase assessed by analysis of spin label incorporation kinetics: Relevance to Alzheimer's disease. J. Neurochem. 1997;68:2451–2457. doi: 10.1046/j.1471-4159.1997.68062451.x. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog. Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Dorman DC, Struve MF, Marshall MW, Parkinson CU, James RA, Wong BA. Tissue manganese concentrations in young male rhesus monkeys following subchronic manganese sulfate inhalation. Toxicol. Sci. 2006;92:201–210. doi: 10.1093/toxsci/kfj206. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Dorman DC, Lash LH, Aschner M. Manganese inhalation by rhesus monkeys is associated with brain regional changes in biomarkers of neurotoxicity. Toxicol. Sci. 2007;97:459–466. doi: 10.1093/toxsci/kfm044. [DOI] [PubMed] [Google Scholar]
- Eriksson H, Gillberg PG, Aquilonius SM, Hedstrom KG, Heilbronn E. Receptor alterations in manganese intoxicated monkeys. Arch. Toxicol. 1992;66:359–364. doi: 10.1007/BF01973632. [DOI] [PubMed] [Google Scholar]
- Gianutsos G, Murray MT. Alterations in brain dopamine and GABA following inorganic or organic manganese administration. Neurotoxicology. 1982;3:75–81. [PubMed] [Google Scholar]
- Guilarte TR. Regional changes in the concentrations of glutamate, glycine, taurine, and GABA in the vitamin B-6 deficient developing rat brain: Association with neonatal seizures. Neurochem. Res. 1989;14:889–897. doi: 10.1007/BF00964820. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, Burton NC, McGlothan JL, Verina T, Zhou Y, Alexander M, Pham L, Griswold M, Wong DF, Syversen T, et al. Impairment of nigrostriatal dopamine neurotransmission by manganese is mediated by pre-synaptic mechanism(s): Implications to manganese-induced parkinsonism. J. Neurochem. 2008a;107:1236–1247. doi: 10.1111/j.1471-4159.2008.05695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR, Burton NC, Verina T, Prabhu VV, Becker KG, Syversen T, Schneider JS. Increased APLP1 expression and neurodegeneration in the frontal cortex of manganese-exposed non-human primates. J. Neurochem. 2008b;105:1948–1959. doi: 10.1111/j.1471-4159.2008.05295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR, Chen MK, McGlothan JL, Verina T, Wong DF, Zhou Y, Alexander M, Rohde CA, Syversen T, Decamp E, et al. Nigrostriatal dopamine system dysfunction and subtle motor deficits in manganese-exposed non-human primates. Exp. Neurol. 2006a;202:381–390. doi: 10.1016/j.expneurol.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, McGlothan JL, Degaonkar M, Chen MK, Barker PB, Syversen T, Schneider JS. Evidence for cortical dysfunction and widespread manganese accumulation in the nonhuman primate brain following chronic manganese exposure: A 1H-MRS and MRI study. Toxicol. Sci. 2006b;94:351–358. doi: 10.1093/toxsci/kfl106. [DOI] [PubMed] [Google Scholar]
- Gulson B, Mizon K, Taylor A, Korsch M, Stauber J, Davis JM, Louie H, Wu M, Swan H. Changes in manganese and lead in the environment and young children associated with the introduction of methylcyclopentadienyl manganese tricarbonyl in gasoline—Preliminary results. Environ. Res. 2006;100:100–114. doi: 10.1016/j.envres.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Gwiazda RH, Lee D, Sheridan J, Smith DR. Low cumulative manganese exposure affects striatal GABA but not dopamine. Neurotoxicology. 2002;23:69–76. doi: 10.1016/s0161-813x(02)00002-5. [DOI] [PubMed] [Google Scholar]
- Hardy IJ, Gillanders L, Hardy G. Is manganese an essential supplement for parenteral nutrition? Curr. Opin. Clin. Nutr. Metab. Care. 2008;11:289–296. doi: 10.1097/MCO.0b013e3282f9e889. [DOI] [PubMed] [Google Scholar]
- Hauser RA, Zesiewicz TA, Martinez C, Rosemurgy AS, Olanow CW. Blood manganese correlates with brain magnetic resonance imaging changes in patients with liver disease. Can. J. Neurol. Sci. 1996;23:95–98. doi: 10.1017/s0317167100038786. [DOI] [PubMed] [Google Scholar]
- Iinuma Y, Kubota M, Uchiyama M, Yagi M, Kanada S, Yamazaki S, Murata H, Okamoto K, Suzuki M, Nitta K. Whole-blood manganese levels and brain manganese accumulation in children receiving long-term home parenteral nutrition. Pediatr. Surg. Int. 2003;19:268–272. doi: 10.1007/s00383-002-0929-6. [DOI] [PubMed] [Google Scholar]
- Jett DA, Guilarte TR. Developmental lead exposure alters N-methyl-D-aspartate and muscarinic cholinergic receptors in the rat hippocampus: An autoradiographic study. Neurotoxicology. 1995;16:7–18. [PubMed] [Google Scholar]
- Josephs KA, Ahlskog JE, Klos KJ, Kumar N, Fealey RD, Trenerry MR, Cowl CT. Neurologic manifestations in welders with pallidal MRI T1 hyperintensity. Neurology. 2005;64:2033–2039. doi: 10.1212/01.WNL.0000167411.93483.A1. [DOI] [PubMed] [Google Scholar]
- Lai JC, Leung TK, Lim L. Brain regional distribution of glutamic acid decarboxylase, choline acetyltransferase, and acetylcholinesterase in the rat: effects of chronic manganese chloride administration after two years. J. Neurochem. 1981;36:1443–1448. doi: 10.1111/j.1471-4159.1981.tb00585.x. [DOI] [PubMed] [Google Scholar]
- Lipe GW, Duhart H, Newport GD, Slikker W, Jr, Ali SF. Effect of manganese on the concentration of amino acids in different regions of the rat brain. J. Environ. Sci. Health B. 1999;34:119–132. doi: 10.1080/03601239909373187. [DOI] [PubMed] [Google Scholar]
- Lucchini R, Apostoli P, Perrone C, Placidi D, Albini E, Migliorati P, Mergler D, Sassine MP, Palmi S, Alessio L. Long-term exposure to “low levels” of manganese oxides and neurofunctional changes in ferroalloy workers. Neurotoxicology. 1999;20:287–297. [PubMed] [Google Scholar]
- Lucchini R, Selis L, Folli D, Apostoli P, Mutti A, Vanoni O, Iregren A, Alessio L. Neurobehavioral effects of manganese in workers from a ferroalloy plant after temporary cessation of exposure. Scand. J. Work Environ. Health. 1995;21:143–149. doi: 10.5271/sjweh.1369. [DOI] [PubMed] [Google Scholar]
- Mergler D, Baldwin M. Early manifestations of manganese neurotoxicity in humans: An update. Environ. Res. 1997;73:92–100. doi: 10.1006/enrs.1997.3710. [DOI] [PubMed] [Google Scholar]
- Mergler D, Huel G, Bowler R, Iregren A, Belanger S, Baldwin M, Tardif R, Smargiassi A, Martin L. Nervous system dysfunction among workers with long-term exposure to manganese. Environ. Res. 1994;64:151–180. doi: 10.1006/enrs.1994.1013. [DOI] [PubMed] [Google Scholar]
- Montes S, Riojas-Rodriguez H, Sabido-Pedraza E, Rios C. Biomarkers of manganese exposure in a population living close to a mine and mineral processing plant in Mexico. Environ. Res. 2008;106:89–95. doi: 10.1016/j.envres.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, Rao KV, Jayakumar AR. Mechanisms of ammonia-induced astrocyte swelling. Metab. Brain Dis. 2005;20:303–318. doi: 10.1007/s11011-005-7911-7. [DOI] [PubMed] [Google Scholar]
- Pan HS, Frey KA, Young AB, Penney JB., Jr Changes in [3H]muscimol binding in substantia nigra, entopeduncular nucleus, globus pallidus, and thalamus after striatal lesions as demonstrated by quantitative receptor autoradiography. J. Neurosci. 1983;3:1189–1198. doi: 10.1523/JNEUROSCI.03-06-01189.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons B, Rainbow TC. Quantitative autoradiography of sodium-dependent [3H]D-aspartate binding sites in rat brain. Neurosci. Lett. 1983;36:9–12. doi: 10.1016/0304-3940(83)90477-9. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Huang X-F, Toga AW. In: The Rhesus Monkey Brain in Stereotaxic Coordinates. Paxinos G, Huang XF, Toga AW, editors. San Diego, CA: Academic Press; 2000. pp. 14–114. [Google Scholar]
- Prohaska JR. Functions of trace elements in brain metabolism. Physiol. Rev. 1987;67:858–901. doi: 10.1152/physrev.1987.67.3.858. [DOI] [PubMed] [Google Scholar]
- Rama Rao KV, Reddy PV, Hazell AS, Norenberg MD. Manganese induces cell swelling in cultured astrocytes. Neurotoxicology. 2007;28:807–812. doi: 10.1016/j.neuro.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Reaney SH, Bench G, Smith DR. Brain accumulation and toxicity of Mn(II) and Mn(III) exposures. Toxicol. Sci. 2006;93:114–124. doi: 10.1093/toxsci/kfl028. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Agudelo Y, Riojas-Rodriguez H, Rios C, Rosas I, Sabido PE, Miranda J, Siebe C, Texcalac JL, Santos-Burgoa C. Motor alterations associated with exposure to manganese in the environment in Mexico. Sci. Total Environ. 2006;368:542–556. doi: 10.1016/j.scitotenv.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Rose C. Effect of ammonia on astrocytic glutamate uptake/release mechanisms. J. Neurochem. 2006;97(Suppl. 1):11–15. doi: 10.1111/j.1471-4159.2006.03796.x. [DOI] [PubMed] [Google Scholar]
- Rose C, Kresse W, Kettenmann H. Acute insult of ammonia leads to calcium-dependent glutamate release from cultured astrocytes, an effect of pH. J. Biol. Chem. 2005;280:20937–20944. doi: 10.1074/jbc.M412448200. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Decamp E, Clark K, Bouquio C, Syversen T, Guilarte TR. Effects of chronic manganese exposure on working memory in non-human primates. Brain Res. 2009;1258:86–95. doi: 10.1016/j.brainres.2008.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Decamp E, Koser AJ, Fritz S, Gonczi H, Syversen T, Guilarte TR. Effects of chronic manganese exposure on cognitive and motor functioning in non-human primates. Brain Res. 2006;1118:222–231. doi: 10.1016/j.brainres.2006.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schor NF. Inactivation of mammalian brain glutamine synthetase by oxygen radicals. Brain Res. 1988;456:17–21. doi: 10.1016/0006-8993(88)90341-1. [DOI] [PubMed] [Google Scholar]
- Seth PK, Hong JS, Kilts CD, Bondy SC. Alteration of cerebral neurotransmitter receptor function by exposure of rats to manganese. Toxicol. Lett. 1981;9:247–254. doi: 10.1016/0378-4274(81)90157-0. [DOI] [PubMed] [Google Scholar]
- Srivastava AK, Gupta BN, Mathur N, Murty RC, Garg N, Chandra SV. An investigation of metal concentrations in blood of industrial workers. Vet. Hum. Toxicol. 1991;33:280–282. [PubMed] [Google Scholar]
- Struve MF, McManus BE, Wong BA, Dorman DC. Basal ganglia neurotransmitter concentrations in rhesus monkeys following subchronic manganese sulfate inhalation. Am. J. Ind. Med. 2007;50:772–778. doi: 10.1002/ajim.20489. [DOI] [PubMed] [Google Scholar]
- Widmer R, Kaiser B, Engels M, Jung T, Grune T. Hyperammonemia causes protein oxidation and enhanced proteasomal activity in response to mitochondria-mediated oxidative stress in rat primary astrocytes. Arch. Biochem. Biophys. 2007;464:1–11. doi: 10.1016/j.abb.2007.03.027. [DOI] [PubMed] [Google Scholar]
- Zwingmann C, Leibfritz D, Hazell AS. Nmr spectroscopic analysis of regional brain energy metabolism in manganese neurotoxicity. Glia. 2007;55:1610–1617. doi: 10.1002/glia.20575. [DOI] [PubMed] [Google Scholar]