Abstract
Chronic (+)-methamphetamine (METH) use during pregnancy increases the health risk for both mother and fetus. To provide insights into these risks, the relationship between changes in METH disposition and METH-induced pharmacological effects were studied in Sprague-Dawley rat dams and litters. Timed-pregnant rats (n = 5–6) were given saline or METH (5.6–17.8 mg/kg/day) by continuous sc infusion from gestational day (GD) 7 (before organogenesis) until GD21 (0–2 days before delivery). By GD11, all rats in the 17.8-mg/kg/day group died or were sacrificed for humane reasons. There were significant (p < 0.05) dose- and gestational time-dependent decreases in maternal body weight in the 10- to 13.2-mg/kg/day groups, which slowly recovered to near normal by GD21. Continued METH dosing in the surviving groups did not affect the mean pups/litter weight at the end of the experiment on GD21. While maternal and fetal METH and (+)-amphetamine serum concentrations were similar on GD21, brain concentrations were significantly greater in the dams (p < 0.05). Importantly, brain-to-serum ratios in the dams were 9:1 and 3:1 in the pups. METH systemic clearance (ClS) in dams significantly (p < 0.05) decreased from 52 ± 14 ml/min/kg on GD10 to 28 ± 6 ml/min/kg on GD21 in all dose groups, indicating late-gestational stage reductions in METH ClS. Overall, these findings suggest that there were two periods of increased susceptibility for dams and fetuses during chronic METH treatment. First was the period after the start of METH dosing in which neuroadaptation and tolerance to METH occurs in the adult. The second was at the end of pregnancy when METH clearance was significantly reduced.
Keywords: methamphetamine, pregnancy, rat, drug abuse, pharmacokinetics
Unlike many drugs of abuse, where the prevalence of use is greater in men than in women, there is a near equal use of (+)-methamphetamine (METH) among men and women in the United States (Cohen et al., 2007). The combination of a high prevalence of use in women and powerful addictive properties increases the probability of METH abuse during pregnancy. Indeed, the Infant Development, Environment, and Lifestyle study reports an estimated 5.2% of women have used METH at least once during pregnancy in geographical areas with high METH use (Arria et al., 2006). The study sample consisted of women who had delivered a baby within 48 h and included nonusers and users of alcohol, tobacco, METH, and other drugs.
Reports from human and animal studies show that prenatal exposure to METH can increase fetal distress (tachy/bradycardia, Stek et al., 1995; meconium staining, Smith et al., 2008), intrauterine growth retardation (Acuff-Smith et al., 1996; Smith et al., 2006), premature delivery (< 37 weeks gestation in humans, Smith et al., 2006; < 22 days in rats, Martin et al., 1976), and postnatal behavioral deficits (Slamberova et al., 2006; Smith et al., 2008). Human data on METH exposures are difficult to interpret due to confounding factors like not knowing the METH dose, small sample sizes, poor maternal nutrition, infrequent prenatal visits, and concomitant drug use. Thus, animal models provide an important tool for determining the impact of METH use on mother and fetus, especially, when studying the higher, unsafe doses used by many abusers.
The rat is an appropriate model for studying human pregnancy because it undergoes similar hemodynamic (Conrad, 1987; Dowell and Kauer, 1997) and plasma volume (Mattison et al., 1991) changes during pregnancy. Furthermore, important placental-blood barrier transporters like the norepinephrine transporter (NET), serotonin transporter (SERT), and organic cation transporter 3 (OCT3) are present in both species (Jayanthi et al., 2002; Ramamoorthy et al., 1995). Rat placentas are analogous to human placentas in several ways, including their type (hemochorial) and shape (discoid) (DeSesso, 1997). While the rat gestation period is 21–23 days and the human is approximately 40 weeks, both pregnancies consist of three trimesters with similar gestational milestones (DeSesso, 1997). Although the timing of neurological development differs between rat and human, both species have rather immature brains until postnatally (Watson et al., 2006).
Pregnancy-related physiological changes are known to alter the disposition of drugs via changes in blood volume, cardiac output, tissue blood flow, and protein binding (Hodge and Tracy, 2007). These changes may have a significant impact on METH disposition and the degree of maternal and fetal exposure. In turn, chronic METH use could have potential impact on the normal homeostasis of developmental physiological processes.
In general, the timing of drug exposure determines which embryonic tissues are affected (DeSesso, 1997). In particular, teratogenic insults more frequently occur during early organogenesis, which is during the first trimester of human pregnancy and between gestational day (GD) 7 to GD12 in the rat (Schmidt and Johnson, 1997). Acuff-Smith et al. (1992) report that a racemic mixture of (±)-METH (sc dose of 50 mg/kg twice daily) administered from GD7 to GD12 produces teratogenic outcomes (anophthalmia and microphthalmia) in Sprague-Dawley fetuses. However, it is highly unlikely that humans would self-administer such a high METH dose; and racemic (±)-METH is not the commonly abused form of the drug (Lee et al., 2007).
Other studies suggest that rat prenatal METH exposure leads to postural motor and cognitive deficits in the offspring (Acuff-Smith et al., 1996; Slamberova et al., 2006). Acuff-Smith et al. (1996) also report significant time-dependent decreases in serotonin concentrations in the nucleus accumbens in rat offspring exposed prenatally to 20 mg/kg METH (sc, twice daily) from GD13 to GD18. This reduction in serotonin concentration is not found in the offspring of rats exposed from GD7 to GD12.
Few studies have examined the pharmacological and toxicological effects of chronic METH exposure in the maternal-fetal rat unit. Thus, for these studies, we hypothesized that METH dose- and gestational time–dependent pharmacological effects in pregnant rats and their litters were related to changes in METH disposition. Pregnant rats received sc METH infusions from GD7 to GD21 to maintain steady-state exposure. This also served to limit the stress to the animals relative to the use of daily iv or sc injections. Maternal METH and (+)-amphetamine (AMP; an active metabolite) pharmacokinetic parameters, behavioral effects, and general health effects were measured at multiple time-points during pregnancy. On GD21 (just prior to delivery), dams and their litters were sacrificed and METH and AMP pharmacokinetics in the sera and brains were determined.
MATERIALS AND METHODS
Drugs and reagents.
METH hydrochloride, AMP sulfate, (±)-4-hydroxy-methamphetamine hydrochloride, and (±)-4-hydroxy-amphetamine hydrobromide were obtained from the National Institute of Drug Abuse (Bethesda, MD). Each drug was prepared in sterile saline. All drug doses and concentrations were expressed as the free base form. (±)-Methamphetamine-d5 and (±)-amphetamine-d11 were purchased from Sigma Aldrich (St Louis, MO) for use as analytical internal standards. [3H]-METH [(+)-[2′, 6′-3H(n)] methamphetamine; 28.3 Ci/mmol; 0.521 mCi/ml] was synthesized with the radiolabel at the 2 and 6 positions of the aromatic ring by the Research Triangle Institute (Research Triangle Park, NC) for the National Institute on Drug Abuse. All other reagents used in these studies were purchased from Fisher Scientific Co. (Fairlawn, NJ), unless specified otherwise.
Animals.
Female Sprague-Dawley rats were impregnated at Charles River Laboratories (Wilmington, MA) and arrived on GD4. Pregnant rat weights ranged from 180 to 270 g on arrival. To prevent undue temperature stress, animals were not shipped during hot summer months. Animals were housed individually and had free access to food and water throughout the experiment. The dams were maintained in an animal care facility with a 12-h light/dark cycle (6:00 A.M.–6:00 P.M.) and a mean ambient temperature of 22°C. Each pregnant rat was handled and acclimatized to its behavioral chamber (2.5 h/day) from arrival until the start of the experiment. The animals were weighed daily between 8:30 and 9:00 A.M. All animal protocols were in accordance with the Guide for the Care and Use of Laboratory Animals, as adopted and promulgated by the National Institutes of Health, and were approved by the Institutional Animal Care and Use Committee of the University of Arkansas for Medical Sciences (Little Rock, AR) prior to beginning the experiments.
Surgical procedures and subcutaneous infusions of METH and AMP.
For the METH dose-response studies and AMP maternal and fetal partitioning studies, dams (n = 5–6 per dose; random assignment) were implanted on GD7 with 14-day sc osmotic minipumps (model 2ML2; ALZET, Cupertino, CA). All pumps were primed in sterile saline for 4 h prior to surgery. Prior to pump implantation, a control sample of blood (200 μl) was collected via tail vein. The activated minipumps were implanted sc into the mid-thoracic dorsal region under isoflurane anesthesia. The minipumps administered saline or moderate to lethal METH doses of 5.6, 10, 13.2, or 17.8 mg/kg/day. Preliminary dose-range–finding experiments showed that a dose of 3.2 mg/kg/day METH had no observable weight or behavioral effects and, thus, was not used in the final studies.
A separate study with chronic sc infusions of 10 mg/kg/day AMP was conducted to further elucidate maternal and fetal partitioning of AMP, resulting from metabolism of METH. METH and AMP doses were calculated based on the weight of the dams on GD6. As the animals gained weight during their pregnancy, the METH infusion rate (dependent on body weight) decreased. Since the minipumps delivered a continuous infusion of METH or AMP from GD7 to GD21, infusion rates were adjusted daily to appropriately calculate the systemic clearance (ClS) values.
General assessments of animals’ well-being.
Once steady-state concentrations of METH were achieved on GD8, the animals underwent daily physical examinations until GD21. General health end points included body weight (due to METH's anorectic properties), presence of chromodacryorrhea (“bloody tears”; a physiological stress indicator due to METH-induced hypertension and capillary leakage around the eyes), and skin lesions (due to intense METH-induced stereotypic licking and gnawing at higher METH doses).
Behavioral activity.
METH's stimulant effects on locomotor activity were monitored in open-top polyethylene monitoring chambers (60 × 45 × 40 cm; United States Plastic Corporation, Lima, OH). Gray clay bedding (Tidy Cats Scoop, 20 lb; Pet Smart, Little Rock, AR) was added to the bottom of each monitoring chamber to provide an absorbent material for animal waste and a nonreflective, contrasting background for the behavioral video imaging system (see below). The animals were placed in the chambers for 2.5 h/day, from approximately 9:00 to 11:30 A.M. The sessions took place just prior to tail vein blood sampling on GD7 (baseline activity; predrug treatment), GD8, GD10, GD13, GD17, and GD20.
Rat locomotor activity was recorded and analyzed with a video imaging system (Ethovision; Noldus Information Technology, Sterling, VA), described in previous studies (Hardin et al., 1998; Rivière et al., 1999). The behavioral parameters measured included total distance traveled (cm) and the total number of times each animal stood on its hind legs (rearing events) during each session in consecutive 4-min time intervals. The first 30 min of the animals’ time in the chamber were not included in the analysis to allow time for the animals to acclimate to the behavioral chamber.
Stereotyped behavior was measured using a rating scale previously developed and validated by our laboratory (Gentry et al., 2004). Using this method, the behavior of each animal was graded for the first 30 s of every 10-min intervals by three trained independent raters who were blinded to the treatment groups. Two of the raters evaluated the behavior for all behavioral study doses (saline, 5.6, 10, and 13.2 mg/kg/day METH); one rater evaluated only for 10 mg/kg/day METH. These raters viewed the same videotapes that were used for the Noldus computer-assisted analysis described in the previous section. As before, the first 30 min of behavior were excluded in the analysis.
METH and AMP concentrations in serum and brain.
Tail vein blood samples were collected (200 μl) on predetermined days of GD8, GD10, GD13, GD17, and GD20 under isoflurane anesthesia. Hematocrits were determined after each blood draw as a measure of health status and an indicator of potential physiological changes associated with pregnancy. Blood samples were allowed to clot at room temperature. The coagulated blood was then centrifuged, and the serum was collected. Serum samples were frozen at − 80°C until analysis.
On GD21, dams were anesthetized with isoflurane, decapitated, and the trunk blood was collected. Brains were removed, rinsed in saline, weighed, and quickly frozen in liquid nitrogen. A laparotomy was immediately performed and the uterine horns were exposed. The pups were removed (starting at the distal left end of the uterus and progressing to the cervix and then to the right distal end) and placed on a heating blanket in the same order. The uterine horns were examined for any resorptions. The number and status (alive or dead) of the pups were determined, and the pups were weighed and quickly examined for any gross physical malformations. After the examination, the pups were decapitated and their brains were removed. The brains from each litter were pooled and weighed. Trunk blood was collected and pooled for each litter. The blood was allowed to clot at room temperature, and the serum was collected after centrifugation. Brain and serum samples were stored at − 80°C until analysis.
Drug analysis and ClS.
Brains were thawed and homogenized in four volumes (wt/vol) of purified water with a tissue homogenizer (7 × 10 mm disposable generator; Fisher Scientific). METH and AMP were extracted from serum and brain samples using a liquid-liquid extraction procedure, as described by Hendrickson et al. (2006). Briefly, a 100-μl aliquot of each homogenized brain and a 25- or 50-μl aliquot of each serum sample were used for extractions. Serum aliquots were brought to 100 μl with drug-free normal rat serum (Pel-Freez Biologicals, Rogers, AR). Calibration and quality control serum samples, also using drug-free normal rat serum, were prepared for quantitation of METH and AMP concentrations in sera and brains. Hendrickson et al. (2006) validated use of serum standards in brain tissue. Calibration and quality control serum samples, also using drug-free normal rat serum, were prepared for quantitation of METH and AMP concentrations. A 10-μl mixture containing (±)-methamphetamine-d5 and (±)-amphetamine-d11 was added as an internal standard to all samples and standards. Precipitation of proteins in the standards and samples was performed using 100 μl of ice-cold 20% (wt/vol) trichloroacetic acid. Samples were vortexed, gently agitated for 15 min, and centrifuged. The supernatant was filtered and the filtrate was injected (20 μl) into a liquid chromatography column (100 × 2.1 mm; Keystone/Thermo-Electron, Belafonte, PA). METH and AMP concentrations were determined using liquid chromatography with tandem mass spectrometry (LC-MS/MS) (Hendrickson et al., 2006).
ClS corrected for the bioavailability or fraction of the drug absorbed (ClS/F) was calculated using the equation, ClS/F = infusion rate/CSS, where CSS is the steady-state drug concentration. Infusion rate was calculated as the dose per day normalized for rat weight on each GD. For this study, F was assumed to be 1.0 in pregnant rats. This assumption was based on a previous study by Gentry et al. (2004), in which F was determined to be 1.0 in male rats dosed with 3 mg/kg sc bolus METH.
METH serum protein binding.
A 96-well high-throughput equilibrium dialysis apparatus (model HTD96b; HT Dialysis LLC, Gales Ferry, CT) was used to determine the serum-free fraction of METH in rats receiving 5.6 or 10 mg/kg/day during pregnancy. There was insufficient serum to run duplicate samples for each rat from GD8 to GD20; thus, two animals per dose group were pooled. For GD21, serum samples from each of the dams and corresponding litters were analyzed in triplicate. The dialysis membrane (6- to 8-kDa cutoff) was conditioned in PBS (pH 7.5–7.6; 1.369M NaCl, 26.8mM KCl, 8.817M KH2PO4, and 64mM Na2HPO4) containing 20% (vol/vol) ethanol, thoroughly rinsed in distilled water, and finally soaked in Sorenson's buffer (0.13M Na2HPO4, 0.13M KH2PO4, pH 7.35) for 15 min. The equilibrium dialysis plate was then assembled with the dialysis membrane separating the two sides of the cells. Subsequently, the cells were loaded with dam or litter serum (30–55 μl) on one side and an equal volume of Sorenson's buffer on the other. Equilibrium was achieved after an overnight incubation at 37°C with gentle agitation. Serum and buffer were then removed from each side of the chamber, and METH concentrations were determined by LC-MS/MS following extraction as described above. The accuracy and reproducibility for determining METH concentrations in Sorenson's buffer were validated using calibration and quality control samples prepared in normal rat serum.
A similar method was used to ascertain the percentage of METH protein binding in normal rat serum versus pooled human serum (IPLA-SER4; Innovative Research, Novi, MI) and human albumin (A-1887; Sigma Aldrich). The human albumin was dissolved in Sorenson's buffer to a physiological concentration of 35 g/l. Drug-free and nonpregnant normal rat serum (gender unspecified by the vendor) or human pooled serum (gender unspecified by the vendor) was spiked with 50, 100, 150, or 200 ng/ml [3H]-METH. The METH concentrations were chosen to be within the range of the METH serum concentrations determined in the pregnant rats infused with 5.6, 10, or 13.2 mg/kg/day (range = 53 ± 20 ng/ml to 231 ± 93 ng/ml). Fifty microliter of each spiked serum or albumin sample (in triplicate) was added to one side of the dialysis cells and an equal volume of Sorenson's buffer was added to the other side. Like before, a 6- to 8-kDa cutoff membrane separated the two sides of the cells. Equilibrium was reached following an overnight incubation at 37°C on a shaking platform. The serum (or albumin) and buffer were removed from each side of the equilibrium dialysis cells and diluted with corresponding rat or human serum or albumin solution to a total volume of 250 μl. Subsequently, a 10-μl aliquot from each of the diluted solutions was added to 4 ml of ScintiVerse scintillation fluid, and the unbound (buffer side) and total (serum or albumin side) [3H]-METH concentrations (in dpm) were quantified by liquid scintillation spectrometry. Once unbound and total [3H]-METH concentrations were corrected for the dilution factor, the percentage of bound METH was calculated by (total concentration − free concentration)/(total concentration) × 100%.
Statistical analysis.
All values are expressed as the mean ± SD. A Grubb’s test was conducted on a few of the experimental data sets to determine the probability of an outlier value in the group. A two-way repeated-measures ANOVA with one within factor (GD) and one between factor (dose) was used to measure any significant differences in the weight, serum concentrations, and behavior of the pregnant rats throughout gestation. The same test detected any significant differences in tissue concentrations of dams and their offspring on GD21. For the litter weight and size data, a one-way ANOVA was used with dose as the main factor. If statistical differences were found, analysis was followed by Bonferroni correction (α = 0.05). Furthermore, a two-way repeated-measures ANOVA with one within factor (GD) and one between factor (dose) was used in the statistical analysis of ClS/F data of dams from GD8 to GD21. To identify if any differences existed between dam and litter brain and serum AMP concentrations on GD21 in the AMP partitioning study, a two-way ANOVA was used. Finally, a two-way ANOVA was also used to detect significance among the percentage of METH bound in dam and fetal serum, as well as rat serum, human serum, and human albumin. For these tests, a Student-Newman-Keuls post hoc test was conducted. A value of p < 0.05 was considered to be significant for all analyses.
RESULTS
Experimental Strategy and Observed Health Effects in Dams
Control (saline treated) rats displayed no overt signs of stress, as measured by their appearance of normalcy and absence of chromodacryorrhea during physical observation. In the 10- and 13.2-mg/kg/day METH dose groups, mild-to-moderate chromodacryorrhea was observed on GD8–GD10. Rats treated with 17.8 mg/kg/day METH exhibited severe chromodacryorrhea from GD8 until their time of death (or sacrifice for humane reasons) on GD9–GD11. The presence of chromodacryorrhea was METH dose dependent with 62, 83, and 100% of the rats exhibiting chromodacryorrhea in the 10-, 13.2-, and 17.8-mg/kg/day groups, respectively.
Another dose-dependent effect of the animals’ well-being was the appearance and severity of skin lesions. These sores were the result of repetitive stereotypic licking and gnawing behavior and were primarily confined to the digits of the rats; however, lesions were also identified on the legs, abdomen, and tail. At the two highest METH doses (13.2 and 17.8 mg/kg/day), 83% of the animals developed skin lesions from excessive licking and gnawing, while 50% of the 10-mg/kg/day METH group developed lesions.
The hematocrits progressively declined as gestation advanced, as expected in pregnancy. Average daily hematocrits ranged from 0.40 to 0.46 for saline-infused rats, 0.37 to 0.44 for 5.6 mg/kg/day METH-infused rats, 0.37 to 0.47 for 10 mg/kg/day METH-infused rats, and 0.36 to 0.46 for 13.2 mg/kg/day METH-infused rats, with no significant differences between dose groups. However, one animal in each of the three higher dose groups appeared to have profound reductions in hematocrit. We treated these as outliers in the group and did not include them in the average hematocrit values; however, we suspected that the more profound anemia in these animals was METH induced. These individual rats in the 10- and 13.2-mg/kg/day groups had hematocrit values of 17 and 13%, respectively, on GD10. The rat in the 17.8-mg/kg/day group had a hematocrit of 28% on GD11. These three rats all showed extreme METH-induced health effects including chromodacryorrhea, weight loss, pale conjunctiva, and lethargy and, thus, were sacrificed for humane reasons on GD10 or GD11. It is unlikely that the low hematocrits of the animals resulted from METH-induced skin lesions since the bleeding was very mild.
The rats were also monitored for signs of infection since chronic METH use is associated with immunosuppression (Friedman et al., 2006). Two rats (in the 10- and 13.2-mg/kg/day groups) developed an abscess at the pump site, which appeared to be confined to the sc minipump implantation site. We assumed that these infections were in part due to local METH-induced vasoconstriction and immunosuppression. Both rats were included in the final data analyses since the infection did not appear to affect the METH and AMP serum or brain concentrations, as determined by a Grubb's outlier test (Z > 1.71).
Most dose groups consisted of n = 6 rats initially, with the exception of 10-mg/kg/day dose group, which originally consisted of n = 8 rats. However, only four animals were included in the final 10-mg/kg/day METH group. One animal was sacrificed on GD11 due to severe anemia, two animals were determined to be outliers (Z > 2.02) due to their high baseline locomotor activity, and one animal chewed her skin at the pump site, exposing the osmotic pump.
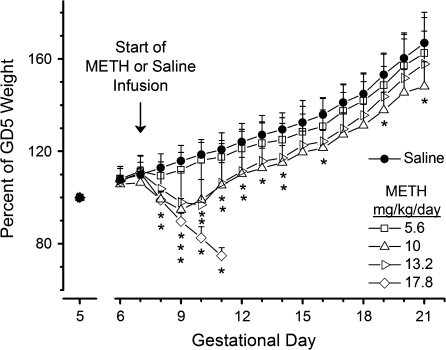
There were no observable adverse health changes in the 5.6-mg/kg/day METH group and no differences in weight gain compared to saline-treated rats (Fig. 1). Rats treated with 10 and 13.2 mg/kg/day METH lost weight soon after pump implantation on GD7, but by GD11, both groups of animals began regaining weight. Among the dams that survived the study, the rats treated with 13.2 mg/kg/day had significantly lower average weights compared to control rats until GD14. Pregnant rats treated with 10 mg/kg/day METH showed the greatest weight difference from control animals throughout their pregnancy. At the highest METH dose (17.8 mg/kg/day), there was significant weight loss between GD7 and GD11 (p < 0.05) with losses of up to 20–25% of their GD6 body weight (Fig. 1). None of these animals survived the study.
FIG. 1.
Time course of maternal weight change expressed as a percentage of each rat's GD5 weight (n = 3–6) from GD5 to GD21. A normalized weight of 100% on GD5 is shown for each dose. Weights were corrected for the weight of the osmotic minipump. The arrow indicates the day of saline or METH treatment. *p < 0.05 compared with saline control's weight on each GD. Stacked * indicates sequential differences from the lowest (10 mg/kg/day) to the highest (17.8 mg/kg/day) dose groups directly above the *. All data points are mean ± SD.
Litter Assessments
On GD21, no apparent METH-induced effects were observed in the litters, that is, the number of fetal resorptions, litter size, and physical appearance did not differ from the saline group. Average litter weights for the METH-infused rats in the 5.6- and 13.2-mg/kg/day groups were significantly greater than the control group (Table 1). Due to the small magnitude of the value, we did not consider this a substantive difference. All pups were alive when they were separated from the uterus during the maternal necropsy on GD21.
TABLE 1.
Litter Profiles on GD21 from Dams Receiving Saline or METH (5.6, 10, and 13.2 mg/kg/day).
| Dose (mg/kg/day) | Litter size | Total litter weight (g) | Pup weight (g) | Resorptions | Observable physical malformations |
| Saline | 13 ± 2 | 47 ± 7 | 3.7 ± 0.3 | 1 | 0 |
| 5.6 | 12 ± 3 | 47 ± 11 | 3.9 ± 0.3* | 0 | 0 |
| 10 | 12 ± 1 | 46 ± 4 | 3.8 ± 0.3 | 1 | 0 |
| 13.2 | 14 ± 1 | 54 ± 4 | 3.9 ± 0.3* | 0 | 0 |
Note. All values are mean ± SD.
“*” Indicates p < 0.05 when compared to saline.
Behavioral Activity Experiments
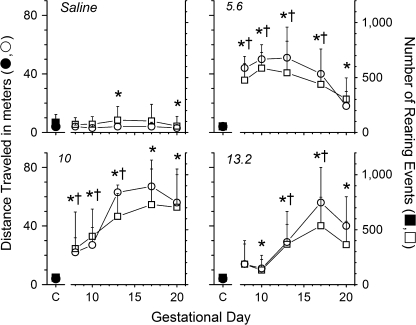
A dose-dependent behavioral effect was observed for distance traveled and rearing events (Fig. 2). Rats in the 5.6- and 10-mg/kg/day groups had a pronounced increase in total distance traveled and rearing events during each session compared to their behavior prior to drug administration; however, there was a smaller initial increase in both behavioral parameters at 10 mg/kg/day. Animals treated with 13.2 mg/kg/day had no significant increase in distance traveled on GD8 or in rearing events on GD8 or GD10. Afterward, there was a steady rise in locomotor activity. In contrast, the 17.8-mg/kg/day METH group showed no increase in locomotor activity on GD8 and GD10 compared to their baseline behavior (data not shown). This lack of METH-induced locomotor activity was apparently due to the profound stereotypic effects and immobility resulting from the very high METH dose.
FIG. 2.
Dose-response summary of (+)-METH-induced distance traveled and rearing events during pregnancy. Baseline behavioral activity prior to chronic infusion of saline or METH (5.6, 10, or 13.2 mg/kg/day) is symbolized as closed symbols for distance traveled and rearing events. After treatment, average distance traveled and rearing events are symbolized as open symbols from GD8 to GD20. Each data point represents the average cumulative distance traveled and number of times the animals reared for each 4-min time interval over 2 h (n = 4–6 per dose group). “*” Indicates a significant increase (p < 0.05) in distance traveled compared to baseline activity for each dose; “†” indicates a significantly greater number of rearing events compared to the baseline activity on GD7.
Particular stereotypic behaviors stood out, including repetitive licking, grooming, and gnawing. These behaviors intensified as the METH dose increased, sometimes leading to cannibalism of the animals’ digits. The GDs that the specific stereotypic behaviors were present correlated to the initial lack of locomotor activity found for animals infused with 10 or 13.2 mg/kg/day METH (Fig. 2). As these behaviors waned, hypermotility and exploratory behaviors increased. Despite this correlation, the stereotypy ratings proved extremely variable (data not shown), especially at 10 mg/kg/day. Thus, statistical differences were not found. Stereotypy ratings were not determined for pregnant rats treated with 17.8 mg/kg/day due to the early-stage lethality of the drug.
METH and AMP Pharmacokinetics
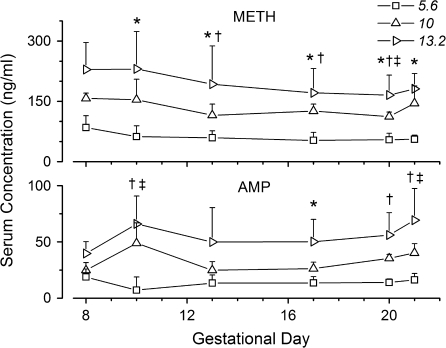
Serum collected from tail vein blood samples after each behavioral experiment showed that METH and AMP concentrations changed as pregnancy advanced (Fig. 3). Although the METH infusion remained constant, the actual dose (per kilo gram body weight) began to slowly decrease after GD11 due to the significant increases in maternal weight as pregnancy progressed. METH serum concentrations surprisingly did not decrease in parallel. For instance, METH concentrations were significantly lower on GD20, in comparison to their initial steady-state concentrations on GD8 but not on GD21 in the 10- and 13.2-mg/kg/day groups.
FIG. 3.
Serum concentrations of METH (top panel) and AMP (bottom panel) during gestation in timed-pregnant Sprague-Dawley rats treated continuously with 5.6, 10, or 13.2 mg/kg/day METH. The symbols *, †, and ‡ represent significant differences (p < 0.05) in METH and AMP concentration compared to values on GD8 in the 5.6-, 10-, and 13.2-mg/kg/day dose groups, respectively. Concentrations were determined by LC-MS/MS. All data points are mean ± SD; n = 4–6 per time-point.
Although serum samples were collected from tail vein blood on GD20 and trunk blood on GD21, we do not think this difference in the blood collection site contributed to any disparities. During preliminary studies, we validated that METH and AMP concentrations determined from the two collection sites at the same time-point were not statistically different. Specifically, METH tail vein serum versus trunk serum concentrations were 138 ± 60 ng/ml and 138 ± 57 ng/ml, respectively, and AMP tail vein serum versus trunk serum concentrations were 10 ± 5 ng/ml and 10 ± 4 ng/ml, respectively.
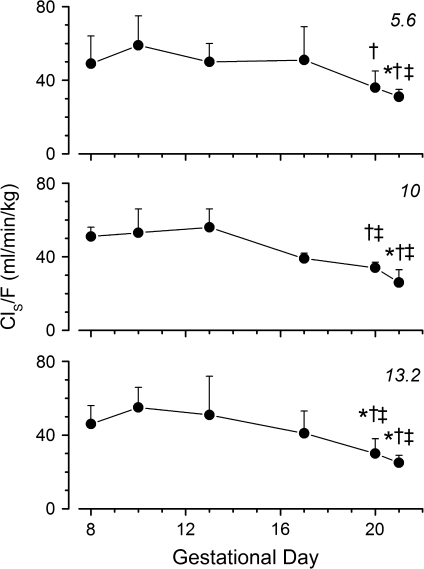
ClS/F values were not significantly different between doses (Fig. 4), suggesting first-order pharmacokinetics. Importantly, ClS/F values at 5.6, 10, and 13.2 mg/kg/day decreased significantly at the end of the experiment (GD20 and GD21) compared to early- and mid-gestation clearance values.
FIG. 4.
Time course of ClS/F of METH from GD8 to GD20 in pregnant Sprague-Dawley rats after chronic infusion of 5.6 (top panel), 10 (middle panel), or 13.2 (bottom panel) mg/kg/day METH. ClS/F values were calculated based on serum METH concentrations, infusion rates, and normalized body weight on each GD. F was assumed to be 1 in pregnant rats. This assumption was based on previous studies conducted in our laboratory, in which F was determined to be 1 in male rats dosed with 3 mg/kg sc bolus METH (Gentry et al., 2004). The symbols *, †, and ‡ indicates a significantly (p < 0.05) lower clearance value compared to clearances on GD8, GD10, or GD13, respectively. All data points are mean ± SD.
Because pregnancy-induced changes in serum protein binding could affect clearance, the METH serum protein binding was determined in the dams throughout gestation and in the fetuses on GD21 (data not shown). METH and AMP serum protein binding remained relatively constant throughout gestation. Average values for METH and AMP serum protein binding for dams were 9 ± 5 and 12 ± 4%, respectively, from GD8 to GD21. METH serum protein binding on GD21 was 11 ± 8% for dams and 5 ± 4% for their pups. AMP serum protein binding was 12 ± 11% and 6 ± 5%, respectively. Thus, no significant or substantive differences in protein binding were found. These results are consistent with METH serum protein binding in adult male Sprague-Dawley rats, in which the protein binding is approximately 10% (Rivière et al., 1999).
METH serum protein binding was determined in serum samples from pregnant rats (∼10%), nonpregnant rat serum (21 ± 4%), pooled human serum (53 ± 6%), and human albumin (50 ± 9%), which contained METH at concentrations within the range of the serum METH concentrations of pregnant rats dosed with 5.6 or 10 mg/kg/day METH from GD7 to GD21. These studies helped to establish METH protein binding in the pregnant rat and how it compares to human serum and albumin protein binding. While the METH protein binding in both species was unaffected by METH concentrations ranging from about 50–200 ng/ml, METH protein binding was significantly lower in the rat serum (p < 0.05) than in the human serum. Interestingly, the data from the human protein binding studies suggest that human albumin could account for all the METH protein binding in human serum.
The 17.8-mg/kg/day METH dose was lethal to three of the six animals from GD9 to GD10, and the remaining three were sacrificed on GD11 for humane reasons. Because the exact time of death could not be determined accurately for the first three animals, final serum and brain concentrations were not included in the pharmacokinetic analyses. However, on GD8, the METH and AMP serum concentrations for all six animals were 495 ± 160 ng/ml and 137 ± 111 ng/ml, respectively. Unfortunately, by GD10, we were unable to collect any blood samples from the animals due to poor blood flow. Although a direct comparison of the METH and AMP serum concentrations on GD10 could not be made to the lower METH dose groups, METH and AMP serum (and brain) concentrations were determined in the three surviving rats that were sacrificed on GD11. METH brain and serum concentrations were 3668 ± 572 ng/g and 641 ± 169 ng/ml, respectively. Average AMP brain and serum concentrations were 977 ± 267 ng/g and 116 ± 25 ng/ml, respectively. The brain-to-serum ratios were 5.7 ± 1 and 8.4 ± 1 for METH and AMP, respectively. The molar ratios of AMP-to-METH were 0.3 for brain and 0.2 for serum. The ClS/F value was not different compared to the animals treated with the three lower doses of METH on GD8 (data not shown).
Comparison of Dam and Litter METH and AMP Concentrations in Serum and Brain on GD21
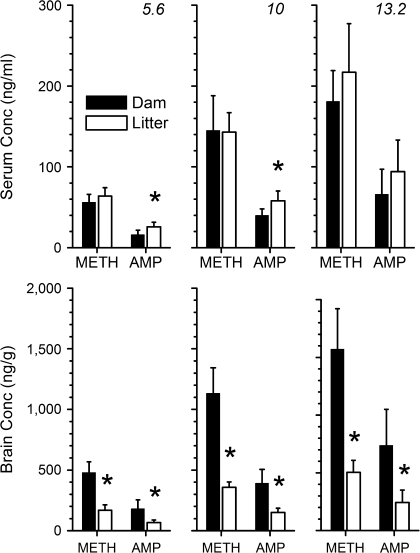
Figure 5 shows the different concentrations of drugs in the sera and brains of dams compared to litters. METH and AMP serum and brain concentrations of dams and their litters on GD21 were dose proportional. Linear regression analysis of METH and AMP brain and serum concentrations versus METH dose data revealed R2 values > 0.97 for dams and their litters.
FIG. 5.
Average serum (upper panel) and brain (lower panel) concentrations of METH and AMP determined in dams and their litters on GD21. Pregnant rats were treated with 5.6 (left panel), 10 (middle panel), or 13.2 (right panel) mg/kg/day of METH via sc osmotic minipump from GD7 to GD21. Both dams and their litters were sacrificed on GD21 to determine serum and brain concentrations by LC-MS/MS. Serum and brain tissues were pooled for the entire litter from each dam for use in determining the METH and AMP concentrations. *p < 0.05 dam versus corresponding litter concentrations. All bars are shown as mean ± SD; n = 4–6 per data point.
The serum concentrations were similar in the dams and their litters (Fig. 5, upper panel). Within dose groups, the serum METH concentrations were not significantly different in the dams compared to the corresponding litters. The litter serum AMP concentrations were statistically higher than the maternal concentrations at the two lower dose groups (p < 0.05). The dam-to-litter serum ratios of METH were 0.9:1, 1:1, and 0.8:1 for 5.6, 10, and 13.2 mg/kg/day, respectively. The dam-to-litter ratios for AMP concentrations were 0.6:1, 0.7:1, and 0.7:1 for the 5.6-, 10-, and 13.2-mg/kg/day METH dose groups, respectively.
The METH and AMP brain concentrations were consistently three times higher in the dams than in the corresponding litter brains at all three doses (Fig. 5, lower panel). These differences were statistically significant for all dose groups (p < 0.05). The METH brain-to-serum ratios in the dams were 8:1, 8:1, and 9:1 at METH doses of 5.6, 10, and 13.2 mg/kg/day, respectively. These values are similar to the 9:1 ratio reported previously for adult male Sprague-Dawley rats by Rivière et al. (2000). In contrast, the brain-to-serum ratios of the litters were 3:1, 3:1, and 2:1 for the 5.6-, 10-, and 13.2-mg/kg/day doses, respectively. AMP brain-to-serum ratios for dams were 11:1, 10:1, and 11:1 for 5.6, 10, and 13.2 mg/kg/day, respectively. The AMP brain-to-serum ratios for litters were 3:1 for all three doses.
At the 5.6-, 10-, and 13.2-mg/kg/day doses, the brain AMP-to-METH molar ratios for dams and litters were 0.4, 0.4, and 0.5, respectively. Conversely, the serum molar ratios were higher in litters compared to dams at all three doses. The serum AMP-to-METH molar ratios of dams were 0.3, 0.3, and 0.4 at doses of 5.6, 10, and 13.2 mg/kg/day, respectively. These ratios for litters were 0.4, 0.4, and 0.5, respectively.
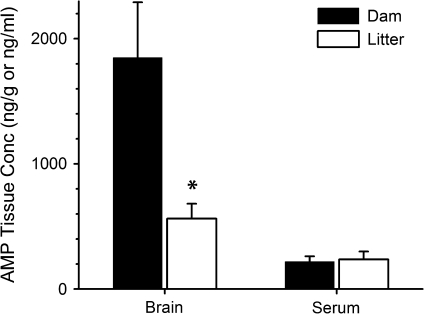
AMP Maternal and Fetal Partitioning Study
To determine whether differences in the metabolic conversion of METH to AMP played a role in the differences observed for METH and AMP serum concentrations in the dams and litters on GD21, a separate group of pregnant rats was dosed with 10 mg/kg/day AMP from GD7 to GD21. On GD21, the brain and serum AMP concentrations were determined (Fig. 6). There was no significant difference in serum concentrations of dams and litters (dam-to-litter ratio = 0.9:1), but as in the METH-dosed groups, AMP brain concentrations were three times higher in dams relative to corresponding litters (p < 0.05). Brain-to-serum AMP ratios for dam and litters were 8:1 and 2:1, respectively. These values, following an AMP infusion, were similar to the previous dam-to-litter ratios determined for METH and AMP when the rats were infused with METH at 10 mg/kg/day.
FIG. 6.
Dam and litter AMP concentrations in brain and serum samples on GD21. Concentrations were determined following a continuous sc infusion of 10 mg/kg/day AMP to pregnant rats from GD7 to GD21. On GD21, 1 day prior to normal delivery, dams and their litters were sacrificed, and AMP concentrations in brain and serum samples were determined by LC-MS/MS. Pooled brain and serum concentrations from each litter represent one unit. *p < 0.05 dam versus corresponding litter concentrations. All data points are mean ± SD; n = 5 per data point.
Concentrations of (+)-4-Hydroxy Methamphetamine and (+)-4-Hydroxy Amphetamine after METH Dosing
Brain and serum concentrations of (+)-4-hydroxy methamphetamine (4-OH METH) were determined in dams and pups on GD21 and in the three rats sacrificed on GD11 in the 17.8-mg/kg/day METH dose group. Maternal 4-OH METH brain (and serum) concentrations on GD21 were 5 ± 0.4 ng/g (below lower limit of quantitation [LLOQ]), 8 ± 1 ng/g (2 ± 0.4 ng/ml), 9 ± 0.7 ng/g (3 ± 0.6 ng/ml) for the 5.6-, 10-, and 13.2-mg/kg/day groups, respectively. For the 17.8-mg/kg/day group on GD11, maternal 4-OH METH concentrations were 19 ± 5.6 ng/g (3 ± 0.2 ng/ml), respectively. The maternal brain-to-serum ratios of 4-OH METH were 3:1, 4:1, and 7:1 at 10, 13.2, and 17.8 mg/kg/day, respectively. Litter 4-OH METH concentrations were below the LLOQ. All brain and serum concentrations of (+)-4-hydroxy amphetamine (4-OH AMP) were also below LLOQ for dams and corresponding litters exposed prenatally to METH. No attempt was made to determine phase II 4-OH METH or 4-OH AMP conjugate concentrations.
DISCUSSION
We hypothesized that METH dose- and gestational time–dependent pharmacological effects in pregnant rats and their litters were related to changes in METH disposition. METH abusers usually smoke, snort, or inject the drug in a binge/crash pattern (Cho and Melega, 2002). However, we chose to chronically infuse METH, which produced relatively stable levels of METH exposure throughout the second and third trimesters. While this route does not accurately mimic human METH use, it minimized the experimental stress to the animals that occurs with frequent iv or sc injections. More importantly, it allowed accurate determination of METH and AMP partitioning and equilibration across the maternal and fetal blood-brain barriers and placenta-blood barrier.
METH Effects on the Dam
The METH dose-dependent changes in weight (Fig. 1), stress, and skin lesions were transient in most animals, but all animals at 17.8 mg/kg/day METH died or were sacrificed by GD11. One animal in the 10- and 13.2-mg/kg/day dose groups also died.
The time to maximum locomotor activity was progressively prolonged as the dose increased (Fig. 2). At the highest dose (13.2 mg/kg/day), the locomotor activity was substantially suppressed from ∼GD8 to GD13. The animals were relatively immobile due to intense METH-induced central nervous system (CNS) effects. This affected their ability to feed and thrive. At the highest doses, the animals displayed excessive gnawing and grooming that resulted in bleeding and self-inflicted wounds to their digits and skin. These behaviors appear equivalent to METH-driven human behaviors like anorexia and compulsive skin “picking” of their face and other sites. While GD7–GD14 was a particularly vulnerable period for lethal effects, as pregnancy (and neuroadaptation) progressed, the animals appeared to develop some tolerance.
A major goal was to determine the impact of pregnancy-induced physiological changes on METH disposition. METH ClS/F remained relatively constant until the end of gestation, at which point it significantly decreased (Fig. 4). Interestingly, this gestational time-dependent change in ClS/F was independent of dose (5.6–13.2 mg/kg/day). It is unlikely serum protein binding affected ClS/F since the maternal serum protein binding for METH and AMP was low (9–12%). However, METH serum protein binding was found to be approximately 2.5–5.3 times higher in human serum than in nonpregnant or pregnant rat serum.
We think late-gestation reductions in ClS/F could be affected by hormonal-induced changes in hemodynamics. For example, renal blood flow is lower in late-gestation rats (Reckelhoff et al., 1992). Additionally, METH may be at least partially eliminated by tubular secretion by the OCT3 (Lee and Kim, 2004). This transporter has an affinity for steroid hormones, especially estradiol (Ganapathy and Prasad, 2005). Increased levels of hormones, like estradiol, prior to delivery could act to competitively inhibit METH binding to OCT3, resulting in decreased METH clearance. Changes in metabolism could also contribute to the decreased clearance near term since some enzymes are inhibited by estradiol (e.g., CYP1A2; Tracy et al., 2005). Neale and Parke (1973) find a 25% decrease in total CYP450 concentrations in late-gestation rats (GD19–GD20) compared to nulliparous females. This reduction is not observed on GD15–GD16. It is also likely that the METH volume of distribution changed during gestation due to increased plasma volume, cardiac output, and/or total body water. We did not attempt to experimentally characterize these potentially interrelated mechanisms in the current studies.
Although pharmacological/toxicological studies of METH in pregnant women would be more insightful than in pregnant rats, this is not possible due to the obvious risk. However, physiological similarities between the rat and the human during pregnancy suggest that the METH disposition changes in late-stage pregnancy in these studies could extrapolate to pregnant women. Hence, determination of METH disposition through analysis of blood and urine samples of pregnant METH addicts are needed to help understand the implications of the risk of METH- and pregnancy-induced physiological changes in the human mother and her fetus.
METH Effects on the Fetus
There were no observable fetal effects at the end of the experiment (GD21). Although some tolerance to METH effects developed by GD21, we were surprised that there were no changes in fetal weight, number of fetal resorptions, litter size, or physical appearance at any dose (Table 1). Nevertheless, we would not rule out the possibility of adverse developmental problems for the pups during the subsequent withdrawal from METH and during the important period of rapid brain development after birth. Slamberova et al. (2006) report a similar lack of effects in litters exposed prenatally to daily sc injections of 5 mg/kg METH throughout gestation. Unlike the current studies, fetal weights in the study by Slamberova et al. were significantly lower in METH-treated rats, which might be attributed to the different routes of administration.
It is known that METH and AMP are transferred across the placenta by the NET and the SERT located on the maternal-facing brush border membrane (Ramamoorthy et al., 1995). However, AMP has greater inhibitor potency for NET and SERT than METH. This difference could explain why METH distributed equally between maternal and fetal compartments (Fig. 5), while AMP serum concentrations were significantly lower in the dams at the 5.6- and 10-mg/kg doses. It is unlikely that ion trapping contributed to this disparity since METH is more likely to be ion trapped than AMP (pKa = 10.4 and 9.9, respectively) in the slightly more acidic fetal tissue and amniotic fluid (0.1–0.15 units more acidic; Reynolds and Knott, 1989).
The developing liver has a limited ability to metabolize drugs due to lower enzyme activity relative to the adult, and the ductus venous shunt serves to decrease the amount of drug reaching the liver (Green et al., 1979). Nevertheless, the fetus can metabolize xenobiotics. For example, meperidine is converted to normeperidine (an N-demethylated metabolite) after fetal administration (Szeto et al., 1978). Since METH is also N-demethylated to form AMP (Caldwell et al., 1972), we administered AMP to the dams to determine how AMP accumulates in fetuses after METH administration. Since no significant differences between maternal and fetal AMP serum concentrations were found (Fig. 6), these data suggested that the higher fetal AMP concentration following METH infusion in dams (Fig. 5) resulted from fetal conversion of METH to AMP. However, these were not profound differences, and more comprehensive studies will be needed to prove this point.
METH Effects on the Dam and Fetus
The METH and AMP brain concentrations were approximately threefold higher in the dams than in the pups at all doses (Figs. 5 and 6). Won et al. (2001) report that METH concentrations are three, two, and five times higher in maternal versus fetal striatum, brainstem, and cortex, respectively, after a single 40-mg/kg sc METH dose on GD14. Brain development in the rat and mouse is not complete until 3 weeks after parturition (Watson et al., 2006); thus, expression of transporters and binding sites in the rat brain are not fully developed on GD21. Although the quantity of SERT and dopamine transporter in the striatum and frontal cortex is lower in fetal brains than in adult brains on GD20, the fetal and maternal transporters have similar affinity for their ligands (Hyde and Bennett, 1994). Hence, lower METH- and AMP-binding capacity for METH sites of action in the fetal brain likely produced the substantially lower pup brain concentrations. We think this mechanism at least partially protected pups from fetal CNS effects but not from the dams’ CNS effects. Other contributing factors might include differences in fetal brain blood-brain barrier permeability, cerebral blood flow, and membrane lipid and fatty acid content (Green et al., 1999; Risau and Wolburg, 1990).
We have observed similar partitioning and constant values for brain-to-serum ratios following infusions of phencyclidine (Proksch et al., 2000). In male rats, a 2.5-mg/kg/day infusion results in a brain-to-serum ratio of 3:1, while 10, 18, and 25 mg/kg/day (near lethal) result in a constant phencyclidine brain-to-serum ratio of 6:1. A major distinction among these doses was that the 10- to 25-mg/kg/doses were pharmacologically active, while the lowest was not.
In conclusion, a steep METH dose-response lethality curve was observed in which most of the pregnant rats treated with doses less than or equal to 13.2 mg/kg/day METH appeared to achieve some level of tolerance to the METH-induced effects, but at a one-quarter log dose higher (17.8 mg/kg/day), all animals died. In the surviving animals, health and behavioral effects in the dams were METH dose- and gestational time dependent. Significant reductions in METH clearance were found in the dams among all doses during late-stage pregnancy. Furthermore, maternal METH and AMP brain concentrations were three times higher than fetal brain concentrations on GD21, while serum concentrations were approximately equal. However, on GD21, the litters appeared to have no observable METH-induced adverse effects nor were there increased premature births, resorptions, or in utero deaths. We do not know how chronic METH exposure during pregnancy, followed by withdrawal, would affect the developing brain after birth. Overall, these findings suggest that there are two periods of increased susceptibility to METH exposure during pregnancy. The first is the period after the start of chronic METH treatment before neuroadaptation and tolerance to METH occurs. The second is at the end of pregnancy when clearance of the drug is significantly reduced and METH concentrations are higher.
FUNDING
National Institute on Drug Abuse (DA07610 to S.M.O); a graduate fellowship (to S.J.W.) from GlaxoSmithKline and the National Institute of Environmental Health Sciences (T32EA07310).
Acknowledgments
The authors thank Melinda Gunnell, Sherri Wood, Sally Huey, Will Atchley, and John Faver for their excellent technical assistance.
References
- Acuff-Smith KD, George M, Lorens SA, Vorhees CV. Preliminary evidence for methamphetamine-induced behavioral and ocular effects in rat offspring following exposure during early organogenesis. Psychopharmacology (Berl.) 1992;109:255–263. doi: 10.1007/BF02245871. [DOI] [PubMed] [Google Scholar]
- Acuff-Smith KD, Schilling MA, Fisher JE, Vorhees CV. Stage-specific effects of prenatal d-methamphetamine exposure on behavioral and eye development in rats. Neurotoxicol. Teratol. 1996;18:199–215. doi: 10.1016/0892-0362(95)02015-2. [DOI] [PubMed] [Google Scholar]
- Arria AM, Derauf C, Lagasse LL, Grant P, Shah R, Smith L, Haning W, Huestis M, Strauss A, Grotta SD, et al. Methamphetamine and other substance use during pregnancy: Preliminary estimates from the infant development, environment, and lifestyle (IDEAL) study. Matern. Child Health J. 2006;10:293–302. doi: 10.1007/s10995-005-0052-0. [DOI] [PubMed] [Google Scholar]
- Caldwell J, Dring LG, Williams RT. Metabolism of (14 C)methamphetamine in man, the guinea pig and the rat. Biochem. J. 1972;129:11–22. doi: 10.1042/bj1290011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho AK, Melega WP. Patterns of methamphetamine abuse and their consequences. J. Addict. Dis. 2002;21:21–34. doi: 10.1300/j069v21n01_03. [DOI] [PubMed] [Google Scholar]
- Cohen JB, Greenberg R, Uri J, Halpin M, Zweben JE. Women with methamphetamine dependence: Research on etiology and treatment. J. Psychoactive Drugs. 2007;Suppl. 4:347–351. doi: 10.1080/02791072.2007.10399896. [DOI] [PubMed] [Google Scholar]
- Conrad KP. Possible mechanisms for changes in renal hemodynamics during pregnancy: Studies from animal models. Am. J. Kidney Dis. 1987;9:253–259. doi: 10.1016/s0272-6386(87)80118-x. [DOI] [PubMed] [Google Scholar]
- DeSesso JM. Comparative embryology. In: Hood RD, editor. Handbook of Developmental Toxicology. Boca Raton, FL: CRC Press; 1997. pp. 111–174. [Google Scholar]
- Dowell RT, Kauer CD. Maternal hemodynamics and uteroplacental blood flow throughout gestation in conscious rats. Methods Find Exp. Clin. Pharmacol. 1997;19:613–625. [PubMed] [Google Scholar]
- Friedman H, Pross S, Klein TW. Addictive drugs and their relationship with infectious diseases. FEMS Immunol. Med. Microbiol. 2006;47:330–342. doi: 10.1111/j.1574-695X.2006.00097.x. [DOI] [PubMed] [Google Scholar]
- Ganapathy V, Prasad PD. Role of transporters in placental transfer of drugs. Toxicol. Appl. Pharmacol. 2005;207:381–387. doi: 10.1016/j.taap.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Gentry WB, Ghafoor AU, Wessinger WD, Laurenzana EM, Hendrickson HP, Owens SM. (+)-Methamphetamine-induced spontaneous behavior in rats depends on route of (+)METH administration. Pharmacol. Biochem. Behav. 2004;79:751–760. doi: 10.1016/j.pbb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Green P, Glozman S, Kamensky B, Yavin E. Developmental changes in rat brain membrane lipids and fatty acids. The preferential prenatal accumulation of docosahexaenoic acid. J. Lipid Res. 1999;40:960–966. [PubMed] [Google Scholar]
- Green TP, O'Dea RF, Mirkin BL. Determinants of drug disposition and effect in the fetus. Annu. Rev. Pharmacol. Toxicol. 1979;19:285–322. doi: 10.1146/annurev.pa.19.040179.001441. [DOI] [PubMed] [Google Scholar]
- Hardin JS, Wessinger WD, Proksch JW, Owens SM. Pharmacodynamics of a monoclonal antiphencyclidine Fab with broad selectivity for phencyclidine-like drugs. J. Pharmacol. Exp. Ther. 1998;285:1113–1122. [PubMed] [Google Scholar]
- Hendrickson H, Laurenzana E, Owens SM. Quantitative determination of total methamphetamine and active metabolites in rat tissue by liquid chromatography with tandem mass spectrometric detection. Aaps. J. 2006;8:E709–E717. doi: 10.1208/aapsj080480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge LS, Tracy TS. Alterations in drug disposition during pregnancy: Implications for drug therapy. Expert Opin. Drug Metab. Toxicol. 2007;3:557–571. doi: 10.1517/17425225.3.4.557. [DOI] [PubMed] [Google Scholar]
- Hyde CE, Bennett BA. Similar properties of fetal and adult amine transporters in the rat brain. Brain Res. 1994;646:118–123. doi: 10.1016/0006-8993(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Jayanthi LD, Vargas G, DeFelice LJ. Characterization of cocaine and antidepressant-sensitive norepinephrine transporters in rat placental trophoblasts. Br. J. Pharmacol. 2002;135:1927–1934. doi: 10.1038/sj.bjp.0704658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Kim RB. Transporters and renal drug elimination. Annu. Rev. Pharmacol. Toxicol. 2004;44:137–166. doi: 10.1146/annurev.pharmtox.44.101802.121856. [DOI] [PubMed] [Google Scholar]
- Lee JS, Yank WK, Han EY, Lee SY, Park YH, Lim MA, Chung HS, Park JH. Monitoring precursor chemicals of methamphetamine through enantiomer profiling. Forensic. Sci. Int. 2007;173:68–72. doi: 10.1016/j.forsciint.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Martin JC, Martin DC, Radow B, Sigman G. Growth, development and activity in rat offspring following maternal drug exposure. Exp. Aging Res. 1976;2:235–251. doi: 10.1080/03610737608257179. [DOI] [PubMed] [Google Scholar]
- Mattison DR, Blann E, Malek A. Physiological alterations during pregnancy: Impact on toxicokinetics. Fundam. Appl. Toxicol. 1991;16:215–218. doi: 10.1016/0272-0590(91)90103-b. [DOI] [PubMed] [Google Scholar]
- Neale MG, Parke DV. Effects of pregnancy on the metabolism of drugs in the rat and rabbit. Biochem. Pharmacol. 1973;22:1451–1461. doi: 10.1016/0006-2952(73)90323-7. [DOI] [PubMed] [Google Scholar]
- Proksch JW, Gentry WB, Owens SM. The effect of rate of drug administration on the extent and time course of phencyclidine distribution in rat brain, testis, and serum. Drug Metab. Dispos. 2000;28:742–747. [PubMed] [Google Scholar]
- Ramamoorthy JD, Ramamoorthy S, Leibach FH, Ganapathy V. Human placental monoamine transporters as targets for amphetamines. Am. J. Obstet. Gynecol. 1995;173:1782–1787. doi: 10.1016/0002-9378(95)90427-1. [DOI] [PubMed] [Google Scholar]
- Reckelhoff JF, Yokota SD, Baylis C. Renal autoregulation in midterm and late-pregnant rats. Am. J. Obstet. Gynecol. 1992;166:1546–1550. doi: 10.1016/0002-9378(92)91632-k. [DOI] [PubMed] [Google Scholar]
- Reynolds F, Knott C. Pharmacokinetics in pregnancy and placental transfer. Oxf. Rev. Reprod. Biol. 1989;11:389–449. [PubMed] [Google Scholar]
- Risau W, Wolburg H. Development of the blood-brain barrier. Trends Neurosci. 1990;13:174–178. doi: 10.1016/0166-2236(90)90043-a. [DOI] [PubMed] [Google Scholar]
- Rivière GJ, Byrnes KA, Gentry WB, Owens SM. Spontaneous locomotor activity and pharmacokinetics of intravenous methamphetamine and its metabolite amphetamine in the rat. J. Pharmacol. Exp. Ther. 1999;291:1220–1226. [PubMed] [Google Scholar]
- Rivière GJ, Gentry WB, Owens SM. Disposition of methamphetamine and its metabolite amphetamine in brain and other tissues in rats after intravenous administration. J. Pharmacol. Exp. Ther. 2000;292:1042–1047. [PubMed] [Google Scholar]
- Schmidt RR, Johnson EM. Principles of teratology. In: Hood RD, editor. Handbook of Developmental Toxicology. Boca Raton, FL: CRC Press; 1997. pp. 3–12. [Google Scholar]
- Slamberova R, Pometlova M, Charousova P. Postnatal development of rat pups is altered by prenatal methamphetamine exposure. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2006;30:82–88. doi: 10.1016/j.pnpbp.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Smith LM, LaGasse LL, Derauf C, Grant P, Shah R, Arria A, Huestis M, Haning W, Strauss A, Della Grotta S, et al. The infant development, environment, and lifestyle study: Effects of prenatal methamphetamine exposure, polydrug exposure, and poverty on intrauterine growth. Pediatrics. 2006;118:1149–1156. doi: 10.1542/peds.2005-2564. [DOI] [PubMed] [Google Scholar]
- Smith LM, Lagasse LL, Derauf C, Grant P, Shah R, Arria A, Huestis M, Haning W, Strauss A, Grotta SD, et al. Prenatal methamphetamine use and neonatal neurobehavioral outcome. Neurotoxicol. Teratol. 2008;30:20–28. doi: 10.1016/j.ntt.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stek AM, Baker RS, Fisher BK, Lang U, Clark KE. Fetal responses to maternal and fetal methamphetamine administration in sheep. Am. J. Obstet. Gynecol. 1995;173:1592–1598. doi: 10.1016/0002-9378(95)90654-1. [DOI] [PubMed] [Google Scholar]
- Szeto HH, Mann LI, Bhakthavathsalan A, Liu M, Inturrisi CE. Meperidine pharmacokinetics in the maternal-fetal unit. J. Pharmacol. Exp. Ther. 1978;206:448–459. [PubMed] [Google Scholar]
- Tracy TS, Venkataramanan R, Glover DD, Caritis SN. Temporal changes in drug metabolism (CYP1A2, CYP2D6 and CYP3A Activity) during pregnancy. Am. J. Obstet. Gynecol. 2005;192:633–639. doi: 10.1016/j.ajog.2004.08.030. [DOI] [PubMed] [Google Scholar]
- Watson RE, DeSesso JM, Hurtt ME, Cappon GD. Postnatal growth and morphological development of the brain: A species comparison. Birth Defects Res. B Dev. Reprod. Toxicol. 2006;77:471–484. doi: 10.1002/bdrb.20090. [DOI] [PubMed] [Google Scholar]
- Won L, Bubula N, McCoy H, Heller A. Methamphetamine concentrations in fetal and maternal brain following prenatal exposure. Neurotoxicol. Teratol. 2001;23:349–354. doi: 10.1016/s0892-0362(01)00151-9. [DOI] [PubMed] [Google Scholar]