Abstract
Objective
To compare the effectiveness of touch screen system with information leaflet for providing women with information on prenatal tests.
Design
Randomised controlled trial; participants allocated to intervention group (given access to touch screen and leaflet information) or control group (leaflet information only).
Setting
Antenatal clinic in university teaching hospital.
Subjects
875 women booking antenatal care.
Interventions
All participants received a leaflet providing information on prenatal tests. Women in the intervention arm also had access to touch screen information system in antenatal clinic.
Main outcome measures
Women's informed decision making on prenatal testing as measured by their uptake of and understanding of the purpose of specific tests; their satisfaction with information provided; and their levels of anxiety.
Results
All women in the trial had a good baseline knowledge of prenatal tests. Women in the intervention group did not show any greater understanding of the purpose of the tests than control women. However, uptake of detailed anomaly scans was significantly higher in intervention group than the control group (94% (351/375) v 87% (310/358), P=0.0014). Levels of anxiety among nulliparous women in intervention group declined significantly over time (P<0.001).
Conclusions
The touch screen seemed to convey no benefit over well prepared leaflets in improving understanding of prenatal tests among the pregnant women. It did, however, seem to reduce levels of anxiety and may be most effective for providing information to selected women who have a relevant adverse history or abnormal results from tests in their current pregnancy.
Key messages
Throughout the NHS, efforts are being made to evaluate traditional methods of conveying information to patients, such as leaflets, and to develop and assess new approaches
This study compared the effectiveness of a touch screen system with a well designed leaflet at providing women with information on prenatal tests
The touch screen conferred no additional benefits over the leaflet when applied to an unselected population of pregnant women
Nulliparous women showed reduced anxiety levels after access to the touch screen, but further research is needed on the measurement of anxiety during pregnancy
Introduction
Informed choice has been an important component of health care in the United Kingdom for almost a decade.1,2 One area in which this principle has long been applied is prenatal testing. Specific initiatives have been launched to promote women's awareness of best evidence on the effectiveness of specific tests and active participation in decisions about their care.3 The number of conditions for which screening is offered continues to grow rapidly, and women consequently face increasingly complex decisions.4 Studies have illuminated many dimensions to this complexity, including the professional and organisational barriers to informed choice,3 the huge variations in the scope and accuracy of information given,5 and the problem of receiving unsolicited and unanticipated information from screening.6 What is also clear is that informed choice depends on an effective partnership between the user, the provider and the communication medium.
Throughout the NHS, efforts are being made to evaluate traditional methods of conveying information, such as leaflets, and to develop and assess new approaches. This paper reports the results of a recent trial to evaluate a touch screen information system for providing information on prenatal tests to women. The primary hypothesis was that access to the system would improve women's informed decision making regarding prenatal tests over and above that achieved by access to an information leaflet alone.
Participants and methods
Study population and setting
Women attending a booking appointment at one of the five antenatal clinics at Aberdeen Maternity Hospital from April 1997 until January 1998 were invited to participate. This large teaching hospital had 4734 deliveries in 1997. The five clinics encompassed women with high and low risk pregnancies. We obtained consent for the trial from the Grampian Health Board and University of Aberdeen Joint Ethical Committee.
Assignment
Women were initially contacted by post and, along with their booking appointment, were given a baseline questionnaire to complete before coming to clinic. At their booking, the women were approached by a research midwife for their verbal consent to participate in the trial. Completed baseline questionnaires were collected, or, if necessary, a further copy was given for the woman to answer at the clinic. Once they had given consent, the women were randomised on a 1:1 ratio into the intervention (touch screen and information leaflet) or control (leaflet only) group. Allocation was made by the research midwife opening consecutive, sealed, opaque envelopes. The randomisation schedule was prepared by AK, who was not involved in recruiting.
Interventions
Both groups of women were given the information leaflet on prenatal tests developed specifically for the trial. Information leaflets already available in the antenatal clinic gave similar information to that provided by the touch screen information system, but none of these matched its scope and detail. The touch screen had been developed by three of the investigators (PS, NS, NH) over the previous two years.7,8 It was a menu driven system with information organised into eight main topics and included video clips and voice overs. Patients accessed the information by means of a touch screen display (fig 1), which is operated by pressing the display with a finger, that was located in the antenatal clinic waiting area. Use of the touch screen was limited to women in the intervention group by means of a password. Privacy in using the system was enhanced by the availability of microphone headsets.
Figure 1.

Menu display of options available on touch screen system. (Other views of system are available on the BMJ's website)
Outcomes measured
The primary outcome assessed in the trial was women's informed decision making on prenatal testing, as measured by their uptake and understanding of the purpose of five tests (ultrasound scan at booking, serum screening, detailed anomaly scan, amniocentesis, and chorionic villus sampling). Secondary outcomes included the women's satisfaction with the information they received and their anxiety levels.
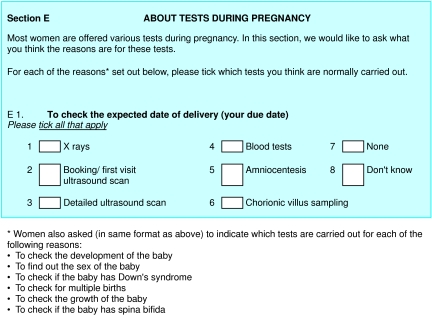
To assess the women's understanding of prenatal tests we gave them questionnaires for self completion at baseline, at around 16 weeks' gestation (after they had accepted or rejected serum screening), and at around 20 weeks' gestation (after they had accepted or rejected a detailed anomaly scan). The questionnaires asked a similar range of questions and were developed from validated schedules of previous studies9,10 and two focus group discussions. The baseline questionnaire was returned by women at recruitment, whereas the other two were sent and returned by post. We sent one reminder after two weeks if we had not received a reply. The women's understanding of prenatal tests was assessed with multiple choice questions, which asked them to look at eight principal reasons for testing and to indicate which of six possible tests were conducted for each purpose (see fig 2). The responses were analysed as a dichotomous variable (correct or incorrect). We used the Spielberger state-trait anxiety inventory (STAI)11 to assess anxiety levels, with the A-state component administered in all three questionnaires and the A-trait included in the first and last questionnaires.
Figure 2.
Extract from questionnaire used to assess women's understanding of prenatal tests
Statistical analysis
We estimated that we needed a sample size of 1000 women, 500 in each arm, to give 90% power to detect at the 5% significance level a difference of 10% in the proportion of women with an understanding of the reasons for serum screening indicative of informed decision making. This calculation assumed a baseline of 60% of the sample being informed9 and allowed for 5-10% to drop out.
All data from the questionnaires were entered into the trial database. We conducted quality control checks on a random sample of 10% of the questionnaires. Statistical analysis was by spss12 on an intention to treat basis. We used independent and paired t tests to compare continuous variables after checking for normal distribution. We compared the outcome variables for the two groups using the χ2 test and McNemar's test for paired data. We give significance levels of differences, and 95% confidence intervals. We used logistic regression to assess understanding of prenatal tests, after adjusting for important confounding factors such as parity and education.
Results
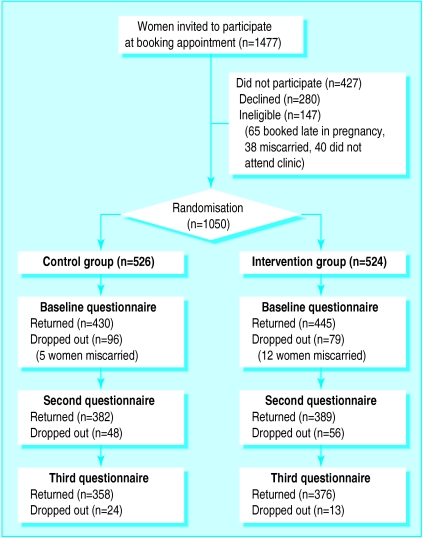
Recruitment—In total, 1477 women were identified as potential participants, of whom 1050 were found to be eligible and consented to take part (fig 3). Of the 427 who did not participate, 147 were ineligible and 280 did not consent. Of the 1050 participants, 670 (64%) returned all three questionnaires, 743 (71%) responded to only the first two questionnaires, and 710 (68%) responded to only the first and last questionnaires. Among the 875 women included in the baseline analysis, there were no significant differences between the characteristics of the intervention and control groups (see extra table on BMJ's website for details), and there were no major differences in the characteristics of the 175 women who did not return the baseline questionnaire and those who did. There were no significant differences between the two groups with regard to the characteristics of the women lost to follow up, nor the reasons for or rate of loss.
Figure 3.
Progress of participants through trial
Use of touch screen and information leaflet—Similar numbers of women in the intervention and control groups reported reading the information leaflet fully (218/380 (57%) and 234/381 (61%) respectively), and 12% in both groups indicated that they had only glanced at it. With regard to the touch screen, 32/374 (9%) women reported that they had never used it, and 342 women (91%) had used it at least once.
Views on prenatal testing—In the baseline questionnaire similar proportions of women in both groups reported that they would accept most tests if offered. The highest level of acceptance was for detailed anomaly scans (98% (405/415) of intervention group, 97% (381/394) of control group), and the lowest acceptance for amniocentesis (40% (160/399) and 42% (163/386) respectively). The only difference in the second questionnaire (after the women had been given information on prenatal testing) was that the acceptability of amniocentesis had increased significantly in both groups (McNemar's test, P=0.030).
Uptake of tests—Fewer than 1% of women did not receive any prenatal tests. Table 1 shows that the only significant difference between the two groups was that more women in the intervention group underwent detailed anomaly scanning (94% v 87%, P=0.0014).
Table 1.
Prenatal tests undergone by pregnant women randomised to control group (information leaflet on prenatal tests) or intervention group (leaflet plus touch screen information system). Values are numbers (percentages) unless stated otherwise
| Prenatal test | Control group (n=358) | Intervention group (n=375)* | % difference (95% CI) | P value |
|---|---|---|---|---|
| None | 3 (1) | 3 (1) | 0 (−1 to 1) | 0.954 |
| Ultrasound scan at booking | 292 (82) | 305 (82) | 0 (−5 to 6) | 0.936 |
| Serum screening | 210 (59) | 226 (60) | −1 (−6 to 9) | 0.658 |
| Detailed anomaly scan | 310 (87) | 351 (94) | −7 (−11 to −3) | 0.001 |
| Amniocentesis | 25 (7) | 35 (9) | −2 (−6 to 2) | 0.246 |
| Chorionic villus sampling | 0 | 2 (1) |
One case excluded from analysis (data missing).
Understanding of prenatal tests—In the baseline questionnaire women showed a high level of understanding of which prenatal tests were carried out for specific reasons, with the exception of chorionic villus sampling. Comparisons of baseline responses with those given by the same women in the second questionnaire showed significant improvements in knowledge for both groups (table 2). The logistic regression confirmed this, with no apparent greater gain in knowledge among women in the intervention arm.
Table 2.
Knowledge of pregnant women about which prenatal tests are undertaken “to check the development of the baby,” before and after they were given information leaflet on prenatal tests (control group) or leaflet plus access to touch screen information system (intervention group). Values are numbers (percentages) unless stated otherwise
| Prenatal test* | Control group (n=361)
|
Intervention group (n=374)
|
|||||
|---|---|---|---|---|---|---|---|
| Before information† | After information‡ | P value of difference | Before information† | After information‡ | P value of difference | ||
| Detailed anomaly scan | 311 (86) | 347 (96) | <0.001 | 348 (93) | 357 (96) | >0.05 | |
| Blood test | 237 (66) | 267 (74) | 0.008 | 246 (66) | 293 (78) | <0.001 | |
| Amniocentesis | 201 (56) | 231 (64) | 0.004 | 228 (61) | 251 (67) | 0.042 | |
| Chorionic villus sampling | 111 (31) | 135 (37) | 0.009 | 121 (32) | 150 (40) | 0.002 | |
Results for x ray and ultrasound scans at booking in were non-significant. †Results from first questionnaire (at baseline).
Results from third questionnaire (at ∼20 weeks' gestation).
Satisfaction with information—Both groups reported high levels of satisfaction with the information leaflet, with over 95% indicating that they would recommend the leaflet to other pregnant women. A similar percentage of the women in the intervention arm reported that they would recommend the touch screen, and over a third (132/347) indicated a preference for the touch screen over the leaflet, while a quarter (91) indicated no preference, a fifth (72) preferred the leaflet, and the rest (52) were “not sure.”
Anxiety levels—Table 3 shows the results of the Spielberger state-trait anxiety inventory. Compared with the results in the baseline questionnaire, both the A-state and A-trait components of the inventory measured in the third questionnaire had declined significantly in the intervention group, mainly among nulliparous women.
Table 3.
Anxiety levels of pregnant women before and after they were given information leaflet on prenatal tests (control group) or leaflet plus access to touch screen information system (intervention group). Values are mean scores for Spielberger state-trait anxiety inventory unless stated otherwise
| Control group (n=317)
|
Intervention group (n=332)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Before information* | After information† | Difference (95% CI) | P value | Before information* | After information† | Difference (95% CI) | P value | ||
| All women | |||||||||
| A-state | 35.15 | 35.67 | −0.52 (−1.54 to 0.50) | 0.317 | 35.58 | 34.20 | 1.38 (0.50 to 2.28) | 0.002 | |
| A-trait | 36.87 | 37.38 | −0.51 (−1.31 to 0.28) | 0.204 | 37.12 | 35.41 | 1.71 (0.87 to 2.56) | <0.001 | |
| Nulliparous women | (n=155) | (n=164) | |||||||
| A-state | 36.03 | 35.97 | 0.06 (−1.39 to 1.49) | 0.947 | 36.42 | 34.22 | 2.20 (0.93 to 3.47) | 0.001 | |
| A-trait | 37.55 | 37.86 | −0.31 (−1.45 to 0.82) | 0.582 | 37.73 | 35.10 | 2.63 (1.38 to 3.88) | <0.001 | |
| Parous women | (n=162) | (n=168) | |||||||
| A-state | 34.30 | 35.37 | −1.07 (−2.53 to 0.39) | 0.150 | 34.77 | 34.17 | 0.60 (−0.65 to 1.84) | 0.348 | |
| A-trait | 36.22 | 36.93 | −0.71 (−1.83 to 0.42) | 0.218 | 36.52 | 35.71 | 0.81 (−0.32 to 1.95) | 0.158 | |
Results from first questionnaire (at baseline). †Results from third questionnaire (at ∼20 weeks' gestation).
Discussion
Antenatal screening is one of the most intensively researched subjects with regard to information for women and their informed choice.13 The principles of equity and quality, so well accepted in screening programmes, are now advocated for the process of giving information,4 but researchers have shown that one of the most serious obstacles to this is health professionals providing the information.3,14 Touch screen information systems have the potential to reduce this barrier by providing consistent information and by being patient driven, thus enabling pregnant women to control information overload.15 Like all new technologies, however, they should be subject to rigorous evaluation.
The touch screen evaluated in this trial conferred no additional benefit to that provided by the more traditional method of an information leaflet. It could be argued that only small effects could be expected in well educated pregnant women whose baseline level of knowledge and “compliance” with prenatal testing are already high. As found in other studies,9,16 both groups of women in our study showed improvements in their knowledge, albeit from a high starting point, which highlights women's receptiveness to information given during pregnancy and, thus, the importance of making it appropriate and reliable.
Interestingly, we observed a significant increase in the uptake of detailed anomaly scanning in the intervention group, and other studies comparing information provided by different methods have noted differential uptake of ultrasonography.15,17 In the case of our touch screen, the use of video clips to show what can be gained from a detailed scan might have helped to reassure women and increase their desire for this investigation.
Women's anxiety
Our trial involved an unselected group of pregnant women, and in such a predominantly healthy population we found, as have other researchers,15 that the information provided did not raise anxiety. In fact, one apparent benefit of the touch screen was to reduce levels of anxiety. The mean score for the A-state component of the Spielberger state-trait anxiety inventory declined significantly, but we also found a significant fall in the mean score for the A-trait component, which is supposed to remain stable over time.11 Other studies have noted this instability in the A-trait when the inventory is applied during pregnancy,18,19 and this effect warrants further investigation. In particular, we need to find the extent to which reduced anxiety could be replicated in a selected group of women with a previous adverse outcome or an abnormal finding from prenatal screening.
Limitations of study
Our findings should be interpreted in the light of two limitations: loss to follow up and the potential for contamination between groups. As with most longitudinal data collection, there was attrition of the number of participants from the point of recruitment to completion of the trial. Although this reduces the statistical power of the study, sub-group analysis showed no major differences in the characteristics of those women who did or did not complete all three questionnaires. As the participants were attending the same antenatal clinics it was not feasible to totally eliminate the risk of contamination between the intervention and control groups, with controls possibly observing the touch screen while it was being used. However, as we had introduced a password system for accessing the touch screen and provided microphone headsets, together with the need to stand right in front of the screen in order to see the images, contamination is likely to have been minimal.
Future studies
Further evaluations of this technology should also consider costs. The touch screen evaluated in this trial incurred initial development costs in 1994-5 of about £25 000, and additional costs can be envisaged in terms of maintenance of hardware and updating of information. A commitment to providing evidence based information must remain the major rationale for any future investment in computer technology.
Acknowledgments
We thank Ms Iciar Frade, Ms Jilly Ireland, Ms Gillian Payne, the medical and midwifery staff of Aberdeen Maternity Hospital, the secretarial staff of the Dugald Baird Centre, and, of course, the women who participated in the study.
Footnotes
Funding: The trial was funded by the NHS Executive programme in evaluating methods to promote the implementation of research and development (project No 4-21).
Competing interests: NH is a non-executive director of Cognetic Creations, which markets the touch screen technology used in the information system.
References
- 1.Department of Health. The patient's charter. London: HMSO; 1991. [Google Scholar]
- 2.Peckham M. Research and development for the National Health Service. Lancet. 1991;338:367–371. doi: 10.1016/0140-6736(91)90494-a. [DOI] [PubMed] [Google Scholar]
- 3.Oliver S, Rajan L, Turner H, Oakley A, Entwistle V, Watt I, et al. Informed choice for users of health services: views on ultrasonography leaflets of women in early pregnancy, midwives, and ultrasonographers. BMJ. 1996;313:1251–1253. doi: 10.1136/bmj.313.7067.1251a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dezateux C, Peckham C. Testing times for pregnant women. Lancet. 1998;352(suppl IV):24. doi: 10.1016/s0140-6736(98)90286-0. [DOI] [PubMed] [Google Scholar]
- 5.Marteau T, Slack J, Kidd J, Shaw R. Presenting a routine screening test in antenatal practice observed. Public Health. 1992;106:131–141. doi: 10.1016/s0033-3506(05)80390-7. [DOI] [PubMed] [Google Scholar]
- 6.Boyd PA, Chamberlain P, Hicks NR. 6 year experience of prenatal diagnosis in an unselected population in Oxford, UK. Lancet. 1998;352:1577–1581. doi: 10.1016/s0140-6736(98)03202-4. [DOI] [PubMed] [Google Scholar]
- 7.Smith APM, Hamilton N, Smith NC. Touchscreen display for prenatal diagnostic tests. The Diplomate. 1996;3:175–178. [Google Scholar]
- 8.Wallace C, Smith PA. Aberdeen: Department of Obstetrics and Gynaecology, University of Aberdeen; 1996. Development of touchscreen display for prenatal tests. . (Unpublished report.) [Google Scholar]
- 9.Marteau T, Johnston M, Plenicar M, Shaw RW, Slack J. Development of a self-administered questionnaire to measure women's knowledge of prenatal screening and diagnostic tests. J Psychosom Res. 1988;32:403–408. doi: 10.1016/0022-3999(88)90023-2. [DOI] [PubMed] [Google Scholar]
- 10.Miedzybrodzka ZH, Mollison J, Templeton A, Russell IT, Dean JC, Kelly KF, et al. Antenatal screening for carriers of cystic fibrosis: randomised trial of stepwise vcouple screening. BMJ. 1995;310:353–357. doi: 10.1136/bmj.310.6976.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spielberger CD, Gorsuch RL, Lushene RE. STAI manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 12.Norusis MJ. SPSS 6.1 Reference Manuals. Chicago: SPSS; 1994. [Google Scholar]
- 13.D'Alton ME, DeCHerney AH. Prenatal diagnosis. N Engl J Med. 1993;328:114–120. doi: 10.1056/NEJM199301143280208. [DOI] [PubMed] [Google Scholar]
- 14.Russel CH, Gordon AJ. How much do health professionals know about serum screening for Down's syndrome? Health Bull. 1998;56:631–634. [Google Scholar]
- 15.Thornton JG, Hewison J, Lilford RJ, Vail A. A randomised trial of three methods of giving information about prenatal testing. BMJ. 1995;311:1127–1130. doi: 10.1136/bmj.311.7013.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reid M. Consumer orientated studies in relation to prenatal screening test. Eur J Obstet Gynaecol Reprod Biol. 1988;28:79–92. [PubMed] [Google Scholar]
- 17.Green J, Statham H, Snowdon C. Screening for fetal abnormalities: attitudes and experiences. In: Chard T, Richards MPM, editors. Obstetrics in the 1990s: current controversies. London: MacKeith Press; 1992. pp. 65–69. [Google Scholar]
- 18.Hundley V, Gurney E, Graham W, Rennie AM. Can anxiety in pregnancy women be measured using the state-trait anxiety inventory. Midwifery. 1998;14:118–121. doi: 10.1016/s0266-6138(98)90009-2. [DOI] [PubMed] [Google Scholar]
- 19.Astbury J. The crisis of childbirth: can information and childbirth education help? J Psychosom Res. 1980;24:9–13. doi: 10.1016/0022-3999(80)90069-0. [DOI] [PubMed] [Google Scholar]