Abstract
In functional magnetic resonance imaging (fMRI), hemispheric dominance is generally indicated by a measure called the laterality index (LI). The assessment of a meaningful LI measure depends on several methodological factors that should be taken into account when interpreting LI values or comparing between subjects. Principally, these include the nature of the quantification of left and right hemispheres contributions, localisation of volumes of interest within each hemisphere, dependency on statistical threshold, thresholding LI values, choice of activation and baseline conditions and reproducibility of LI values. This review discusses such methodological factors and the different approaches that have been suggested to deal with them. Although these factors are common to a range of fMRI domains, they are discussed here in the context of fMRI of the language system.
Keywords: Functional MRI, Laterality index, Hemispheric dominance, Language system, Statistical threshold
1. Introduction
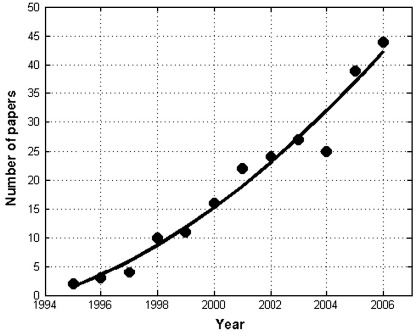
Asymmetric processing of sensory, affective and cognitive information has long been one of the intriguing properties of human brain function [1–5]. Although the two hemispheres are in continual communication with each other, differences between the left (LH) and right (RH) hemispheres have commonly been reported in numerous studies with functional neuroimaging. The emergence of noninvasive functional techniques, such as functional magnetic resonance imaging (fMRI), is now providing a very interesting characterisation of this neural property, both for theoretical or clinical purposes in several domains, including language (e.g., Ref. [6]), vision (e.g., Ref. [7]), audition [8] and memory [9]. Fig. 1 demonstrates that the number of studies investigating hemispheric laterality with fMRI has increased at least linearly over the last few years, as measured by a PubMed search with “fMRI” and “laterality|dominance” as words in the title or abstract of the paper.
Fig. 1.
Number of papers published per year with title or abstract containing “fMRI” AND (“laterality” OR “dominance”).
The most widely studied domain is undoubtedly language. Since the pioneering observations of Paul Broca, left hemisphere dominance is factually assumed for language processing. More recent studies have explored language dominance in populations with different demographic characteristics, including handedness [10,11], age [12–14], gender [15,16], multilinguism [6,17,18] and the presence of diseases [19–22]. Critically, measures of language hemisphere dominance, assessed with fMRI, have been shown to be concordant with those from other techniques, including the clinical Wada test [21,23,24], functional transcranial Doppler ultrasonography [25,26] and neuropsychological tests [27,28]. These findings have supported the usefulness of fMRI for the assessment of language dominance for clinical purposes (e.g., Ref. [29]).
The hemispheric dominance in fMRI is generally indicated by a measure called the laterality index (LI). Other groups have used the term Asymmetry Index (e.g., Refs. [12,14,22]); here, LI is used throughout this review. The major rational for using the LI value is to facilitate the description of hemispheric dominance from functional activation patterns because it is easier to manipulate one value per subject/contrast than thousands of voxels. However, LI assessment depends on several methodological factors that should be taken into account when interpreting LI values or comparing between subjects. Here, I attempt to present a succinct review of such factors and the different approaches that have been suggested to deal with them. These factors are valid for all fMRI domains but will be discussed here in the context of the language domain.
2. The LI formula
Generally, the LI value is computed using the following classic formula [23,24,30]:
| (1) |
where QLH and QRH are representative quantities measured by fMRI for the LH and RH contributions, respectively. The factor f is a scaling factor that defines the range of LI values (i.e., LI varies continuously from −f for pure RH dominance to +f for pure LH dominance). Usually, f is held to 1 (i.e., LI varies between −1 to +1) or 100 (e.g., [31–33]) in which case LI varies from −100 to 100 as a percent ratio measure. Other values like 200 [27,34] or −1 [35] have also been used. Note that this formula was initially defined by assuming that all measures are positive (QLH≥0; QRH≥0; QLH+QRH>0).
Furthermore, it is interesting to examine the linearity and the sensitivity of this formula for representing differences between LH and RH contributions. Theoretically, LI can be related to the ratio between left and right contributions (QLH and QRH, respectively). Specifically, the relative difference R between QLH and QRH can be defined as:
| (2) |
with R∈[−1, +∞[. Accordingly, we can easily express LI as:
| (3) |
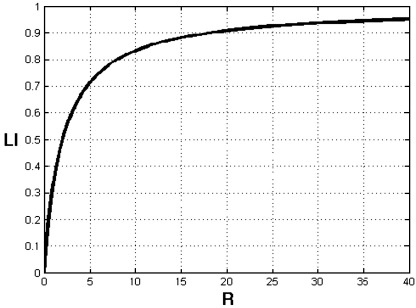
Fig. 2 shows that LI increases approximately linearly with R when R is less than 5 (i.e., QLH less than six times QRH) but saturates towards a plateau for high R values (e.g., R>20). This has an important implication for the sensitivity of the LI measure to differences between LH and RH contributions. For instance, if a subject with two tests (e.g., pre- and postsurgical fMRI evaluation) shows double the increase in LH hemisphere involvement for the first than second test, then this difference will be revealed by the LI value with high sensitivity when QLH increases from two to four times QRH but very low sensitivity when QLH increases from 10 to 20 QRH.
Fig. 2.
LI as a function of the relative difference (R) between QLH and QRH quantities (with f=1).
3. The nature of QLH and QRH
Commonly, LI is assessed by counting the number of voxels that survive a fixed threshold within LH and RH regions of interest (e.g., Refs. [23,30]). Consequently, QLH and QRH are positive quantities. However, some studies have shown that this measure does not adequately reflect the differences between both hemispheres, as intensity differences are not taken into account. For instance, if QLH and QRH are identical, LI will be equal to zero even when voxels in the left hemisphere are statistically higher than those of the right hemisphere. To take these statistical differences into account, other authors have presented alternative measures (for more details, see Refs. [33,36]). Benson et al. [34] used signal change amplitude as a measure for QLH and QRH quantities. Practically, a histogram between the number of voxels N and statistics [−log(p)] is first assessed; then QLH and QRH are set equal to the weighted sum , where −log(p) and N are the mean of the statistics (i.e., probability) and the number of voxels within the ith bin of the histogram, respectively [34]. Therefore, LI can also reflect statistical differences between both hemispheres.
In addition, Fernandez et al. [37] have defined QLH and QRH as a sum of all t values above a predefined individual threshold. Practically, they defined the mean of the 5% most activated voxels within each hemisphere or region of interest (ROI) and then summed the t values of all voxels above 50% of this mean [37,38]. LI was then directly influenced by the t values in each hemisphere. Others have suggested that QLH and QRH can be quantified using the average of correlation coefficients [27], weighted t values [33,39,40], mean signal change [41,42], or statistical F values [43]. Alternatively, Baciu et al. [44] have proposed that QLH and QRH can be statistically compared by directly contrasting between correct “right side images” with “mirror images” (flipped hemispheres) of the same subjects.
One of the problems with using these statistical measures is that QLH and QRH might be negative (e.g., sum of negative t values), leading to some misinterpretation of LI values (e.g., Ref. [36]). In this case, users might modify their statistical threshold or move their regions of interest. It is also possible to employ a modified expression of Eq. (1) to take into account the sign of QLH and QRH quantities, as in the following formula:
| (4) |
Note also that some of these alternative measures for QLH and QRH quantities are not always superior than the standard way (i.e., QLH and QRH set to the number of voxels above a predefined threshold) because they rely on several other factors during LI assessment (for more details, see Refs. [33,36]).
4. LI and ROI selection
Obviously, LI values depend on the cortical volume used for their assessment. While some studies measure QLH and QRH across the whole hemisphere (i.e., global measures), others use ROIs (regional measures). Principally, language studies have focused on ROIs within the inferior frontal gyrus (Broca's area), prefrontal cortex, temporoparietal cortex, middle/superior temporal gyrus, angular gyrus, or fusiform gyrus (e.g., Refs. [38,42,45–47]). Although other studies have suggested that both global and regional ROIs yield concordant LI values (e.g., [38]), LI values with regional ROIs (frontal and temporoparietal) were found to correspond better with Wada scores than LIs with whole hemispheres [47]. Specifically, higher reliability for lateralization was obtained using ROI within the frontal lobe as compared to other temporoparietal regions (e.g., Refs. [46,48]; but see Ref. [42]).
Nevertheless, when computing one LI value per subject, the choice of ROI localisation and volume can lead to different conclusions about language laterality (see illustration in Fig. 3). This is particularly problematic in subjects with crossed language dominance. For example, Jansen et al. [49] reported a healthy normal subject performing a verbal fluency task, with LH dominance for frontal regions and RH dominance for temporal regions. Likewise, Baciu et al. [50] and Ries et al. [51] reported epileptic patients who had a negative LI value (right hemispheric dominance) in the frontal ROI and a positive LI value (left hemispheric dominance) in the temporal ROI.
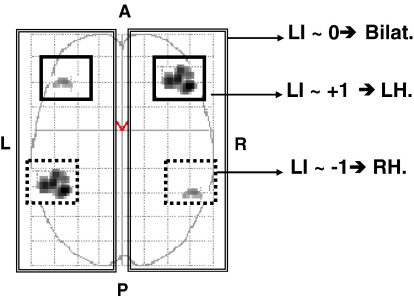
Fig. 3.
Influence of the ROI selection. Global ROI (whole hemisphere), frontal ROI and temporal ROI are illustrated on a schematic activation map.
Furthermore, the inclusion of the cerebellum is also delicate and is generally omitted when using whole hemisphere regions (but see Ref. [33]). This is due to the fact that some language components have a crossed cerebral and cerebellar representations of laterality (e.g., Ref. [52]), which may yield exaggerated bilateral laterality values. In addition, due to their localisation near the interhemispheric fissure, mesial regions are usually not considered when using global or regional ROIs for LI assessment, thereby limiting inferences concerning reorganisation mechanisms (i.e., intra- or interhemispheric) in brain-damaged patients when mesial regions are the best signatures of such mechanisms (e.g., supplementary motor area region, [53,54]).
Purposely, in order to depict a complete picture of language laterality for a given task, it might be more informative to use both regional (i.e., frontal and temporoparietal regions) and global ROIs for LI assessment in each subject.
5. LI threshold for hemispheric dominance
Hemispheric dominance is typically determined by the size of LI compared to a predefined threshold (LITH) according to the following rule:
-
–
LI>LITH, left hemispheric dominance;
-
–
LI<−LITH, right hemispheric dominance;
-
–
|LI|≤LITH, bilateral dominance.
When f is held to 1, the threshold LITH value is usually set to 0.2 [46,55], but values like 0.1 [56], 0.15 [57], 0.25 [10,57] and even 0.3 [31] have also been used in previous work.
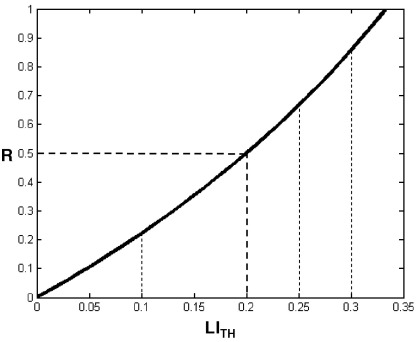
Consequently, language dominance is critically dependent on the LITH value, particularly when LI is compared to other clinical tests such as the Wada (e.g., [48,58]). As shown above in Eq. (3), LITH can be related to the relative ratio R between left and right contributions (QLH and QRH, respectively). In this case, LITH could be chosen according to the desired R value. For instance, for left hemispheric dominance (i.e., LI>LITH), QLH will be at least 50% more than QRH if LITH is set to 0.2. Fig. 4 illustrates the different possible values of LITH relative to R values. When using a fixed LITH, the value 0.2 seems to be reasonable for attributing language dominance.
Fig. 4.
The relative difference (R) as function of the threshold (LITH) on LI values (with f=1).
Instead of using an absolute and fixed LITH value, other groups have proposed variable and adapted LITH values that depend on the task and the group of subjects (e.g., Refs. [41,59]). Practically, all individual LI values are first assessed, and the mean (meanLI) and standard deviation (SDLI) are calculated. Then, the LITH value is set to meanLI−2SDLI if meanLI>0, or to meanLI+2SDLI if meanLI<0. This approach has been shown very useful when comparing patients to control subjects (e.g., is lateralisation in patients and controls identical?) by determining LITH according to the distribution of LI values in control subjects [39,41,60]. However, this approach is only appropriate for tasks with high lateralising power (i.e., high |LI| values) and showing low variability in controls (e.g., 2SDLI<|meanLI|).
6. LI and the statistical threshold
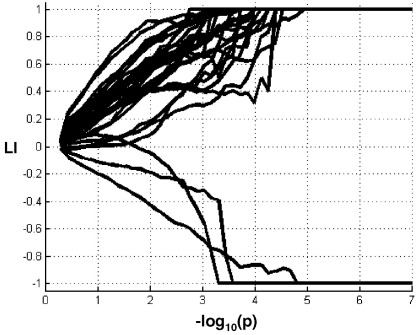
One inherent issue in laterality assessment is the dependency between the statistical threshold and the LI value. This means that the LI value is not unique but can vary with the statistical threshold. Generally, the quantities QLH and QRH are determined at a specific threshold (e.g., number of activated voxels at P<.001, uncorrected, or P<.05, corrected). Different ways have been proposed to take into account the influence of statistical threshold on LI. A simple way is to compute LI at several thresholds [33,41,61]. However, several LI values per subject may not be easy to interpret. A more general approach is to assess the curves of LI(P) (i.e., LI as a function of the statistical threshold P) [23,46,59,62]. Fig. 5 illustrates LI(P) curves in 30 healthy subjects during a semantic decision task relative to perceptual matching, evidently with bilateral dominance (i.e., LI≅0) for low statistical thresholds and high LIs (in absolute value) for conservative thresholds. Using these curves, some authors have tried to find the best representative threshold to assess LI, for instance, by choosing a statistical threshold between the starting period (LI towards 0) and the plateau (LI towards ±1) (e.g., Refs. [23,59].
Fig. 5.
LI as a function of the threshold P (with f=1). Illustration with 30 subjects performing a semantic task.
Moreover, Nagata et al. [62] have presented an alternative way to minimise the influence of the threshold on LI assessment. The idea is to identify empirically the dependency of the quantities QLH and QRH with the threshold (e.g., z score, t or P value) and then search for the best regression function to fit such dependency. For example, with individual t maps, we can write LI as:
| (5) |
where A, B, α and β are constant values. By assuming that α and β are equal and fixed to a typical value of −4 [62], coefficients A and B are then determined by nonlinear regression, and thus, the LI value will be independent from the statistical threshold. Other values for α and β between −3 and −8 are also plausible depending on the data set and the contrast used for LI assessment. On the other hand, the quantities QLH and QRH may show different relationships with the threshold in some data sets (i.e., α and β are very different). In this case, other methods can alternatively be used (see below). Note that the regression function may depend on the statistical interval (e.g., range of t values) used during curve fitting, and it is critical to keep the same statistical interval during nonlinear regression when comparing LI between tasks or subjects.
In the same way, Branco et al. [39] have proposed a similar way to assess LI independently from the threshold using the estimated distribution of weighted t values in specific ROIs. This approach has been suggested to be less variable even in patients. Recently, a combined bootstrap/histogram analysis approach was proposed to generate threshold-free LI values [40]. By performing bootstrap analysis at different statistical thresholds within predefined ROIs, this study showed that LI assessment was more robust and stable compared to standard methods [40]. According to these reports, it is recommended to use one of these approaches that allow the influence of the statistical threshold on LI assessment to be minimised [39,40,62].
Alternatively, the threshold can be defined not on the statistical height but on the extent of activation [63]. In this case, for each subject, the threshold is adapted to obtain a certain predefined number of voxels and then LI is computed on these selected voxels (e.g., Refs. [36,64]). However, as the optimal number of voxels for a given task is usually unknown, the utility of this extent-based approach is limited.
7. LI and task selection
LI depends on the task chosen, even within a domain such as language (e.g., Refs. [36,59,61]). Thus, to obtain the best estimation of LI, previous studies have employed several tasks in the same subjects and then assessed LI for each task. In this way, each subject is characterised not only by one LI value but with a vector of LI values, which may be pertinent from a clinical perspective. On the other hand, clinicians may also be interested in estimating the best representative LI value over tasks for a given subject, particularly when confronting the LI value with other clinical or behavioural measures. In this case, an alternative approach is to analyse all tasks conjointly and combine these measures into one representative LI value. This combined task analysis approach, proposed by Ramsey et al. [61], was initially used with verb generation, synonyms generation, and categorisation tasks. Results indicated the strong lateralisation power of this approach and an improvement in the detection of language areas [61,65].
Several factors related to task selection may also influence language laterality. For instance, it has been suggested that task repetition can affect fMRI-based measures of language lateralization and lead to pseudoincreases in bilaterality [66]. Moreover, task difficulty can also affect the normal activity patterns with language paradigms (e.g., Refs. [67–70]), thereby perturbing language laterality particularly when LI is calculated across whole hemispheres. The input modality, visual or auditory, might also influence the hemispheric distribution of activated regions (e.g., Refs. [71,72], but see Ref. [73]). For instance, in healthy subjects and epileptic patients, LI values were found to be significantly stronger for visual than for auditory task presentation [74]. Other factors, including the use of words or pictures, silent or overt tasks, passive or active tasks and receptive and expressive language tasks, should also be taken into account when assessing LI within and between groups.
8. LI and baseline condition
Discrepancies in laterality assessment across studies may be attributable, in part, to differences in baseline tasks. The subtractive logic used in functional neuroimaging attributes the same “influence” to baseline and activation conditions. Different studies have emphasized the importance of baseline selection when interpreting functional maps (e.g., Refs. [75–77]). For instance, when a phoneme discrimination task was compared to three baseline conditions (rest, tone monitoring and passive listening), the baseline conditions systematically affected the amount of activation in different language areas [78], demonstrating different LI values with different baseline conditions. In addition, a recent study has investigated the most appropriate baseline task (picture naming, or passive viewing of nonsense objects) to isolate syntactic processes [79]. This showed that activity in language areas (e.g., Broca's area) was dependant on the baseline used, with more activation in these areas when the baseline was passive viewing of nonsense objects than picture naming. Moreover, using a low-level baseline condition (e.g., rest or fixation condition) may lead to LI values near to zero (i.e., more bilateral functional patterns), whereas a perceptual baseline may lead to higher LI values due to the elimination of nonrelevant regions involved in early perceptual and sensory processing [73]. Accordingly, control conditions that are close to the activation task might be more appropriate when nonrelevant low level areas need to be excluded from LI assessment [80]. Ideally, control conditions should have bilateral activation patterns (i.e., |LI|≅0) because if control activation has for example a strong RH dominance, then the activation task might be artificially lateralised to the LH (LI towards +1 as all right activations have been removed by the control condition).
9. Variability and reproducibility of LI
LI values are not absolute measures but can present some degree of variability across sessions and subjects. This is obviously related to the commonly observed variability of functional maps with language paradigms (e.g., Refs. [81,82]). Particularly, in the context of longitudinal fMRI studies, assessing the reliability and the reproducibility of LI values is imperative for the usefulness of such measures. For this aim, different studies have attempted to quantify the reproducibility of LI measures; however, as detailed above, these assessments are inherently related to the task used, the statistical threshold and the localisation of the regions of interest (e.g., Ref. [36]). For instance, recently, by computing laterality within inferior frontal and temporoparietal regions of interest for six different receptive and expressive language tasks, LI values were shown to be reproducible across sessions in both inferior frontal and temporoparietal regions [43]. In this study, the reproducibility of LI values was higher for (i) verb generation tasks, (ii) inferior frontal ROIs and (iii) QLH and QRH computed with F statistics rather than number of voxels [43]. In addition, for a wide range of statistical thresholds, LI values were compared across sessions, tasks, subjects and two a priori defined volumes of interest (classical language regions versus whole hemisphere) [65]. LI values were found to be reproducible for verb generation tasks and combined task analysis, with higher reproducibility when using regions of interest centred on language areas [65]. Moreover, LI reproducibility within and between session has been explored in a group of patients undergoing evaluation for epilepsy surgery [38]. LI had higher reliability within and across sessions when using one global (whole hemisphere) and three regional regions of interest (Broca's area, remaining prefrontal cortex, temporoparietal area).
10. Other considerations
Although it is possible to minimise the influence of these methodological factors with carefully conducted experiments, it is important to consider additional issues that might compromise the meaningfulness of the LI measure. Critically, the LI value is globally related to haemodynamic changes (i.e., physiological nature) detected by fMRI. Recently, Krach et al. [83] have shown that differences might exist between behavioural and physiological indicators of laterality, which might be problematic in some circumstances when confronting LI values with behavioural scores in patients. In addition, the risk of alteration of fMRI signal in the presence of brain insult (e.g., [84,85]) can lead to errors during the assessment of the quantities QLH and QRH. Furthermore, the LI measure should be considered as representative of one facet of hemispheric dominance that is mainly related to gray matter activity. Other interesting facets are thus not taken into account in the LI measure, including, for instance, laterality of white matter pathways [86,87] and functional connectivity (e.g., Ref. [88]).
11. Conclusion
This review has highlighted the importance of controlling different methodological factors in order to ensure meaningful LI values. Without taking into account these factors, the LI measure might be seriously misguiding, which has, in some situations, discouraged some groups to use it and to rely on pure visual laterality rating (e.g., Refs. [14,89]). Critically, the quantification of QLH and QRH, localisation of ROIs, task selection, the dependency on the statistical threshold and reproducibility are major issues that should be considered when using LI as a clinical measure for hemispheric dominance. I believe such factors warrant further investigations in order to establish a valid and unified methodological protocol for LI assessment with fMRI in different sensory and cognitive domains.
Acknowledgments
The author would like to thank Cathy Price for her valuable suggestions on the manuscript.
Footnotes
This work was funded by the Wellcome Trust.
References
- 1.Strauss E., Kosaka B., Wada J. The neurobiological basis of lateralized cerebral function. A review. Hum Neurobiol. 1983;2:115–127. [PubMed] [Google Scholar]
- 2.Galaburda A.M., Rosen G.D., Sherman G.F. Individual variability in cortical organization: its relationship to brain laterality and implications to function. Neuropsychologia. 1990;28:529–546. doi: 10.1016/0028-3932(90)90032-j. [DOI] [PubMed] [Google Scholar]
- 3.Hugdahl K., Davidson R.J., editors. The asymmetrical brain. MIT Press; Cambridge (MA): 2002. [Google Scholar]
- 4.Hutsler J., Galuske R.A. Hemispheric asymmetries in cerebral cortical networks. Trends Neurosci. 2003;26:429–435. doi: 10.1016/S0166-2236(03)00198-X. [DOI] [PubMed] [Google Scholar]
- 5.Springer S.P., Deutsch G. Freeman; New York: 1998. Left brain, right brain: perspectives from cognitive neuroscience. [Google Scholar]
- 6.Hull R., Vaid J. Laterality and language experience. Laterality. 2006;11:436–464. doi: 10.1080/13576500600691162. [DOI] [PubMed] [Google Scholar]
- 7.Seghier M.L., Vuilleumier P. Functional neuroimaging findings on the human perception of illusory contours. Neurosci Biobehav Rev. 2006;30:595–612. doi: 10.1016/j.neubiorev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Tervaniemi M., Hugdahl K. Lateralization of auditory-cortex functions. Brain Res Rev. 2003;43:231–246. doi: 10.1016/j.brainresrev.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Detre J.A., Maccotta L., King D.W., Alsop D.C., Glosser G., D'Esposito M. Functional MRI lateralization of memory in temporal lobe epilepsy. Neurology. 1998;50:926–932. doi: 10.1212/wnl.50.4.926. [DOI] [PubMed] [Google Scholar]
- 10.Pujol J., Deus J., Losilla J.M., Capdevila A. Cerebral lateralization of language in normal left-handed people studied by functional MRI. Neurology. 1999;52:1038–1043. doi: 10.1212/wnl.52.5.1038. [DOI] [PubMed] [Google Scholar]
- 11.Szaflarski J.P., Binder J.R., Possing E.T., McKiernan K.A., Ward B.D., Hammeke T.A. Language lateralization in left-handed and ambidextrous people: fMRI data. Neurology. 2002;59:238–244. doi: 10.1212/wnl.59.2.238. [DOI] [PubMed] [Google Scholar]
- 12.Ahmad Z., Balsamo L.M., Sachs B.C., Xu B., Gaillard W.D. Auditory comprehension of language in young children: neural networks identified with fMRI. Neurology. 2003;60:1598–1605. doi: 10.1212/01.wnl.0000059865.32155.86. [DOI] [PubMed] [Google Scholar]
- 13.Szaflarski J.P., Holland S.K., Schmithorst V.J., Byars A.W. fMRI study of language lateralization in children and adults. Hum Brain Mapp. 2006;27:202–212. doi: 10.1002/hbm.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wood A.G., Harvey A.S., Wellard R.M., Abbott D.F., Anderson V., Kean M. Language cortex activation in normal children. Neurology. 2004;63:1035–1044. doi: 10.1212/01.wnl.0000140707.61952.ca. [DOI] [PubMed] [Google Scholar]
- 15.Frost J.A., Binder J.R., Springer J.A., Hammeke T.A., Bellgowan P.S., Rao S.M. Language processing is strongly left lateralized in both sexes. Evidence from functional MRI. Brain. 1999;122:199–208. doi: 10.1093/brain/122.2.199. [DOI] [PubMed] [Google Scholar]
- 16.Plante E., Schmithorst V.J., Holland S.K., Byars A.W. Sex differences in the activation of language cortex during childhood. Neuropsychologia. 2006;44:1210–1221. doi: 10.1016/j.neuropsychologia.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 17.Vingerhoets G., Van Borsel J., Tesink C., van den Noort M., Deblaere K., Seurinck R. Multilingualism: an fMRI study. Neuroimage. 2003;20:2181–2196. doi: 10.1016/j.neuroimage.2003.07.029. [DOI] [PubMed] [Google Scholar]
- 18.Briellmann R.S., Saling M.M., Connell A.B., Waites A.B., Abbott D.F., Jackson G.D. A high-field functional MRI study of quadri-lingual subjects. Brain Lang. 2004;89:531–542. doi: 10.1016/j.bandl.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Sommer I.E., Ramsey N.F., Kahn R.S. Language lateralisation in schizophrenia, an fMRI study. Schizophr Res. 2001;52:57–67. doi: 10.1016/s0920-9964(00)00180-8. [DOI] [PubMed] [Google Scholar]
- 20.Billingsley R.L., McAndrews M.P., Crawley A.P., Mikulis D.J. Functional MRI of phonological and semantic processing in temporal lobe epilepsy. Brain. 2001;124:1218–1227. doi: 10.1093/brain/124.6.1218. [DOI] [PubMed] [Google Scholar]
- 21.Woermann F.G., Jokeit H., Luerding R., Freitag H., Schulz R., Guertler S. Language lateralization by Wada test and fMRI in 100 patients with epilepsy. Neurology. 2003;61:699–701. doi: 10.1212/01.wnl.0000078815.03224.57. [DOI] [PubMed] [Google Scholar]
- 22.Anderson D.P., Harvey A.S., Saling M.M., Anderson V., Kean M., Abbott D.F. FMRI lateralization of expressive language in children with cerebral lesions. Epilepsia. 2006;47:998–1008. doi: 10.1111/j.1528-1167.2006.00572.x. [DOI] [PubMed] [Google Scholar]
- 23.Binder J.R., Swanson S.J., Hammeke T.A., Morris G.L., Mueller W.M., Fischer M. Determination of language dominance using functional MRI: a comparison with the Wada test. Neurology. 1996;46:978–984. doi: 10.1212/wnl.46.4.978. [DOI] [PubMed] [Google Scholar]
- 24.Desmond J.E., Sum J.M., Wagner A.D., Demb J.B., Shear P.K., Glover G.H. Functional MRI measurement of language lateralization in Wada-tested patients. Brain. 1995;118:1411–1419. doi: 10.1093/brain/118.6.1411. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt P., Krings T., Willmes K., Roessler F., Reul J., Thron A. Determination of cognitive hemispheric lateralization by “functional” transcranial Doppler cross-validated by functional MRI. Stroke. 1999;30:939–945. doi: 10.1161/01.str.30.5.939. [DOI] [PubMed] [Google Scholar]
- 26.Deppe M., Knecht S., Papke K., Lohmann H., Fleischer H., Heindel W. Assessment of hemispheric language lateralization: a comparison between fMRI and fTCD. J Cereb Blood Flow Metab. 2000;20:263–268. doi: 10.1097/00004647-200002000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Fernandes M.A., Smith M.L., Logan W., Crawley A., McAndrews M.P. Comparing language lateralization determined by dichotic listening and fMRI activation in frontal and temporal lobes in children with epilepsy. Brain Lang. 2006;96:106–114. doi: 10.1016/j.bandl.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Hund-Georgiadis M., Lex U., Friederici A.D., von Cramon D.Y. Non-invasive regime for language lateralization in right- and left-handers by means of functional MRI and dichotic listening. Exp Brain Res. 2002;145:166–176. doi: 10.1007/s00221-002-1090-0. [DOI] [PubMed] [Google Scholar]
- 29.Medina L.S., Bernal B., Ruiz J. Role of functional MR in determining language dominance in epilepsy and nonepilepsy populations: a Bayesian analysis. Radiology. 2007;242:94–100. doi: 10.1148/radiol.2421050677. [DOI] [PubMed] [Google Scholar]
- 30.Hinke R.M., Hu X., Stillmann A.E. Functional magnetic resonance imaging of Broca's area during internal speech. Neuroreport. 1993;4:675–678. doi: 10.1097/00001756-199306000-00018. [DOI] [PubMed] [Google Scholar]
- 31.Benbadis S.R., Binder J.R., Swanson S.J., Fischer M., Hammeke T.A., Morris G.L. Is speech arrest during Wada testing a valid method for determining hemispheric representation of language? Brain Lang. 1998;65:441–446. doi: 10.1006/brln.1998.2018. [DOI] [PubMed] [Google Scholar]
- 32.van der Kallen B.F., Morris G.L., Yetkin F.Z., van Erning L.J., Thijssen H.O., Haughton V.M. Hemispheric language dominance studied with functional MR: preliminary study in healthy volunteers and patients with epilepsy. AJNR Am J Neuroradiol. 1998;19:173–177. [PMC free article] [PubMed] [Google Scholar]
- 33.Chlebus P., Mikl M., Brazdil M., Pazourkova M., Krupa P., Rektor I. fMRI evaluation of hemispheric language dominance using various methods of laterality index calculation. Exp Brain Res. 2007;179:365–374. doi: 10.1007/s00221-006-0794-y. [DOI] [PubMed] [Google Scholar]
- 34.Benson R.R., FitzGerald D.B., LeSueur L.L., Kennedy D.N., Kwong K.K., Buchbinder B.R. Language dominance determined by whole-brain functional MRI patients with brain lesions. Neurology. 1999;52:798–809. doi: 10.1212/wnl.52.4.798. [DOI] [PubMed] [Google Scholar]
- 35.Fernandez B., Cardebat D., Demonet J.F., Joseph P.A., Mazaux J.M., Barat M. Functional MRI follow-up of language processes in health subjects and during recovery in a case of aphasia. Stroke. 2004;35:2171–2176. doi: 10.1161/01.STR.0000139323.76769.b0. [DOI] [PubMed] [Google Scholar]
- 36.Jansen A., Menke R., Sommer J., Forster A.F., Bruchmann S., Hempleman J. The assessment of hemispheric lateralization in functional MRI — robustness and reproducibility. Neuroimage. 2006;33:204–217. doi: 10.1016/j.neuroimage.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 37.Fernandez G., de Greiff A., von Oertzen J., Reuber M., Lun S., Klaver P. Language mapping in less than 15 minutes: real-time functional MRI during routine clinical investigation. Neuroimage. 2001;14:585–594. doi: 10.1006/nimg.2001.0854. [DOI] [PubMed] [Google Scholar]
- 38.Fernandez G., Specht K., Weis S., Tendolkar I., Reuber M., Fell J. Intrasubject reproducibility of presurgical language lateralization and mapping using fMRI. Neurology. 2003;60:969–975. doi: 10.1212/01.wnl.0000049934.34209.2e. [DOI] [PubMed] [Google Scholar]
- 39.Branco D.M., Suarez R.O., Whalen S., O'Shea J.P., Nelson A.P., da Costa J.C. Functional MRI of memory in the hippocampus: laterality indices may be more meaningful if calculated from whole voxel distributions. Neuroimage. 2006;32:592–602. doi: 10.1016/j.neuroimage.2006.04.201. [DOI] [PubMed] [Google Scholar]
- 40.Wilke M., Schmithorst V.J. A combined bootstrap/histogram analysis approach for computing a lateralization index from neuroimaging data. Neuroimage. 2006;33:522–530. doi: 10.1016/j.neuroimage.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 41.Adcock J.E., Wise R.G., Oxbury J.M., Oxbury S.M., Matthews P.M. Quantitative fMRI assessment of the differences in lateralization of language-related brain activation in patients with temporal lobe epilepsy. Neuroimage. 2003;18:423–438. doi: 10.1016/s1053-8119(02)00013-7. [DOI] [PubMed] [Google Scholar]
- 42.Bethmann A., Tempelmann C., de Bleser R., Scheich H., Brechmann A. Determining language laterality by fMRI and dichotic listening. Brain Res. 2007;1133:145–157. doi: 10.1016/j.brainres.2006.11.057. [DOI] [PubMed] [Google Scholar]
- 43.Harrington G.S., Buonocore M.H., Farias S.T. Intrasubject reproducibility of functional MR imaging activation in language tasks. AJNR Am J Neuroradiol. 2006;27:938–944. [PMC free article] [PubMed] [Google Scholar]
- 44.Baciu M., Juphard A., Cousin E., Bas J.F. Evaluating fMRI methods for assessing hemispheric language dominance in healthy subjects. Eur J Radiol. 2005;55:209–218. doi: 10.1016/j.ejrad.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 45.Harrington G.S., Tomaszewski Farias S., Buonocore M.H., Yonelinas A.P. The intersubject and intrasubject reproducibility of FMRI activation during three encoding tasks: implications for clinical applications. Neuroradiology. 2006;48:495–505. doi: 10.1007/s00234-006-0083-2. [DOI] [PubMed] [Google Scholar]
- 46.Deblaere K., Boon P.A., Vandemaele P., Tieleman A., Vonck K., Vingerhoets G. MRI language dominance assessment in epilepsy patients at 1.0 T: region of interest analysis and comparison with intracarotid amytal testing. Neuroradiology. 2004;46:413–420. doi: 10.1007/s00234-004-1196-0. [DOI] [PubMed] [Google Scholar]
- 47.Spreer J., Arnold S., Quiske A., Wohlfarth R., Ziyeh S., Altenmuller D. Determination of hemisphere dominance for language: comparison of frontal and temporal fMRI activation with intracarotid amytal testing. Neuroradiology. 2002;44:467–474. doi: 10.1007/s00234-002-0782-2. [DOI] [PubMed] [Google Scholar]
- 48.Lehéricy S., Cohen L., Bazin B., Samson S., Giacomini E., Rougetet R. Function MR evaluation of temporal and frontal language dominance compared with the Wada test. Neurology. 2000;54:1625–1633. doi: 10.1212/wnl.54.8.1625. [DOI] [PubMed] [Google Scholar]
- 49.Jansen A., Deppe M., Schwindt W., Mohammadi S., Sehlmeyer C., Knecht S. Interhemispheric dissociation of language regions in a healthy subject. Arch Neurol. 2006;63:1344–1346. doi: 10.1001/archneur.63.9.1344. [DOI] [PubMed] [Google Scholar]
- 50.Baciu M.V., Watson J.M., McDermott K.B., Wetzel R.D., Attarian H., Moran C.J. Functional MRI reveals an interhemispheric dissociation of frontal and temporal language regions in a patient with focal epilepsy. Epilepsy Behav. 2003;4:776–780. doi: 10.1016/j.yebeh.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 51.Ries M.L., Boop F.A., Griebel M.L., Zou P., Phillips N.S., Johnson S.C. Functional MRI and Wada determination of language lateralization: a case of crossed dominance. Epilepsia. 2004;45:85–89. doi: 10.1111/j.0013-9580.2004.04403.x. [DOI] [PubMed] [Google Scholar]
- 52.Hubrich-Ungureanu P., Kaemmerer N., Henn F.A., Braus D.F. Lateralized organization of the cerebellum in a silent verbal fluency task: a functional magnetic resonance imaging study in healthy volunteers. Neurosci Lett. 2002;319:91–94. doi: 10.1016/s0304-3940(01)02566-6. [DOI] [PubMed] [Google Scholar]
- 53.Krainik A., Duffau H., Capelle L., Cornu P., Boch A.L., Mangin J.F. Role of the healthy hemisphere in recovery after resection of the supplementary motor area. Neurology. 2004;62:1323–1332. doi: 10.1212/01.wnl.0000120547.83482.b1. [DOI] [PubMed] [Google Scholar]
- 54.Karbe H., Thiel A., Weber-Luxenburger G., Herholz K., Kessler J., Heiss W.-D. Brain plasticity in poststroke aphasia: what the contribution of the right hemisphere? Brain Lang. 1998;64:215–230. doi: 10.1006/brln.1998.1961. [DOI] [PubMed] [Google Scholar]
- 55.Springer J.A., Binder J.R., Hammeke T.A., Swanson S.J., Frost J.A., Bellgowan P.S. Language dominance in neurologically normal and epilepsy subjects: a functional MRI study. Brain. 1999;122:2033–2046. doi: 10.1093/brain/122.11.2033. [DOI] [PubMed] [Google Scholar]
- 56.Yuan W., Szaflarski J.P., Schmithorst V.J., Schapiro M., Byars A.W., Strawsburg R.H. fMRI shows atypical language lateralization in pediatric epilepsy patients. Epilepsia. 2006;47:593–600. doi: 10.1111/j.1528-1167.2006.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baciu M.V., Watson J.M., Maccotta L., McDermott K.B., Buckner R.L., Gilliam F.G. Evaluating functional MRI procedures for assessing hemispheric language dominance in neurosurgical patients. Neuroradiology. 2005;47:835–844. doi: 10.1007/s00234-005-1431-3. [DOI] [PubMed] [Google Scholar]
- 58.Baciu M., Kahane P., Minotti L., Charnallet A., David D., Le Bas J.F. Functional MRI assessment of the hemispheric predominance for language in epileptic patients using a simple rhyme detection task. Epileptic Disord. 2001;3:117–124. [PubMed] [Google Scholar]
- 59.Seghier M.L., Lazeyras F., Pegna A.J., Annoni J.M., Zimine I., Mayer E. Variability of fMRI activation during a phonological and semantic language task in healthy subjects. Hum Brain Mapp. 2004;23:140–155. doi: 10.1002/hbm.20053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khateb A., Martory M.-D., Annoni J.-M., Lazeyras F., de Tribolet N., Pegna A.J. Transient crossed aphasia evidenced by functional brain imagery. Neuroreport. 2004;15:785–790. doi: 10.1097/00001756-200404090-00009. [DOI] [PubMed] [Google Scholar]
- 61.Ramsey N.F., Sommer I.E.C., Rutten G.J., Kahn R.S. Combined analysis of language tasks in fMRI improves assessment of hemispheric dominance for language functions in individual subjects. Neuroimage. 2001;13:719–733. doi: 10.1006/nimg.2000.0722. [DOI] [PubMed] [Google Scholar]
- 62.Nagata S., Uchimura K., Hirakawa W., Kuratsu J. Method for quantitatively evaluating the lateralization of linguistic function using functional MR imaging. AJNR Am J Neuroradiol. 2001;22:985–991. [PMC free article] [PubMed] [Google Scholar]
- 63.Moser E., Teichtmeister C., Diemling M. Reproducibility and postprocessing of gradient-echo fMRI to improve localization of brain activity in the human visual cortex. Magn Reson Imaging. 1996;14:567–579. doi: 10.1016/0730-725x(96)00095-1. [DOI] [PubMed] [Google Scholar]
- 64.Knecht S., Jansen A., Frank A., van Randenborgh J., Sommer J., Kanowski M. How atypical is atypical language dominance? Neuroimage. 2003;18:917–927. doi: 10.1016/s1053-8119(03)00039-9. [DOI] [PubMed] [Google Scholar]
- 65.Rutten G.J., Ramsey N.F., van Rijen P.C., van Veelen C.W. Reproducibility of fMRI-determined language lateralization in individual subjects. Brain Lang. 2002;80:421–437. doi: 10.1006/brln.2001.2600. [DOI] [PubMed] [Google Scholar]
- 66.Lohmann H., Deppe M., Jansen A., Schwindt W., Knecht S. Task repetition can affect functional magnetic resonance imaging-based measures of language lateralization and lead to pseudoincreases in bilaterality. J Cereb Blood Flow Metab. 2004;24:179–187. doi: 10.1097/01.WCB.0000100066.36077.91. [DOI] [PubMed] [Google Scholar]
- 67.Just M.A., Carpenter P.A., Keller T.A., Eddy W.F., Thulborn K.R. Brain activation modulated by sentence comprehension. Science. 1996;274:114–116. doi: 10.1126/science.274.5284.114. [DOI] [PubMed] [Google Scholar]
- 68.Fridriksson J., Morrow L. Cortical activation and language task difficulty in aphasia. Aphasiology. 2005;19:239–250. doi: 10.1080/02687030444000714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Desai R., Conant L.L., Waldron E., Binder J.R. FMRI of past tense processing: the effects of phonological complexity and task difficulty. J Cogn Neurosci. 2006;18:278–297. doi: 10.1162/089892906775783633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Drager B., Jansen A., Bruchmann S., Forster A.F., Pleger B., Zwitserlood P. How does the brain accommodate to increased task difficulty in word finding? A functional MRI study. Neuroimage. 2004;23:1152–1160. doi: 10.1016/j.neuroimage.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 71.Chee M.W., O'Craven K.M., Bergida R., Rosen B.R., Savoy R.L. Auditory and visual word processing studied with fMRI. Hum Brain Mapp. 1999;7:15–28. doi: 10.1002/(SICI)1097-0193(1999)7:1<15::AID-HBM2>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Burton M.W., Locasto P.C., Krebs-Noble D., Gullapalli R.P. A systematic investigation of the functional neuroanatomy of auditory and visual phonological processing. Neuroimage. 2005;26:647–661. doi: 10.1016/j.neuroimage.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 73.Hund-Georgiadis M., Lex U., von Cramon Y. Language dominance assessment by means of fMRI: contributions from task design, performance, and stimulus modality. J Magn Reson Imaging. 2001;13:668–675. doi: 10.1002/jmri.1094. [DOI] [PubMed] [Google Scholar]
- 74.Carpentier A., Pugh K.R., Westerveld M., Studholme C., Skrinjar O., Thompson J.L. Functional MRI of language processing: dependence on input modality and temporal lobe epilepsy. Epilepsia. 2001;42:1241–1254. doi: 10.1046/j.1528-1157.2001.35500.x. [DOI] [PubMed] [Google Scholar]
- 75.Stark C.E., Squire L.R. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci U S A. 2001;98:12760–12766. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gusnard D.A., Raichle M.E. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 77.Binder J.R., Frost J.A., Hammeke T.A., Bellgowan P.S., Rao S.M., Cox R.W. Conceptual processing during the conscious resting state. A functional MRI study. J Cogn Neurosci. 1999;11:80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- 78.Newman S.D., Tweig D.B., Carpenter P.A. Baseline conditions and subtractive logic in neuroimaging. Hum Brain Mapp. 2001;14:228–235. doi: 10.1002/hbm.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peck K.K., Wierenga C.E., Moore A.B., Maher L.M., Gopinath K., Gaiefsky M. Comparison of baseline conditions to investigate syntactic production using functional magnetic resonance imaging. Neuroimage. 2004;23:104–110. doi: 10.1016/j.neuroimage.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 80.Brandt T. How to see what you are looking for in fMRI and PET — or the crucial baseline condition. J Neurol. 2006;253:551–555. doi: 10.1007/s00415-006-0087-1. [DOI] [PubMed] [Google Scholar]
- 81.Brannen J.H., Badie B., Moritz C.H., Quigley M., Meyerand M.E., Haughton V.M. Reliability of functional MR imaging with word-generation tasks for mapping Broca's area. AJNR Am J Neuroradiol. 2001;22:1711–1718. [PMC free article] [PubMed] [Google Scholar]
- 82.Otzenberger H., Gounot D., Marrer C., Namer I.J., Metz-Lutz M.N. Reliability of individual functional MRI brain mapping of language. Neuropsychology. 2005;19:484–493. doi: 10.1037/0894-4105.19.4.484. [DOI] [PubMed] [Google Scholar]
- 83.Krach S., Chen L.M., Hartje W. Comparison between visual half-field performance and cerebral blood flow changes as indicators of language dominance. Laterality. 2006;11:122–140. doi: 10.1080/13576500500384975. [DOI] [PubMed] [Google Scholar]
- 84.Roux F.E., Boulanouar K., Lotterie J.A., Mejdoubi M., LeSage J.P., Berry I. Language functional magnetic resonance imaging in preoperative assessment of language areas: correlation with direct cortical stimulation. Neurosurgery. 2003;52:1335–1345. doi: 10.1227/01.neu.0000064803.05077.40. [DOI] [PubMed] [Google Scholar]
- 85.Jayakar P., Bernal B., Santiago Medina L., Altman N. False lateralization of language cortex on functional MRI after a cluster of focal seizures. Neurology. 2002;58:490–492. doi: 10.1212/wnl.58.3.490. [DOI] [PubMed] [Google Scholar]
- 86.Barrick T.R., Lawes I.N., Mackay C.E., Clark C.A. White matter pathway asymmetry underlies functional lateralization. Cereb Cortex. 2007;17:591–598. doi: 10.1093/cercor/bhk004. [DOI] [PubMed] [Google Scholar]
- 87.Powell H.W., Parker G.J.M., Alexander D.C., Symms M.R., Boulby P.A., Wheeler-Kingshott C.A.M. Hemispheric asymmetries in language-related pathways: a combined functional MRI and tractography study. Neuroimage. 2006;32:388–399. doi: 10.1016/j.neuroimage.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 88.Stephan K.E., Fink G.R., Marshall J.C. Mechanisms of hemispheric specialization: insights from analyses of connectivity. Neuropsychologia. 2007;45:209–228. doi: 10.1016/j.neuropsychologia.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Benke T., Koylu B., Visani P., Karner E., Brenneis C., Bartha L. Language lateralization in temporal lobe epilepsy: a comparison between fMRI and the Wada Test. Epilepsia. 2006;47:1308–1319. doi: 10.1111/j.1528-1167.2006.00549.x. [DOI] [PubMed] [Google Scholar]