Abstract
When attacked by herbivorous insects, plants emit volatile compounds that attract natural enemies of the insects. It has been proposed that these volatile signals can be manipulated to improve crop protection. Here, we demonstrate the full potential of this strategy by restoring the emission of a specific belowground signal emitted by insect-damaged maize roots. The western corn rootworm induces the roots of many maize varieties to emit (E)-β-caryophyllene, which attracts entomopathogenic nematodes that infect and kill the voracious root pest. However, most North American maize varieties have lost the ability to emit (E)-β-caryophyllene and may therefore receive little protection from the nematodes. To restore the signal, a nonemitting maize line was transformed with a (E)-β-caryophyllene synthase gene from oregano, resulting in constitutive emissions of this sesquiterpene. In rootworm-infested field plots in which nematodes were released, the (E)-β-caryophyllene-emitting plants suffered significantly less root damage and had 60% fewer adult beetles emerge than untransformed, nonemitting lines. This demonstration that plant volatile emissions can be manipulated to enhance the effectiveness of biological control agents opens the way for novel and ecologically sound strategies to fight a variety of insect pests.
Keywords: tritrophic interaction, biological control, transgenic crops, Zea mays, entomopathogenic nematodes
Plants synthesize and emit blends of volatile organic compounds in response to damage from herbivorous arthropods (1). The induced volatiles are proposed to serve a variety of physiological and ecological functions (2), including the attraction of natural enemies of herbivores, which is termed “indirect defense” (3–7). Recent advances in plant biotechnology have allowed investigators to manipulate plant volatile emissions and demonstrate their defensive function in laboratory studies with model plants (8–11). It has been suggested that these same advances should also be applicable to crop plants and help enhance specific volatile signals to increase the effectiveness of natural enemies in reducing damage from herbivore pests (12–15).
The western corn rootworm (WCR), Diabrotica virgifera virgifera LeConte (Coleoptera: Chrysomelidae), is the most severe pest of maize in the United States. It was introduced into Europe ≈15 years ago, where it is now a serious problem in the Balkan region and is rapidly spreading to other countries (16, 17). In areas without crop rotation, WCR is exceedingly difficult to control, and pesticide applications are relatively expensive, environmentally unfriendly, and not always effective (18). The use of genetically modified maize lines that carry bacterial-derived genes coding for Bt toxins shows promise (19–21), but resistance traits are likely to develop (22). Biological control of WCR with entomopathogenic nematodes (EPNs) could provide an effective and more sustainable alternative.
(E)-β-Caryophyllene (EβC), a sesquiterpene olefin, is emitted by the roots of maize (Zea mays L.) in response to feeding by larvae of WCR (23). EβC diffuses readily in the gaseous phase of sand and soil (24) and is highly attractive to the EPN Heterorhabditis megidis (Poinar) (Rhabditida: Heterorhabditidae), which parasitizes and kills WCR larvae within a few days (23, 25). Attempts to control WCR with nematodes have been largely ineffective or required extraordinary large numbers of nematodes (26, 27). A possible explanation for these failures is the inability of most North American maize lines to emit EβC (23, 28). We recently identified the caryophyllene synthase gene tps23, which is responsible for EβC production in maize. North American maize lines possess a fully functional tps23, but EβC production is absent because of the lack of tps23 transcript (29). The absence of EβC seems to dramatically reduce attraction of nematodes, as was apparent from a field experiment in which we found a 5-fold higher infection by nematodes near a variety producing EβC than near a variety without this signal (23). To “restore” EβC emission and test its importance for root protection under realistic field conditions, a maize variety that normally does not emit this compound was transformed with a gene from oregano (Origanum vulgare). We then compared WCR-inflicted root damage and beetle survival on transformed and nontransformed lines in the presence and absence of EPNs (25, 30).
Results and Discussion
Transformation of Maize with an EβC Synthase Gene from Oregano.
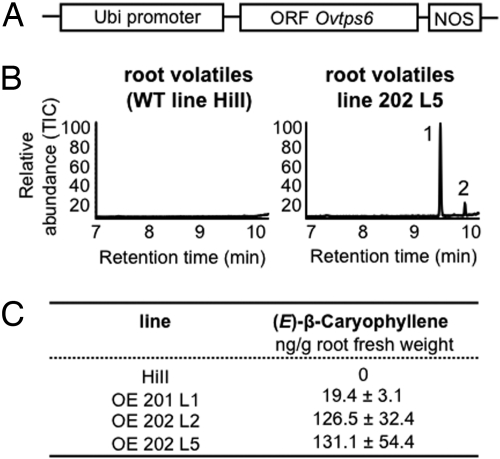
The maize variety HiII was transformed with an EβC synthase gene from Origanum vulgare L. (31) under the control of a maize ubiquitin promoter (Fig. 1A). The transformation resulted in plants that produced EβC constitutively (Fig. 1B), supporting the observation that loss of EβC production in most American maize lines is due to the loss of EβC synthase activity (29). We selected 3 independently transformed lines with EβC concentrations in their roots that were either similar (line 201 L1) or 6-fold higher (lines 202 L2, 202 L5) than what is typically present in our model European maize line Delprim (23) (Fig. 1C). Sesquiterpene hydrocarbons such as EβC appear to be emitted from maize by passive diffusion, and only a small proportion (<1%) accumulates in plant tissue; most is released directly from plant surfaces (32). Therefore, the roots of transgenic maize containing higher concentrations of EβC should release proportionally higher levels of this compound. In the following field experiments, selfed T1 generations of the 3 transformed lines were tested. As control plants, we used F1 progeny of the selfed nontransformed HiII, as well as the T1 progeny of a transgenic line (197 L1) with no expression of the EβC synthase gene, both with very high genetic similarity to the transformed plants.
Fig. 1.
Insertion of an EβC synthase gene from Origanum vulgare L. in maize variety HiII results in a constitutive production of EβC. (A) Terpene synthase overexpression construct (for details, see Materials and Methods). (B) A typical chromatogram obtained for the volatiles emitted by roots of the hybrid variety HiII line alongside a chromatogram for one of the transformed lines. Peak 1 is EβC and peak 2 is α-humulene, a side-product of EβC synthase. (C) Average quantities of EβC present in the roots of the untransformed HiII and the 3 transformed lines that were used in the field experiments (n = 8).
Field Experiments Comparing Transformed and Control Lines.
Field tests were conducted to determine whether the EβC emission of the transgenic plants enhanced the ability of H. megidis to find and kill WCR under realistic conditions and so reduce plant damage. In a 20 × 70 m maize field, 30 experimental plots were planted, each consisting of 1 row with 8 plants of a transformed line alongside a row with 8 plants of a control line (Fig. S1a). Two weeks after planting, all plots were infested with WCR eggs, and another 3 weeks later, ≈600,000 H. megidis were applied between the 2 rows of each plot. In addition to these 2-row experimental plots, we planted 2 types of control plots that did not receive nematodes, but one-half of these plots did receive WCR eggs (Fig. S1 b and c) (for details, see Materials and Methods). The effectiveness of the EPN in controlling WCR was evaluated by measuring root damage and emergence of WCR beetles.
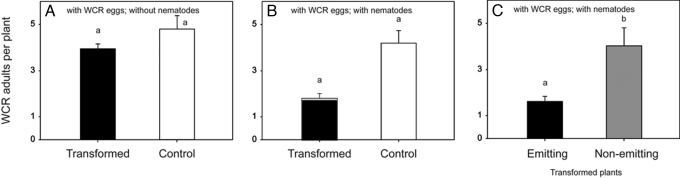
Damage to roots in experimental plots without WCR was minimal (Fig. 2A), whereas considerable root damage was found in plots that had received WCR eggs but no nematodes (Fig. 2B). In the absence of nematodes, there was no difference in WCR-caused root damage between transformed plants and the control plants (Fig. 2B); the number of emerging WCR beetles (approximately 4 per plant) was the same for both (Fig. 3A). However, in plots where nematodes were released, the transformed plants emitting EβC received significantly less root damage than the controls (Fig. 2C). Consistent with this, adult WCR emergence per transformed plant was reduced to less than one-half compared with control plants (Fig. 3B).
Fig. 2.
Transformants releasing EβC suffered less damage than control lines when EPNs were present. (A) Root damage measured on plants that had received neither WCR eggs nor nematodes was minimal, and there was no difference between transformed and nontransformed plants (n = 5, P = 0.87). (B) Root damage on plants that received only WCR eggs, but no nematodes, was substantial. Again, no significant difference was found between the transformed and nontransformed plants (n = 5, P = 0.18). (C) In plots that received WCR eggs and H. megidis, roots from transformed plants (pooled) had significantly less damage than roots from control lines (n = 30, P = 0.007). Approximately one-quarter of the transformed plants were found not to emit EβC. Removing these plants from the statistical analysis did not significantly affect the results. The letters above the bars indicate significant differences within a graph. Error bars indicate standard errors.
Fig. 3.
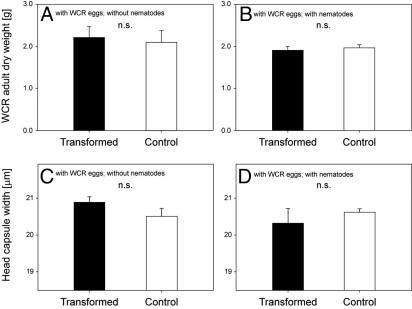
Fewer adult WCR beetles emerged near EβC-releasing transformants when nematodes were applied. (A) Adult emergence for plants from the plots that received WCR eggs only, but no nematodes. No significant difference was found between the transformed and nontransformed plants (n = 5, P = 0.47). (B) Adult emergence for plants that received both WCR eggs and nematodes was significantly different between transformed plants (pooled) and control plants (n = 30, P < 0.0001). Approximately one-quarter of the transformed plants were found not to emit EβC. Removing these plants from the statistical analysis slightly reduced the average emergence near the transformed plants (black bar) and increased the difference with untransformed control plants to 60%. (C) Significantly fewer adults emerged near EβC-producing transformed plants (black bar) than near transformed plants that were not emitting EβC (gray bar; P = 0.023). The letters above the bars indicate significant differences within a graph. Error bars indicate standard errors. No WCR adults were recovered from plots that did not receive WCR eggs.
Because all seeds used for the experiments were produced by selfed plants, the transformed lines segregated 3:1 for the transgene and therefore one-quarter of the plants did not produce EβC. This provided us with an additional fully blind control. We checked for EβC emission from leaf clippings harvested from all plants the day before the evaluation of root damage (see Materials and Methods) and excluded the results of the plants that did not produce EβC (26.8%) from the data analyses. This correction had no effect on the average for root damage, but the average adult emergence near EβC-emitting plants was reduced by another 5%, making it 60% less than near nontransformed plants (Fig. 3B). Moreover, there was a striking difference in the average number of adults that emerged near EβC-producing plants compared with the quarter of their sister plants that did not produce EβC in the same rows (1.79 vs. 3.80 beetles per plant) (Fig. 3C), whereas in plots without nematodes there was no such difference (3.92 vs. 4.25).
For each emerging beetle, the head capsule width, the sex, and dry weight were determined, but no differences for these parameters were found among treatments (Fig. S2). Emergence of beetles started in mid-July and lasted until mid-August (Fig. S3). Comparison of the emergence over time between plots with and without H. megidis shows that the nematode mostly suppressed WCR emergence at the end of the season (Fig. S3). This late effect is likely due to increasing numbers of H. megidis, because a new generation of infective juveniles will emerge within 2 weeks after infection, allowing the nematode to multiply over several generations during the season.
The data are consistent with the hypothesis that the transformation with the EβC synthase gene from oregano resulted in increased attraction of applied H. megidis and thereby enhanced WCR mortality and root protection. However, we considered and tested 2 additional explanations for increased root protection: that EβC attracted native nematodes that protected the plants or that the EβC-releasing transformants had a direct negative effect on the performance of WCR larvae.
Eliminating Alternative Explanations.
To evaluate the possible effects of native nematodes on the results, we placed waxmoth larvae in soil from the plots before and after the application of H. megidis (see Materials and Methods). In soil collected before H. megidis release, 4.9% of the larvae were infected, whereas in soil from after release 59.2% were infected. These results imply that there was indeed a small effect of native EPNs, which could explain the tendency of reduced root damage (Fig. 2B) and beetle emergence (Fig. 3A) near transgenic plants in the WCR plots without H. megidis. This effect was only apparent at the end of the season (Fig. S3), when the native nematode population can be expected to have had built up high numbers thanks to the large number of WCR hosts in the soil.
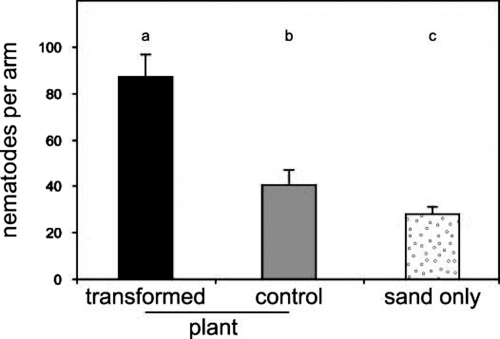
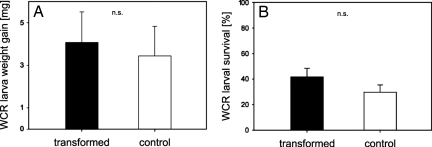
To test for a possible direct negative effect of plant transformation on WCR performance, as well as to confirm the enhanced attractiveness of transformed plants to H. megidis, we conducted an additional laboratory experiment with the use of 6-arm below-ground olfactometers [for details, see Materials and Methods and Rasmann et al. (23)]. When nematodes were given the choice between the volatiles of a WCR-infested transformed plant and a WCR-infested nontransformed plant, they were significantly more attracted to transformed plants (Fig. 4). Moreover, the transformation had no direct effect on the performance of WCR larvae. Average weight increase and survival were the same for larvae that had fed for 5 days on transformed plants compared with larvae that had fed the same period on nontransformed plants (Fig. 5). Thus, the results support the conclusion that the improved control of WCR near transformed plants in the field experiments was due to the enhanced attractiveness of the roots to nematodes and not to a direct negative effect on the WCR larvae.
Fig. 4.
Confirmation of nematode attraction to transformed maize lines. H. megidis nematodes were more strongly attracted to transformed plants than to untransformed controls in 6-arm olfactometers. The graph depicts the average number of nematodes recovered from olfactometer arms connected to pots containing either a WCR-infested transformed plant (line 202 L2; black bar), a WCR-infested control plant (gray bar), or only moist sand (white bar). The plants were each infested with 5 second-instar WCR larvae. For each replicate, the total number of nematodes found in the 4 moist sand-only control pots (white bar) were summed and divided by 4. The attraction to transformed plants was significantly higher than to the control plants (n = 11, P < 0.001). The letters above the bars indicate significant differences. Error bars indicate standard errors.
Fig. 5.
There was no direct effect of the transformation on WCR performance. (A) Average weight gain of WCR larvae fed for 5 days on transformed (line 202 L2) or control plants. No statistical difference was found (n = 13, P = 0.75). (B) Survival of WCR larvae after 5 days on transformed or nontransformed plants. No statistical difference was found (n = 13, P = 0.18). Error bars indicate standard errors.
Conclusions
Besides irrefutably demonstrating that the presence of a specific volatile signal is of essential importance for the attraction of a major natural enemy of soil herbivores, the current study shows that plant-produced chemical signals can be modified to improve the search and killing efficiency of natural enemies of pest insects in an agricultural setting. The 60% reduction in adult WCR emergence achieved by nematodes on plants transformed to release EβC approaches the efficacy of synthetic pesticides that have been used to control this important pest (33, 34) and is more environmentally friendly. Moreover, this considerable effect on the pest was accomplished with a much lower nematode dose than is commonly used (26, 27, 35–37). It should be noted that the transformed lines are not of commercial value. Because the EβC signal is already present in many maize lines, as well as in ancestral wild relatives, optimizing the production of EβC should be possible through classical breeding. However, a transgenic approach might be favorable, because it could be faster and minimize changes of desirable traits in existing lines. Such an approach also allows the combination of direct resistance traits, like the production of a Bt-toxin, with the indirect resistance tested here. The use of EβC to enhance WCR control with EPNs might be improved by making the emission inducible when insects feed on the roots, thereby guiding the nematodes specifically to those plants that are actually under insect attack. The current study reveals that the hotly debated genetic manipulation of crop plants (21, 38) can be used to enhance biological control.
Materials and Methods
Transformation.
The general procedure for the transformation of the maize line HiII has been described by Frame et al. (39). The Ti-plasmid pTF101.1 was kindly provided by the Plant Transformation Facility (Iowa State University, Ames, IA). Plasmid pTF101.1 was derived from pTF102. It lacked the β-glucuronidase reporter gene and instead expressed the phosphoinothricin acetyltransferase from Streptomyces hygroscopicus gene via a double cauliflower mosaic virus 35S promoter. Inserted in this vector was the EβC synthase tps6 from Origanum vulgare under control of a maize ubiquitin promoter (40, 41). The structural gene was followed by a terminator from nopaline synthase of the Agrobacterium tumefaciens Ti plasmid. The transgenic lines 202 L1, 202 L2, and 202 L5 were regenerated from 3 independent transgenic calli generated in 2 independent transformation experiments. Selfed T1 generations of the 3 transformed lines were used in the experiments. As nontransformed controls, the selfed F1 progeny of HiII as well as the T1 progeny of a transgenic line with no expression of the EβC synthase gene, 197 L1, were used.
Field Experiment.
All experimental plots were hand planted on May 22, 2007, at the Bradford Research and Extension Center (Columbia, MO). Each plot had rows of 8 plants of a particular line with 43-cm spacing between plants and 76-cm spacing between rows. For the main experiment, there was a total of 30 plots. Each plot had a row of control plants and a row of a transformed line (Fig. S1a). There were 10 plots for each of the 3 transformed lines and all plots received feral WCR eggs (French Agricultural Research) as well as nematodes (Becker Underwood). In addition, we planted 10 control plots that did not receive nematodes. One-half of the control plots were infested with WCR. Each control plot had 4 rows: 3 rows with each 8 plants of a particular transformed line and 1 row with 8 control plants (Fig. 2 B and C). Plots were randomly distributed in the field, with each plot surrounded by 2 rows of maize plants of the variety Pioneer 3394, serving as a buffer between plots. The above plantings and infestation procedures resulted in 3 types of plots (Fig. S1): (i) 30 principal experimental plots with WCR infestation and nematode release, (ii) 5 plots that had only WCR and no nematodes, and (iii) 5 plots without WCR and without nematodes.
On June 4, all plots except one-half (5) of the control plots were infested with WCR eggs. Eggs were mixed into a solution of water and 0.15% agar and each plant received ≈400 viable eggs in each of 2 10-cm deep holes at a distance of 5 cm from the plant (≈800 eggs per plant). On June 28, ≈600,000 H. megidis (Becker Underwood) were applied in between the 2 rows of the 30 experimental plots. Nematodes were mixed in 0.5 L of water, and the solution was poured into a 2-cm-deep trench that was dug between the rows. The number of EPNs that was used is equivalent to 250,000 EPNs per square meter. No nematodes were applied to the 10 control plots. Plans had been made to irrigate before and after infection with EPNs, but 5.9 cm of rain fell starting the day before infection, extending 3 days after infection, including 3.76 cm on the day of infection.
On July 11, root damage was assessed by digging out one-half of the plants in each plot (4 plants per row). Plants were immediately and carefully removed from the field to avoid nematode and WCR contamination of the neighboring plants and plots. The root systems were washed, and damage from WCR larval feeding was rated by using a 0–3 root scale (42).
The 400 remaining plants stayed in the field and emergence cages (78 × 36 cm) modified after Pierce and Gray (43) were placed over each plant on July 11. Traps were checked 3 times per week during the peak of WCR emergence and twice per week during periods when few adults were emerging. The last cage check was performed on September 7. All beetles collected were placed in individually labeled scintillation vials (J&H Berge) containing 95% ethanol and brought to laboratory for processing. First, beetles were counted and sexed by emergence date. Second, the head capsule width of each beetle was measured by using an ocular micrometer (10×/21; Wild) mounted on a microscope (M3Z; Wild). On these 2 measurements, beetles were placed in a desiccating oven (Thelco Model 16; GCA/Precision Scientific) at 60 °C for 48 h. The dried beetles were then placed on an analytical scale (Model AB135-S FACT; Metler), and total dry weight was recorded.
PROC MIXED of the statistical package SAS (SAS Institute 1990) was used for data analyses. Data were analyzed as a randomized complete block with treatments arranged in a 5 × 2 factorial (5 maize genotypes × 2 experiments) as outlined by Steel et al. (44). One-half of the blocks contained a randomization of the main experimental plots (WCR plus nematodes) and the control plots with WCR and the other one-half contained a randomization of the main experimental plots and control plots without WCR. Consequently, 2 separate groups of analyses were performed for plant damage, adult emergence, beetle dry weight, and head capsule width. First, a comparison among the 3 transformed lines revealed no significant differences within the datasets. Therefore, these lines were pooled for further analyses. A separate comparison between the 2 isolines sources of the original nontransformed line also revealed no significant differences in any of the above factors, and they were also pooled for subsequent analyses. Finally, selected contrasts were made between specific treatments within each type of plots following the methods outlined by Littell et al. (45). Difference in WCR adult emergence between transformed plants producing EβC or not was analyzed with SPSS 14.0. Because homogeneity of variance test failed, means were compared with the nonparametric Mann–Whitney test.
Screening for the Presence of Native EPN.
Three soil samples along the application trench were taken 2 days before (June 26) and again 7 days after the nematode H. megidis application (July 5). Each sample contained ≈200 g of soil from an area of 5 cm2 and was kept at 4 °C until it was used for the next step. Detection of nematodes was done with larvae of the waxmoth (Galleria mellonella L., Lepidoptera: Galleridae) (46). Soil was homogenized by hand and each soil sample was placed in a 350-mL glass jar (7 cm diameter, 12 cm deep) with 3 final instar larvae of G. mellonella (Pet Centre). After the larvae had been added, the jars were stored upside-down at 25 °C in darkness. Five days later, larvae were examined for nematode infection. Larvae with the typical red color, indicative of the Heterorhabiditidae bacterial symbiont introduced into the host by EPN (47), were recorded as infected. Because other families of EPN could also have infected the larvae, the remaining cadavers were dissected and checked for EPN presence/absence. In soil collected before H. megidis release, 4.9% of the larvae were infected, whereas in soil from after release 59.2% were infected.
EβC Emission Screens.
Leaf clippings (≈25 cm2) were sampled from all plants the day before harvesting plants used for root damage rating (on July 10). These clippings were frozen in the field by placing them in liquid nitrogen and subsequently stored in a −80 °C freezer. Before chemical analyses, individual leaf samples were ground in liquid nitrogen and ≈0.3 g of leaf powder was placed in a 20-mL glass vial. Using a multipurpose sampler (MPS2; Gerstel), a 100-μm polydimethylsiloxane solid-phase microextraction fiber (Supelco) was inserted in the vial through a septum in the lid and exposed for 60 min at 40 °C. The compounds adsorbed on the fiber were analyzed with an Agilent 6890 Series (G1530A) gas chromatograph coupled to a quadrupole-type mass-selective detector (Agilent 5973; transfer line, 230 °C; source, 230 °C; ionization potential, 70 eV). The fiber was inserted into the injector port (230 °C, 50 mm), desorbed for 3 min, and chromatographed on a DB5-MS column (30 m, 0.25-mm internal diameter, 0.25-μm film thickness; J&W Scientific). Helium at a constant pressure of 18.55 lb·in−2 (127.9 kPa) was used for carrier gas flow. After fiber insertion, the column temperature was maintained at 60 °C for 1 min and then increased to 270 °C at 10 °C min−1 and ended with a final step of 5 min at 270 °C. Chromatograms were analyzed by using ChemStation D.00.00.38.
Olfactometer Assays.
Below-ground olfactometer assays were modified after Rasmann et al. (23). Olfactometers consisted of 6 glass pots connected via glass tubes to a central pot. Each system of 7 pots was filled with sand moistened to 10% water by weight (Migros). A 12-day-old transformed plant (line 202 L2) that had been grown in a climate chamber (Weiss Technik; 16:8 light/dark hours, 25:15 °C day/night temperature, and 60% humidity) in a mix of potted soil and sand was transplanted in sand in 1 of the outer pots (5 cm diameter, 11 cm deep). A similar nontransformed plant (line 197 L1) was placed in another sand-filled pot. The 4 remaining pots only contained sand. The pots with a plant each received 5 second-instar WCR larvae that were weighed as a group just before on a microscale (Mettler-Toledo GmbH). Two days after WCR infestation, the posts were connected to the olfactometer central pot (8 cm diameter, 11 cm deep) by using a glass connector (8 cm long; 24/29 male connector on both sides; all glassware from VQT-Verre Quartz Technique) and a Teflon connector (24/29 female to 29/31 male) containing an ultrafine mesh metal screen (2300 mesh; Small Parts), which prevented the nematodes from entering the odor source pots. One day later, ≈2,000 H. megidis nematodes (Becker Underwood) were released in the central pot, and the following day the olfactometers were disassembled. Sand contained in the glass and Teflon connectors was separately placed on cotton filters (19 cm diameter; Hoeschele). Filters and sand were placed on a Bearmann extractor (48, 49), and recovered nematodes were counted the following day.
To evaluate the performance of the WCR larvae on the plants, the larvae were left in the pots for 2 additional days. Then they were recovered from the pots by passing sand through a 0.6-mm sieve (Eijkelkamp). Survival was determined, and recovered WCR larvae were again weighed.
The nematodes' choices among the arms of the olfactometers were examined with a log linear model. The entity computing a repetition in the statistical analysis corresponds to the response of a group of nematodes released, which was shown to follow a multinomial distribution (50). Because the data did not conform to simple variance assumptions implied in using the multinomial distribution, we used quasi-likelihood functions to compensate for the overdispersion of nematodes within the olfactometer (51). WCR larvae performances differences were tested with SPSS 14.0. Because normality and homogeneity of variance tests passed, survival and weigh means were compared with a t test.
Supplementary Material
Acknowledgments.
We are grateful to the U.S. Department of Agriculture field crew assistance during the field experiment and especially to Julie Barry for her help and advice. We thank Neil Villard for his valuable technical assistance with laboratory experiments and analyses. This work was supported by the Swiss National Center of Competence in Research Plant Survival, the German National Science Foundation (SFB648), and the Max-Planck Society.
Footnotes
Conflict of interest statement: A patent for the transformation with the oregano (E)-β-caryophyllene synthase gene has been filed. Some of the authors and their institutions may financially benefit from this patent.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906365106/DCSupplemental.
References
- 1.Paré PW, Tumlinson JH. Plant volatiles as a defense against insect herbivores. Plant Physiol. 1999;121:325–331. [PMC free article] [PubMed] [Google Scholar]
- 2.Dudareva N, Negre F, Nagegowda DA, Orlova I. Plant volatiles: Recent advances and future perspectives. CRC Crit Rev Plant Sci. 2006;25:417–440. [Google Scholar]
- 3.De Moraes CM, Lewis WJ, Pare PW, Alborn HT, Tumlinson JH. Herbivore-infested plants selectively attract parasitoids. Nature. 1998;393:570–573. [Google Scholar]
- 4.Dicke M, Sabelis MW. How plants obtain predatory mites as bodyguards. Neth J Zool. 1988;38:148–165. [Google Scholar]
- 5.Hilker M, Meiners T. Induction of plant responses to oviposition and feeding by herbivorous arthropods: A comparison. Entomol Exp Appl. 2002;104:181–192. [Google Scholar]
- 6.Turlings TCJ, Tumlinson JH, Lewis WJ. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science. 1990;250:1251–1253. doi: 10.1126/science.250.4985.1251. [DOI] [PubMed] [Google Scholar]
- 7.Heil M. Indirect defence via tritrophic interactions. New Phytol. 2008;178:41–61. doi: 10.1111/j.1469-8137.2007.02330.x. [DOI] [PubMed] [Google Scholar]
- 8.Beale MH, et al. Aphid alarm pheromone produced by transgenic plants affects aphid and parasitoid behavior. Proc Natl Acad Sci USA. 2006;103:10509–10513. doi: 10.1073/pnas.0603998103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kappers IF, et al. Genetic engineering of terpenoid metabolism attracts, bodyguards to Arabidopsis. Science. 2005;309:2070–2072. doi: 10.1126/science.1116232. [DOI] [PubMed] [Google Scholar]
- 10.Schnee C, et al. The products of a single maize sesquiterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores. Proc Natl Acad Sci USA. 2006;103:1129–1134. doi: 10.1073/pnas.0508027103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng AX, et al. The rice (E)-β-caryophyllene synthase (OsTPS3) accounts for the major inducible volatile sesquiterpenes. Phytochemistry. 2007;68:1632–1641. doi: 10.1016/j.phytochem.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Degenhardt J, Gershenzon J, Baldwin IT, Kessler A. Attracting friends to feast on foes: Engineering terpene emission to make crop plants more attractive to herbivore enemies. Curr Opin Biotechnol. 2003;14:169–176. doi: 10.1016/s0958-1669(03)00025-9. [DOI] [PubMed] [Google Scholar]
- 13.Aharoni A, Jongsma MA, Bouwmeester HJ. Volatile science? Metabolic engineering of terpenoids in plants. Trends Plants Sci. 2005;10:594–602. doi: 10.1016/j.tplants.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Turlings TCJ, Ton J. Exploiting scents of distress: The prospect of manipulating herbivore-induced plant odours to enhance the control of agricultural pests. Curr Opin Plant Biol. 2006;9:421–427. doi: 10.1016/j.pbi.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Pickett JA, Poppy GM. Switching on plant genes by external chemical signals. Trends Plants Sci. 2001;6:137–139. doi: 10.1016/s1360-1385(01)01899-4. [DOI] [PubMed] [Google Scholar]
- 16.Vidal S, Kuhlmann U, Edwards CR, editors. Western Corn Rootworm: Ecology and Management. London: CABI; 2005. 310 pp. [Google Scholar]
- 17.Miller N, et al. Multiple transatlantic introductions of the western corn rootworm. Science. 2005;310:992. doi: 10.1126/science.1115871. [DOI] [PubMed] [Google Scholar]
- 18.Levine E, Oloumisadeghi H. Management of diabroticite rootworms in corn. Annu Rev Entomol. 1991;36:229–255. [Google Scholar]
- 19.Moellenbeck DJ, et al. Insecticidal proteins from Bacillus thuringiensis protect corn from corn rootworms. Nat Biotechnol. 2001;19:668–672. doi: 10.1038/90282. [DOI] [PubMed] [Google Scholar]
- 20.Vaughn T, et al. A method of controlling corn rootworm feeding using a Bacillus thuringiensis protein expressed in transgenic maize. Crop Sci. 2005;45:931–938. [Google Scholar]
- 21.Romeis J, Meissle M, Bigler F. Transgenic crops expressing Bacillus thuringiensis toxins and biological control. Nat Biotechnol. 2006;24:63–71. doi: 10.1038/nbt1180. [DOI] [PubMed] [Google Scholar]
- 22.Meihls LN, et al. Increased survival of western corn rootworm on transgenic corn within three generations of on-plant greenhouse selection. Proc Natl Acad Sci USA. 2008;105:19177–19182. doi: 10.1073/pnas.0805565105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasmann S, et al. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature. 2005;434:732–737. doi: 10.1038/nature03451. [DOI] [PubMed] [Google Scholar]
- 24.Hiltpold I, Turlings TCJ. Belowground chemical signaling in maize: When simplicity rhymes with efficiency. J Chem Ecol. 2008;34:628–635. doi: 10.1007/s10886-008-9467-6. [DOI] [PubMed] [Google Scholar]
- 25.Kurtz B, Hiltpold I, Turlings TCJ, Kuhlmann U, Toepfer S. Comparative susceptibility of larval instars and pupae of the western corn rootworm to infection by three entomopathogenic nematodes. Biocontrol. 2009;54:255–262. [Google Scholar]
- 26.Jackson JJ. Field performance of entomopathogenic nematodes for suppression of western corn rootworm (Coleoptera: Chrysomelidae) J Econ Entomol. 1996;89:366–372. doi: 10.1093/jee/91.2.410. [DOI] [PubMed] [Google Scholar]
- 27.Journey AM, Ostlie KR. Biological control of the western corn rootworm (Coleoptera: Chrysomelidae) using the entomopathogenic nematode, Steinernema carpocapsae. Environ Entomol. 2000;29:822–831. [Google Scholar]
- 28.Degen T, Dillmann C, Marion-Poll F, Turlings TCJ. High genetic variability of herbivore-induced volatile emission within a broad range of maize inbred lines. Plant Physiol. 2004;135:1928–1938. doi: 10.1104/pp.104.039891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Köllner TG, et al. A maize (E)-β-caryophyllene synthase implicated in indirect defense responses against herbivores is not expressed in most American maize varieties. Plant Cell. 2008;20:482–494. doi: 10.1105/tpc.107.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toepfer S, Peters A, Ehlers RU, Kuhlmann U. Comparative assessment of the efficacy of entomopathogenic nematode species at reducing western corn rootworm larvae and root damage in maize. J Appl Entomol. 2008;132:337–348. [Google Scholar]
- 31.Crocoll C, Asbach J, Novak J, Gershenzon J, Degenhardt J. The terpene synthases of oregano (Origanum vulgare L.) and their roles in the pathway and regulation of terpene biosynthesis. Plant Mol Biol. 2009 doi: 10.1007/s11103-010-9636-1. in press. [DOI] [PubMed] [Google Scholar]
- 32.Köllner TG, Schnee C, Gershenzon J, Degenhardt J. The sesquiterpene hydrocarbons of maize (Zea mays) form five groups with distinct developmental and organ-specific distributions. Phytochemistry. 2004;65:1895–1902. doi: 10.1016/j.phytochem.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 33.Cox WJ, Shields E, Cherney DJR, Cherney JH. Seed-applied insecticides inconsistently affect corn forage in continuous corn. Agron J. 2007;99:1640–1644. [Google Scholar]
- 34.French BW, Chandler LD, Riedell WE. Effectiveness of corn rootworm (Coleoptera: Chrysomelidae) areawide pest management in South Dakota. J Econ Entomol. 2007;100:1542–1554. doi: 10.1603/0022-0493(2007)100[1542:eocrcc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 35.Jackson JJ, Hesler LS. Placement and application rate of the nematode Steinernema carpocapsae (Rhabditida: Steinernematidae) for suppression of the western corn rootworm (Coleoptera: Chrysomelidae) J Kans Entomol Soc. 1995;68:461–467. [Google Scholar]
- 36.Nishimatsu T, Jackson JJ. Interaction of insecticides, entomopathogenic nematodes, and larvae of the western corn rootworm (Coleoptera: Crysomelidae) J Econ Entomol. 1998;91:410–418. doi: 10.1093/jee/91.2.410. [DOI] [PubMed] [Google Scholar]
- 37.Wright RJ, Witkowski JF, Echtenkamp G, Georgis R. Efficacy and persistence of Steinernema carpocapsae (Rhabditida, Steinernematidae) applied through a center-pivot irrigation system against larval corn rootworms (Coleoptera, Chrysomelidae) J Econ Entomol. 1993;86:1348–1354. [Google Scholar]
- 38.Romeis J, et al. Assessment of risk of insect-resistant transgenic crops to nontarget arthropods. Nat Biotechnol. 2008;26:203–208. doi: 10.1038/nbt1381. [DOI] [PubMed] [Google Scholar]
- 39.Frame BR, et al. Agrobacterium tumefaciens-mediated transformation of maize embryos using a standard binary vector system. Plant Physiol. 2002;129:13–22. doi: 10.1104/pp.000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christensen AH, Quail PH. Ubiquitin promoter-based vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. Transgenic Res. 1996;5:213–218. doi: 10.1007/BF01969712. [DOI] [PubMed] [Google Scholar]
- 41.Torney F, et al. Heritable transgene expression pattern imposed onto maize ubiquitin promoter by maize adh-1 matrix attachment regions: Tissue and developmental specificity in maize transgenic plants. Plant Cell Rep. 2004;22:931–938. doi: 10.1007/s00299-004-0779-x. [DOI] [PubMed] [Google Scholar]
- 42.Oleson JD, Park YL, Nowatzki TM, Tollefson JJ. Node-injury scale to evaluate root injury by corn rootworms (Coleoptera: Chrysomelidae) J Econ Entomol. 2005;98:1–8. doi: 10.1093/jee/98.1.1. [DOI] [PubMed] [Google Scholar]
- 43.Pierce CMF, Gray ME. Population dynamics of a western corn rootworm (Coleoptera: Chrysomelidae) variant in East Central Illinois commercial maize and soybean fields. J Econ Entomol. 2007;100:1104–1115. doi: 10.1603/0022-0493(2007)100[1104:pdoawc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 44.Steel RD, Torrie JH, Dickey DA. Principles and Procedures of Statistics: A Biometrical Approach. 3rd Ed. New York: McGraw Hill; 1997. [Google Scholar]
- 45.Littell RC, Henry PR, Ammerman CB. Statistical analysis of repeated measures data using SAS procedures. J Anim Sci. 1998;76:1216–1231. doi: 10.2527/1998.7641216x. [DOI] [PubMed] [Google Scholar]
- 46.Bedding RA, Akhurst RJ. A simple technique for the detection of insect parasitic rhabditid nematodes in soil. Nematologica. 1975;21:109–110. [Google Scholar]
- 47.Forst S, Clarke D. Bacteria-nematode symbiosis. In: Gaugler R, editor. Entomopathogenic Nematology. Wallingford, UK: CABI; 2002. pp. 57–77. [Google Scholar]
- 48.Curran J. Influence of application method and pest population size on the field efficacy of entomopathogenic nematodes. J Nematol. 1992;24:631–636. [PMC free article] [PubMed] [Google Scholar]
- 49.Hass B, Griffin CT, Downes MJ. Persistence of Heterorhabditis infective juveniles in soil: Comparison of extraction and infectivity measurements. J Nematol. 1999;31:508–516. [PMC free article] [PubMed] [Google Scholar]
- 50.Ricard I, Davison AC. Statistical inference for olfactometer data. J R Stat Soc Ser C Appl Stat. 2007;56:479–492. [Google Scholar]
- 51.Turlings TCJ, Davison AC, Tamo C. A six-arm olfactometer permitting simultaneous observation of insect attraction and odour trapping. Physiol Entomol. 2004;29:45–55. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.