Abstract
Dopamine has a crucial role in the modulation of neurocognitive function, and synaptic dopamine activity is normally regulated by the dopamine transporter (DAT) and catechol-O-methyltransferase (COMT). Perturbed dopamine function is a key pathophysiological feature of schizophrenia. Our objectives were (i) to examine epistasis between the DAT 3′ UTR variable number of tandem repeats (VNTR) and COMT Val158Met polymorphisms on brain activation during executive function, and (ii) to then determine the extent to which such interaction is altered in schizophrenia. Regional brain response was measured by using blood-oxygen-level-dependent fMRI during an overt verbal fluency task in 85 subjects (44 healthy volunteers and 41 patients with DSM-IV schizophrenia), and inferences were estimated by using an ANOVA in SPM5. There was a significant COMT × DAT nonadditive interaction effect on activation in the left supramarginal gyrus, irrespective of diagnostic group (Z-score = 4.3; family-wise error (FWE) p = 0.03), and in healthy volunteers alone (Z-score = 4.7; FWEp = 0.006). In this region, relatively increased activation was detected only when COMT Met-158/Met-158 subjects also carried the 9-repeat DAT allele, or when, reversely, Val-158/Val-158 subjects carried the 10/10-repeat genotype. Also, there was a significant diagnosis × COMT × DAT nonadditive interaction in the right orbital gyrus (Z-score = 4.3; FWEp = 0.04), where, only within patients, greater activation was only associated with a 9-repeat allele and Val-158 conjunction, and with a 10-repeat and Met-158 conjunction (Z-score = 4.3; FWE p = 0.04). These data demonstrate that COMT and DAT genes interact nonadditively to modulate cortical function during executive processing, and also, that this effect is significantly altered in schizophrenia, which may reflect abnormal dopamine function in the disorder.
The dopamine transporter (DAT or SLC6A3) and the catechol-O-methyl transferase (COMT) regulate synaptic levels of dopamine in the brain, and thus, modulate central dopaminergic function. The DAT is a presynaptic neuronal membrane protein, which removes dopamine from the synaptic cleft (1) and is thought to be particularly important as a regulator of phasic dopamine release in subcortical regions where DAT is most abundant (2–4). In cortical regions, where DAT is less abundant, postsynapatic intracellular degradation by the COMT enzyme is an important regulator of dopamine function (5–9). In the cortex, DAT is mainly extrasynaptic, and thus, seems better situated to regulating dopamine diffusion to the extrasynaptic space (10–12).
Within the healthy population, the activity of COMT and DAT is affected by at least 2 well-studied polymorphisms. The enzymatic activity of COMT is altered by a guanine (G) to adenine (A) SNP change (known as Val158Met or rs4680) in the gene, which translates into a Val-to-Met amino acid change in codon 158 of the protein. This polymorphism is codominant and causes a 3- to 4-fold decrease in the molecular thermo-stability of the protein, which decreases its abundance and enzymatic activity, inclusively in the human brain (13–17). The DAT gene displays a polymorphic 40-bp variable number of tandem repeats (VNTR) in a UTR, which yields alleles ranging from 3 to 11 repeats, 9- and 10-repeat alleles being the most common (18). This polymorphism has been found to act as a modulator of gene transcription: the 10-repeat allele being associated with higher levels of DAT expression (19–22).
To date, the impact of these dopamine-regulating genes on cognitive function has been assessed in studies within the healthy population. Variation in the functional Val158Met polymorphism within COMT is associated with significant differences in both the performance of cognitive tasks (23–26), including verbal fluency (27), and the pattern of cortical activation during their execution (24, 27–32). Similarly, functional neuroimaging studies of the DAT 3′ UTR VNTR in healthy subjects suggest that this gene modulates cortical activation during working memory (12) and verbal fluency (84). Also, 2 neuroimaging studies have reported additive interactions between the effects of COMT Val158Met and of DAT 3′ UTR VNTR on activation in the prefrontal cortex during working memory tasks (12, 33). These studies found a linear relationship between the effects of the 2 polymorphisms; both the Met-158 and the 10-repeat alleles were associated with relatively less activation when simultaneously present (and the opposite applied to the Val-158 and the 9-repeat alleles). Nonadditive (i.e., multiplicative) interactions, where an effect of an allele is different, depending on the presence of another allele, have also been reported for the same genetic polymorphisms in the ventral striatum during a reward-sensitive guessing task (34), in the hippocampus (35), and tentatively in the prefrontal cortex (35), during 2 memory tasks, in healthy subjects.
Schizophrenia is associated with abnormalities in dopaminergic function in the cerebral cortex (36–41) and the striatum (42–45). This observation suggests that the impact of variation in the COMT and DAT genes on regional brain activation might differ in patients compared with healthy controls. We have recently verified this hypothesis for both COMT and DAT, with variation in each gene associated with a different pattern of activation during verbal fluency in patients and controls (27, 84). Interestingly, both DAT and COMT genes are strong candidate susceptibility genes for schizophrenia given their biological role, but there is lack of consensus between genetic association studies with a similar number of positive and negative studies reported for each gene. One reason behind the inconsistencies that relate to one gene may be the lack of assessment of the other gene, especially if they interact in a nonadditive manner to influence disease outcome and symptomatology.
The aims of the present study were to examine (i) the existence of a nonadditive interaction between 2 distinct genes, DAT and COMT, on brain activation during a verbal fluency task, and (ii) the extent to which this effect is modified in schizophrenia. We used 2 well-characterized functional polymorphisms (COMT Val158Met and DAT 3′ UTR VNTR) and functional MRI to study healthy volunteers and patients with schizophrenia, with genotype subgroups of sufficient size to detect interactive effects of both genes on activation by using an ANOVA. Subjects were scanned while they performed an overt phonological verbal fluency paradigm, which is associated with activation in the frontal, cingulate, and temporal cortex in healthy volunteers (46–54), and with impaired performance (55, 56) and altered activation (51, 54, 57–60) in schizophrenia. On the basis of evidence that variation in DAT (at the 3′ UTR VNTR) and in COMT (at the Val158Met SNP) separately modulate fronto-temporal activation during verbal fluency, and that these effects are altered in patients with schizophrenia (27, 84), we predicted that there would be an epistatic interaction between their effects on regional activation in the same sample and paradigm. We further tested the hypothesis that this genotype × genotype interaction on activation would be significantly different in patients with schizophrenia compared with healthy volunteers, reflecting the abnormal cortical and striatal dopamine function associated with the disorder.
Results
Behavioral Data.
As expected, there was a significant main effect of task demand on the number of incorrect responses (F = 45.36; P < 0.0001) as there was for diagnosis, with patients making more errors than controls (F = 9.71; P = 0.003). There were no significant 2-way or 3-way interactions.
Main Effect of Task.
In both diagnostic groups, word generation (irrespective of task difficulty or genotype) was associated with activation in a distributed network that included, bilaterally, the inferior frontal, insular, and dorsal anterior cingulate cortex, the caudate and the thalamus, and the left middle frontal, superior temporal, and inferior parietal cortex [family-wise error (FWE) p < 0.05] Conversely, repetition was associated with greater engagement of the rostral anterior cingulate gyrus, precuneus, and occipital cortex. These data were reported in detail in an earlier study (27).
Main Effect of Diagnostic Group.
Activation in the left inferior frontal gyrus (−44 18 30; Z = 3.4), anterior insula (−34 14 8, Z = 3.3), and frontal operculum (−36 14 12; Z = 3.6) was greater in patients than in healthy volunteers (P < 0.001, uncorrected). There were no areas more activated in healthy volunteers than in patients, and there were no between group differences in deactivation. These data were reported in detail in an earlier study (27).
Individual Effects of DAT and COMT Genotypes.
A significant diagnosis by genotype interaction was detected in the right peri-Sylvian cortex, reflecting greater activation in Val-158/Val-158 than Met-158/Met-158 schizophrenia patients, and the opposite in healthy volunteers (40 6 14, Z = 4.3; 34 14 6, Z = 4.0; 50 −24 18, Z = 4.0; 54 −10 −14, Z = 3.9), as reported earlier (27). In respect to DAT 3′ UTR VNTR, significant main effects of genotype were found in the activation of the left anterior insula (−30 6 16, Z = 3.5 and −34 14 −6, Z = 3.2) and the right caudate nucleus (22 4 20, Z = 3.2) reflecting greater activation in 10/10-repeat than 9-repeat carriers. A significant diagnosis by genotype interaction was detected in the left middle frontal gyrus (−42 44 24, Z = 2.8) and the nucleus accumbens (−6 6 −10, Z = 3.1), with greater activation in 9-repeat than 10/10-repeat carriers in schizophrenia, but not in healthy controls (84).
DAT × COMT Interaction Irrespective of Diagnostic Group.
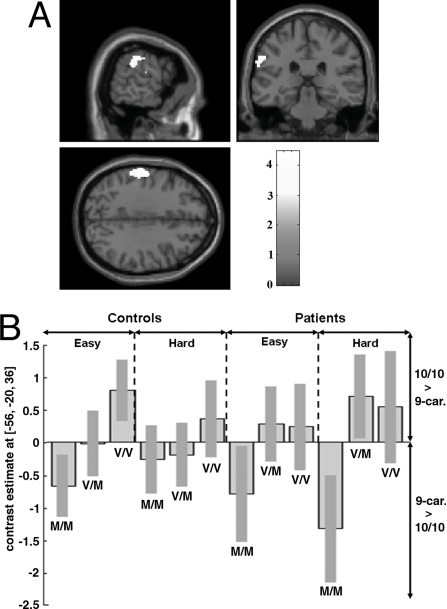
There was a significant interaction between the effects of the COMT and DAT genotypes on activation in all subjects, irrespective of diagnostic group, in the left supramarginal gyrus, part of the inferior parietal lobule (FWEp = 0.03) (Table 1). In this region, DAT 9-repeat carriers activated >10/10-repeat subjects within the Met-158/Met-158 group, whereas the opposite (10/10-repeat activating >9-repeat carriers) was evident in Val-158/Val-158 subjects (Table 1 and Fig. 1). This effect accounted for 13% of the interindividual variance in activation (ηp2 = 13%). Although in patients alone this interaction effect was not significant, in controls, it was highly significant (FWE = 0.006), accounting for 25% of the interindividual variance (ηp2 = 25%). Exploration of the plot in Fig. 1 shows that the effect of DAT in COMT heterozygotes was generally intermediate (across task versions and diagnostic groups) between that in the 2 COMT homozygote groups. There was no evidence for an interaction in the opposite direction (DAT 9-repeat carriers activating >10/10-repeat subjects within the Val-158/Val-158 group, with the reverse in the Met-158/Met-158 group).
Table 1.
Interaction between effects of DAT 3′ UTR VNTR and COMT Val158Met genotypes on regional activation across diagnostic groups
| Brain area | Controls,DAT × COMT interaction | SCZ patients, DAT × COMT interaction | Controls and SCZ patients |
||
|---|---|---|---|---|---|
| 9-c.>10/10,Met/Met | 10/10 > 9-c.,Val/Val | DAT × COMT interaction | |||
| L supramarginal/postcentral gyrus | −60 −16 34 (4.5) FWEp = 0.006 | −56 −20 35 (3.1) | −60 −28 36 (4.0) | −56 −16 32 (2.7) | −56 −20 36 (4.3) FWEp = 0.03 |
All inferences significant at FWE P < 0.05 for a DAT × COMT effect irrespective of diagnostic group (last column) are reported (Z-scores in brackets). FWE correction is also shown for other effects that survive significance at the same threshold. SCZ, schizophrenia.
Fig. 1.
Nonadditive interaction between effects of COMT Val158Met and DAT 3′ UTR VNTR on activation in the left supramarginal/postcentral gyrus, irrespective of diagnostic group (FWE, P < 0.05; plotted in B). (A) Threshold for image is set at P < 0.001 (uncorrected) for visualization purposes. (B) Plot showing DAT 9-repeat carriers activated >10/10-repeat subjects within the Met-158/Met-158 group, whereas the opposite (10/10-repeat activating >9-repeat carriers) was evident in Val-158/Val-158 subjects in this region. Negative values represent activation in 9-repeat carriers greater than that of 10/10-repeat subjects, whereas positive bars represent 10/10-repeat subjects activating >9-repeat carriers. M, 158Met; V, 158Val.
Group × DAT × COMT Interaction.
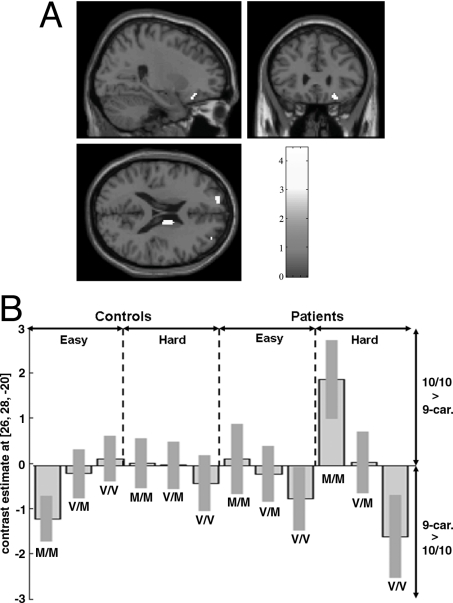
An interaction between diagnosis and the effect of DAT genotype on the effect of COMT was detected in the right medial/posterior orbital gyrus (FWEp = 0.04) (Table 2). In this region, controls who where 9-repeat carriers activated >10/10-repeat controls in the Met-158/Met-158 group, whereas the opposite applied for Val-158/Val-158 subjects; the converse was evident in patients, with 10/10-repeat subjects activating >9-repeat carriers in the Met-158/Met-158 group, and the opposite in the Val-158/Val-158 groups (Table 2 and Fig. 2). This group × COMT × DAT interaction accounted for 9% of the variance in activation in this region (ηp2 = 9%). Although in controls it was not, the COMT × DAT interaction effect in patients was significant (FWE P = 0.04), accounting for 16% of the interindividual variation (ηp2 = 16%). Again, the DAT effect in COMT heterozygotes was, in general, intermediate between that in the COMT homozygote groups (Fig. 2). Also, the interaction in the patient group was generally stronger than that in the control group (Fig. 2). No other 3-way interactions were detected at P < 0.001, uncorrected.
Table 2.
Interaction between effects of DAT 3′ UTR VNTR and COMT Val158Met genotypes on regional activation conditioned by diagnostic group
| Brain area | Controls, 10/10 > 9-car. in Val/Val > Met/Met | SCZ patients, 9-car. > 10/10 in Val/Val > Met/Met | Controls and SCZ patients, diagnosis × COMT × DAT |
|---|---|---|---|
| R media/posterior orbital gyrus | 26 30 −16 (2.2) | 26 28 −20 (4.3) FWEp = 0.04 | 26 28 −20 (4.3) FWEp = 0.04 |
All inferences significant at FWE P < 0.05 for a DAT × COMT effect in interaction with diagnostic group (last column) are reported (Z-scores in brackets). FWE correction is also shown for other effects that survive significance at the same threshold. SCZ, schizophrenia.
Fig. 2.
Nonadditive interaction between effects of DAT 3′ UTR VNTR, COMT Val158Met, and diagnostic group on activation. (A) Threshold for image was set at P < 0.001 (uncorrected) to show the significant effect in the right medial/posterior orbital gyrus (FWE, P < 0.05; plotted in B), and trends in the left superior frontal gyri and the right caudate nucleus. (B) The response profile in the right orbital gyrus showing a significant COMT × DAT interaction in schizophrenia patients and a trend for an interaction in the opposite direction in controls.
Effects of Potentially Confounding Factors on Activation.
Within the patient sample, there were no significant differences in the dose, duration, or type (first vs. second generation) of antipsychotic treatment between the genotype subgroups. Also, whole-brain regression analyses indicated that activation in the areas where there were significant effects of COMT genotype and/or of DAT genotype was not related to either the dose, type (first vs. second generation), or the duration of antipsychotic treatment, even at a liberal statistical threshold (P = 0.01, uncorrected). Also, when these medication variables were entered in each analysis as covariates of no interest, they did not change the foci of maximal significance or reduce the associated Z-scores. Similarly, introducing IQ, years of education, gender, and ethnicity as covariates of no interest did not alter any of the results.
Discussion
We have previously reported the separate effects of COMT Val158Met and DAT 3′ UTR VNTR genotype on fronto-temporal cortical activation during verbal fluency and the main effect of task and of diagnosis in this sample (27, 84). Both genes regulate brain dopaminergic transmission, and both polymorphisms are functional, in that they lead to differences in gene expression and/or protein function (13–17, 19–22). Therefore, the effect of a given polymorphism on fronto-temporal cortical activation during a cognitive task may not be the same across individuals who carry different variants of a second polymorphism.
The present data provide evidence for a significant interaction of DAT × COMT genotype (independent of diagnostic group) in the left parietal cortex. This interaction was also significant when the healthy volunteers group were analyzed alone, but not in the patient group. The supramarginal gyrus is thought to be involved in the integration of somatosensory, auditory, and visual input (61), and in the phonological and articulatory processing of words (62). It has consistently been engaged in previous studies of verbal fluency (47, 50, 53, 63, 64); it also has a role in semantic representation (65) and in decision making under conditions of uncertainty (66). In this area, subjects with the Met-158/Met-158 COMT genotype who carried the 9-repeat DAT allele showed more activation than Met-158/Met-158 subjects who carried 2 copies of the 10-repeat allele. Conversely, in Val-158 homozygotes, activation was greater in 10/10-repeat than 9-repeat carriers. These data suggest that, in addition to the main effects of each polymorphism on cortical function (27, 84), there are other effects that are conditional on a particular combination of COMT and DAT polymorphisms.
Additive effects of the COMT and DAT genotypes on activation in the precentral, anterior cingulate (12), and dorsolateral prefrontal cortex have previously been described in the context of a working memory task (12, 33). In those studies, linear regression analyses indicated that the Val-158 allele in combination with the 9-repeat was associated with the greatest activation, whereas the Met-158 allele combined with the 10-repeat was associated with the lowest. In the present study, a nonadditive (nonlinear) interaction was demonstrated, because both the combination of the Met-158 and 10-repeat alleles and the combination of the Val-158 and 9-repeat alleles were associated with relatively low left parietal activation. A nonadditive interaction between the effects of these 2 polymorphisms on prefrontal activation during a working memory task was recently suggested by a regression analysis, which included an independent factor predicting a priori different weights for COMT-DAT genotype combinations (35). We used a different method, an ANOVA to test for nonadditive effects. ANOVA avoids the need to assume the weights or relative effect sizes of the effects of DAT-COMT genotype groups on activation a priori. Another difference between the present study and previous studies of COMT × DAT interactions (12, 33–35, 84) is that we did not use a region-of-interest approach, but examined putative interactions corrected across the whole brain, in view of the lack of previous studies of nonadditive interactive effects of these genotypes on activation during verbal fluency. Thus, the statistical threshold we used was relatively more conservative.
The DAT 9-repeat allele has previously been associated with increased activation in the prefrontal cortex. This effect was interpreted as being secondary to effects of DAT on striatal function, given that the expression of DAT in the prefrontal cortex is relatively low (12, 33–35). However, DAT is generally present in higher concentrations in the parietal than the prefrontal cortex (4, 6, 67). This observation raises the possibility that variation in DAT may have relatively greater influence on the availability of dopamine in the synaptic cleft of dopaminergic terminals in the parietal cortex compared with cortical regions where removal of synaptic dopamine is primarily a function of COMT (5, 6, 9, 68). Thus, it would be reasonable to relate the COMT by DAT interaction to a local effect of DAT, although we cannot exclude the possibility that it was driven (instead or in addition) by an effect of DAT in another site connected to this region. In cortical areas that have relatively high levels of DAT, the contribution of the 2 enzymes to this process may be more similar, providing more scope for interactions between the effects of COMT and DAT genotypes. In these areas, if DAT is relatively more effective in removing dopamine, its action may negate the impact of a relatively ineffective variant of COMT, and vice versa. For example, whereas Val-158 is associated with high COMT activity and, thus, lower dopamine levels, the 9-repeat allele is associated with low DAT activity and, thus, higher dopamine levels. The converse would apply for the Met-158 and 10-repeat alleles. Thus, both combinations (Val-158–9-repeat and Met-158–10-repeat) may be associated with similar dopamine levels in the supramarginal gyrus, and a similar degree of cortical “efficiency” (or signal-to-noise ratio) (69, 70). However, other COMT–DAT combinations may exaggerate the effects of each allele on cortical dopamine levels. Thus, the combination of the 2 genotypes associated with the most active forms of COMT and DAT (Val-158 and 10-repeat) would be associated with maximal dopamine removal and the lowest dopamine levels. Conversely, the Met-158–9-repeat combination would be associated with the least effective dopamine removal and maximal cortical dopamine levels. We found that both these latter combinations were associated with increased activation in the supramarginal gyrus, consistent with the notion that cortical function becomes “inefficient” when local dopamine activity is either unusually low or unusually high (69, 70), with an inverted U-shaped relationship between cortical efficiency and local dopamine activity (Fig. S1).
There was an additional significant interaction between the effects of COMT and DAT in the right medial/posterior orbital gyrus that was specific to patients with schizophrenia and was not evident in healthy subjects. The interaction was significantly different in patients and healthy subjects, with a group × COMT × DAT interaction that accounted for 22% of the variance in this region. In patients, both the Val-158–9-repeat combination and the Met-158–10-repeat combination were associated with higher activation, whereas in healthy subjects, there was a trend in the opposite direction. Although its reports of association with verbal fluency and differential activation in schizophrenia have not been as frequent as those in the adjacent inferior frontal gyrus (46, 47, 50, 51, 53, 63, 64), the right orbitofrontal cortex has shown abnormal volume in schizophrenic patients (71–75) and significant activation during verbal fluency in females (50); it has also been linked to semantic processing (76, 77). An interaction between diagnostic group and genetic variations that influence dopamine transmission in the orbitofrontal cortex is also consistent with the presence of dopaminergic inputs to the prefrontal cortex, and with the putative disruption of dopaminergic inputs to prefrontal cortex in schizophrenia (36–40). Because there are relatively low levels of DAT in the prefrontal cortex (4, 6, 67), the effect of DAT genotype on activation in this region may reflect an effect at a site where DAT levels are higher that is connected to prefrontal cortex, such as the striatum, as suggested in previous studies (12, 33). However, it was not possible to test this possibility in the present study. As such, the 9-repeat allele, which is relatively ineffective at removing dopamine from striatal synapses, could have induced lower signal-to-noise (or lower cortical efficiency) in prefrontal pyramidal networks, compared with the 10-repeat. Schizophrenia is associated with an increase in presynaptic dopamine function (42–45). In patients with the DAT 9-repeat allele, the increased striatal dopamine activity associated with the disorder may be even more exaggerated, which may exacerbate the impaired efficiency of the prefrontal cortex also associated with schizophrenia (36–40). When the 9-repeat allele is combined with the Val-158 version of COMT, which is relatively ineffective at removing dopamine in the cortex, cortical dopamine levels will tend to decrease and reduce cortical efficiency even further in schizophrenia patients. This reasoning would explain why, in the present data, the Val-158–9-repeat combination is associated with increased activation in patients (for the same level of performance), but not in healthy subjects. Conversely, the Met-158–10-repeat combination was also associated with the same high level of activation in patients. This observation could mean that the putatively associated high levels of noise were inducing a lower prefrontal signal-to-noise ratio in patients. Thus, in schizophrenia, Val-158–9-repeat and the Met-158–10-repeat combinations may both be facilitating inefficient activation by decreasing the signal-to-noise ratio.
In addition to the finding in the orbitofrontal cortex, we detected trends (at P < 0.001, uncorrected) for similar group differences in the nature of the COMT × DAT interaction in the left superior frontal gyri (−16 62 20; Z = 3.9) and the right caudate nucleus (14 −2 18; Z = 3.5) (Fig. 2A). The prefrontal and striatal location of these interactions is of particular interest, because they are thought to be key sites of dopaminergic dysfunction in schizophrenia (36–40, 42–45). However, because these interactions were only evident at an uncorrected threshold (P < 0.001), they must be interpreted with caution. In sum, the interaction between the effects of variation in COMT and DAT may, thus, be different in patients from healthy subjects as a result of the perturbation of central dopamine function associated with schizophrenia. In schizophrenia, elevated striatal dopamine activity combined with reduced prefrontal dopamine activity may result in interactions between DAT and COMT that are not evident in healthy subjects, with variation in DAT influencing activity in the striatum and, thus indirectly, the cortex, and variation in COMT having direct effect in the cortex.
In general, the effect of DAT genotype in the findings discussed above was intermediate in the Val158Met heterozygote groups compared with the homozygote genotype groups, as evident in the plots of Figs. 1 and 2. This observation is in accordance with findings of allelic codominance in the Val158Met polymorphism (13–17).
Because all of the patients had been treated with antipsychotic medication, we cannot exclude the possibility that differences in the effects of genotype in patients compared with controls were related to medication (because these drugs have an antagonistic effect on central dopamine receptors), as opposed to an effect of schizophrenia. This issue could be addressed by repeating the study in medication naive patients, although this design would be logistically difficult. However, within the patient sample, neither the dose, type, nor duration of treatment differed between the genotype subgroups or were correlated with activation at the sites of group effects. Also, introducing these medication variables as covariates of no interest did not alter the peak foci of activation or the Z-scores. Thus, although effects of medication cannot be completely excluded, we could not find any evidence that the findings were attributable to effects of treatment. Because DAT and specifically the 3′ UTR VNTR has also been implicated, although not consistently, in the vulnerability to substance abuse disorders (78, 79), it is possible that the DAT subgroups differed with respect to smoking and alcohol consumption, which might have influenced the fMRI results. In the present study, we excluded subjects who met DSM-IV criteria for a substance misuse disorder, but did not measure the frequency of substance use among those who were included (SI Materials and Methods). We cannot exclude an additional effect of the noradrenaline transporter (NET) on cortical activation, given that it has been shown to have some affinity to synaptic dopamine (80, 81). Last, we acknowledge that the relatively small number of subjects in some cells may have limited our sensitivity and prevented us from detecting additional effects to those reported.
Although the data suggest that there is significant epistasis between the effects of DAT and COMT on cortical activation, the precise mechanisms underlying these interactions are unclear and will require cellular and molecular studies. Further levels of complexity relate to the effects of regional variation in the densities and types of dopamine receptors, the influence of tonic vs. phasic dopamine transmission, and the extent to which these factors are altered by a pathological afferent dopamine activity such as that in schizophrenia. Our findings suggest that an epistatic effect of COMT and DAT on brain function is more evident in certain areas than others, possibly reflecting regional variation in the relative levels of the 2 proteins. The different epistatic effect hereby found in schizophrenia may help explain why the results from genetic association studies of the genes for DAT and COMT in schizophrenia have been inconsistent, despite the biological plausibility of a role for these genes in the pathophysiology of a disorder of central dopamine function. In studies of DAT and COMT and dopamine function more generally, it may be advisable to assess both genotypes together, rather than either in isolation.
In conclusion, these data suggest that there are interactions between the effects of variations in the genes for different dopamine-regulating agents that affect regional brain function. Also, the nature of these interactions may be different in patients with schizophrenia and healthy volunteers. The latter may reflect the alteration in brain dopamine function associated with the disorder, although interactions between these genes and other effects of the disorder or with risk factors for the disorder (such as other genes) could also contribute.
Materials and Methods
Subjects.
A total of 85 subjects, all native English speakers, participated. Patients (n = 41) had established schizophrenia as defined by DSM-IV criteria, and healthy volunteers (n = 44) had no history of mental illness. For subject recruitment and demographic details, see SI Materials and Methods. All subjects, 90% of whom were Caucasian, were genotyped for the rs4680 SNP, which encodes the Val158Met polymorphism of COMT, and for the VNTR in the 3′ UTR of the DAT gene (as described in refs. 27 and 84). The participants comprised the following groups: within healthy subjects, there were 18 9-repeat carriers (of which 5 were Met-158/Met-158, 8 Met-158-Val-158, and 6 Val-158/Val-158), and 26 10-repeat homozygotes (9 Met-158/Met-158, 11 Met-158-Val-158, and 6 Val-158/Val-158). Among the patient sample, there were 18 9-repeat carriers (6 Met-158/Met-158, 7 Met-158-Val-158, and 5 Val-158/Val-158) and 23 10-repeat homozygotes (6 Met-158/Met-158, 10 Met-158-Val-158, and 7 Val-158/Val-158). There were no significant differences (P < 0.05) between the patient and healthy control groups in age, ethnicity, or handedness, but patients had a lower mean IQ, fewer years of education, and a higher proportion of males (Table S1). Between the genotype subgroups of either polymorphism, either in each diagnostic group or across the whole sample, there were no significant differences (P < 0.05) for any of the demographic, psychopathological, or medication variables (Tables S2 and S3). Two previous studies, investigating the effect of one SNP in the NRG1 gene and of the DISC1 gene, respectively, have used samples partially overlapping with those in the present study (85, 86).
Verbal Fluency Task and Image Acquisition.
The task and image acquisition was performed as described (53); for details, see SI Materials and Methods. Briefly, during a “generation” condition, subjects were visually presented with a series of letters and required to overtly articulate a word beginning with the presented letter. This condition was contrasted with a “repetition” condition, in which subjects were presented with the word “rest” and were required to say rest out loud. Task difficulty was manipulated by presenting separate sets of “easy” and “hard” letters (53). Verbal responses were recorded allowing us to identify “incorrect” trials, in which the subject did not generate any response or generated repetitions, derivatives, or grammatical variations of a previous word.
Behavioral Analysis.
The effect of task load, genotype (DAT and COMT), diagnosis, and their interaction on the level of accuracy of verbal responses (measured by the number of incorrect responses during scanning) were assessed by using a multivariate 2 × 2 × 2 ANOVA in SPSS (version 15.0), with diagnosis and genotype as between-subjects factors and task load as a within-subject factor.
Neuroimaging Analysis.
Analysis was performed with SPM5 software (www.fil.ion.ucl.ac.uk/spm) (82), running under Matlab 6.5 (Mathworks). To minimize movement-related artifacts, all volumes from each subject were realigned and unwarped (using the first as reference resliced with sinc interpolation), normalized to a standard MNI-305 template, and spatially smoothed with an 8-mm FWHM isotropic Gaussian kernel. First, the statistical analysis of regional responses was performed in a subject-specific fashion by convolving each onset time with a synthetic haemodynamic response function (HRF). To minimize performance confounds, we modeled correct and incorrect trials separately by using an event-related model. This design resulted in a total of 4 experimental conditions: (i) easy generation, (ii) hard generation, (iii) repetition, and (iv) incorrect responses. The latter condition was excluded from the group analysis to control for effects of group (genotype or diagnostic) differences in task performance on activation. Correct responses among the generation events (35 in the hard or 35 in the easy version) were contrasted with 70 repetition events. To remove low-frequency drifts, the data were high-pass filtered by using a set of discrete cosine basis functions with a cut-off period of 128 s. The parameter estimates were calculated for all brain voxels by using the general linear model, and contrast images for “easy generation > repetition” and “hard generation > repetition” were computed in a subject-specific fashion. Second, to permit inferences at the population level (83), the subject-specific contrast images were entered into a full-factorial ANOVA with 6 experimental groups based on the COMT genotype (i.e., Met-158/Met-158 controls; Val-158/Met-158 controls; Val-158/Val-158 controls; Met-158/Met-158 patients; Val-158/Met-158 patients; Val-158/Val-158 patients). To allow testing for multiplicative interactions between the 2 polymorphisms, DAT 3′ UTR VNTR genotype was added as an interacting covariate expressed with the values −1 (9-repeat carriers) and 1 (10-repeat homozygotes) in each of the 6 experimental groups. This design allowed us to test for differences in activation between the DAT genotype groups in each COMT group and for nonadditive interaction effects between genes, either group-independent, group-specific, or group-dependent. We did not restrict our search to areas that expressed significant activation for “word generation > repetition,” but considered the whole brain. Statistical inferences were made using by t tests at P < 0.05, corrected for multiple comparisons across the whole brain with voxelwise FWE rate. The t-images for each contrast at the second level were transformed into statistical parametric maps of the Z-statistic. We modeled task load to minimize error variance, but report results for the hard and easy conditions combined. To assess how much of the interindividual variance in blood-oxygen-level-dependent activation was explained by the genetic variation, we used the ηp2 measure of effect size in SPSS after extracting the subjects' β-measure at the voxel of peak activation from SPM5. To confirm that demographic and medication variables did not bias our analyses, we performed additional analyses by using them as covariates of no interest and a whole-brain regression analysis with each medication variable as a covariate.
Supplementary Material
Acknowledgments.
D.P.P. was funded by the Portuguese Fundacao para a Ciencia e Tecnologia. C.H.F. was funded by a Wellcome Traveling Fellowship. M.P. was funded by a Wellcome Trust Training Fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0903007106/DCSupplemental.
References
- 1.Masson J, et al. Unexpected localization of the Na+/Cl-dependent-like orphan transporter, Rxt1, on synaptic vesicles in the rat central nervous system. Eur J Neurosci. 1999;11:1349–1361. doi: 10.1046/j.1460-9568.1999.00540.x. [DOI] [PubMed] [Google Scholar]
- 2.Ciliax BJ, et al. The dopamine transporter: Immunochemical characterization and localization in brain. J Neurosci. 1995;15:1714–1723. doi: 10.1523/JNEUROSCI.15-03-01714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sesack SR, Carr DB. Selective prefrontal cortex inputs to dopamine cells: Implications for schizophrenia. Physiol Behav. 2002;77:513–517. doi: 10.1016/s0031-9384(02)00931-9. [DOI] [PubMed] [Google Scholar]
- 4.Lewis DA, et al. Dopamine transporter immunoreactivity in monkey cerebral cortex: Regional, laminar, and ultrastructural localization. J Comp Neurol. 2001;432:119–136. doi: 10.1002/cne.1092. [DOI] [PubMed] [Google Scholar]
- 5.Karoum F, Chrapusta SJ, Egan MF. 3-Methoxytyramine is the major metabolite of released dopamine in the rat frontal cortex: Reassessment of the effects of antipsychotics on the dynamics of dopamine release and metabolism in the frontal cortex, nucleus accumbens, and striatum by a simple two pool model. J Neurochem. 1994;63:972–979. doi: 10.1046/j.1471-4159.1994.63030972.x. [DOI] [PubMed] [Google Scholar]
- 6.Sesack SR, Hawrylak VA, Guido MA, Levey AI. Cellular and subcellular localization of the dopamine transporter in rat cortex. Adv Pharmacol. 1998;42:171–174. doi: 10.1016/s1054-3589(08)60720-6. [DOI] [PubMed] [Google Scholar]
- 7.Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ. Catechol-O-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci. 2004;24:5331–5335. doi: 10.1523/JNEUROSCI.1124-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huotari M, et al. Brain catecholamine metabolism in catechol-O-methyltransferase (COMT)-deficient mice. Eur J Neurosci. 2002;15:246–256. doi: 10.1046/j.0953-816x.2001.01856.x. [DOI] [PubMed] [Google Scholar]
- 9.Gogos JA, et al. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci USA. 1998;95:9991–9996. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cragg SJ, Rice ME. DAncing past the DAT at a DA synapse. Trends Neurosci. 2004;27:270–277. doi: 10.1016/j.tins.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-O-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol Psychiatry. 2006;60:141–151. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 12.Bertolino A, et al. Additive effects of genetic variation in dopamine regulating genes on working memory cortical activity in human brain. J Neurosci. 2006;26:3918–3922. doi: 10.1523/JNEUROSCI.4975-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saito S, et al. Identification of 197 genetic variations in six human methyltranferase genes in the Japanese population. J Hum Genet. 2001;46:529–537. doi: 10.1007/s100380170035. [DOI] [PubMed] [Google Scholar]
- 14.Spielman RS, Weinshilboum RM. Genetics of red cell COMT activity: Analysis of thermal stability and family data. Am J Med Genet. 1981;10:279–290. doi: 10.1002/ajmg.1320100311. [DOI] [PubMed] [Google Scholar]
- 15.Lotta T, et al. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: A revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34:4202–4210. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- 16.Lachman HM, et al. Human catechol-O-methyltransferase pharmacogenetics: Description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Chen J, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): Effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75:807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vandenbergh DJ, et al. Human dopamine transporter gene (DAT1) maps to chromosome 5p15.3 and displays a VNTR. Genomics. 1992;14:1104–1106. doi: 10.1016/s0888-7543(05)80138-7. [DOI] [PubMed] [Google Scholar]
- 19.Fuke S, et al. The VNTR polymorphism of the human dopamine transporter (DAT1) gene affects gene expression. Pharmacogenomics J. 2001;1:152–156. doi: 10.1038/sj.tpj.6500026. [DOI] [PubMed] [Google Scholar]
- 20.Mill J, et al. Expression of the dopamine transporter gene is regulated by the 3′ UTR VNTR: Evidence from brain and lymphocytes using quantitative RT-PCR. Am J Med Genet. 2002;114:975–979. doi: 10.1002/ajmg.b.10948. [DOI] [PubMed] [Google Scholar]
- 21.Heinz A, et al. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology. 2000;22:133–139. doi: 10.1016/S0893-133X(99)00099-8. [DOI] [PubMed] [Google Scholar]
- 22.VanNess SH, Owens MJ, Kilts CD. The variable number of tandem repeats element in DAT1 regulates in vitro dopamine transporter density. BMC Genet. 2005;6:55–66. doi: 10.1186/1471-2156-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diamond A, Briand L, Fossella J, Gehlbach L. Genetic and neurochemical modulation of prefrontal cognitive functions in children. Am J Psychiatry. 2004;161:125–132. doi: 10.1176/appi.ajp.161.1.125. [DOI] [PubMed] [Google Scholar]
- 24.Egan MF, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldberg TE, et al. Executive subprocesses in working memory: Relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Arch Gen Psychiatry. 2003;60:889–896. doi: 10.1001/archpsyc.60.9.889. [DOI] [PubMed] [Google Scholar]
- 26.Malhotra AK, et al. A functional polymorphism in the COMT gene and performance on a test of prefrontal cognition. Am J Psychiatry. 2002;159:652–654. doi: 10.1176/appi.ajp.159.4.652. [DOI] [PubMed] [Google Scholar]
- 27.Prata DP, et al. Opposite effects of catechol-O-methyltransferase Val158Met on cortical function in healthy subjects and patients with schizophrenia. Biol Psychiatry. 2008;65:473–480. doi: 10.1016/j.biopsych.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 28.Bertolino A, et al. Interaction of COMT (Val(108/158)Met) genotype and olanzapine treatment on prefrontal cortical function in patients with schizophrenia. Am J Psychiatry. 2004;161:1798–1805. doi: 10.1176/ajp.161.10.1798. [DOI] [PubMed] [Google Scholar]
- 29.Bertolino A, et al. Prefrontal-hippocampal coupling during memory processing is modulated by COMT Val158Met genotype. Biol Psychiatry. 2006;60:1250–1258. doi: 10.1016/j.biopsych.2006.03.078. [DOI] [PubMed] [Google Scholar]
- 30.Bertolino A, et al. Prefrontal dysfunction in schizophrenia controlling for COMT Val158Met genotype and working memory performance. Psychiatry Res. 2006;147:221–226. doi: 10.1016/j.pscychresns.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Blasi G, et al. Effect of catechol-O-methyltransferase Val158Met genotype on attentional control. J Neurosci. 2005;25:5038–5045. doi: 10.1523/JNEUROSCI.0476-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winterer G, et al. COMT genotype predicts BOLD signal and noise characteristics in prefrontal circuits. NeuroImage. 2006;32:1722–1732. doi: 10.1016/j.neuroimage.2006.05.058. [DOI] [PubMed] [Google Scholar]
- 33.Caldu X, et al. Impact of the COMT Val108/158 Met and DAT genotypes on prefrontal function in healthy subjects. NeuroImage. 2007;37:1437–1444. doi: 10.1016/j.neuroimage.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 34.Yacubian J, et al. Gene-gene interaction associated with neural reward sensitivity. Proc Natl Acad Sci USA. 2007;104:8125–8130. doi: 10.1073/pnas.0702029104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bertolino A, et al. Epistasis between dopamine regulating genes identifies a nonlinear response of the human hippocampus during memory tasks. Biol Psychiatry. 2008;64:226–234. doi: 10.1016/j.biopsych.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Akil M, et al. Catechol-O-methyltransferase genotype and dopamine regulation in the human brain. J Neurosci. 2003;23:2008–2013. doi: 10.1523/JNEUROSCI.23-06-02008.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weinberger DR, Berman KF, Chase TN. Mesocortical dopaminergic function and human cognition. Ann NY Acad Sci. 1988;537:330–338. doi: 10.1111/j.1749-6632.1988.tb42117.x. [DOI] [PubMed] [Google Scholar]
- 38.Weinberger DR, et al. Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry. 2001;50:825–844. doi: 10.1016/s0006-3223(01)01252-5. [DOI] [PubMed] [Google Scholar]
- 39.Abi-Dargham A, et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci. 2002;22:3708–3719. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akil M, et al. Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am J Psychiatry. 1999;156:1580–1589. doi: 10.1176/ajp.156.10.1580. [DOI] [PubMed] [Google Scholar]
- 41.Okubo Y, et al. Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature. 1997;385:634–636. doi: 10.1038/385634a0. [DOI] [PubMed] [Google Scholar]
- 42.Meyer-Lindenberg A, et al. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat Neurosci. 2002;5:267–271. doi: 10.1038/nn804. [DOI] [PubMed] [Google Scholar]
- 43.Bertolino A, et al. The relationship between dorsolateral prefrontal neuronal N-acetylaspartate and evoked release of striatal dopamine in schizophrenia. Neuropsychopharmacology. 2000;22:125–132. doi: 10.1016/S0893-133X(99)00096-2. [DOI] [PubMed] [Google Scholar]
- 44.Grace AA. Gating of information flow within the limbic system and the pathophysiology of schizophrenia. Brain Res Brain Res Rev. 2000;31:330–341. doi: 10.1016/s0165-0173(99)00049-1. [DOI] [PubMed] [Google Scholar]
- 45.Laruelle M. The role of endogenous sensitization in the pathophysiology of schizophrenia: Implications from recent brain imaging studies. Brain Res Brain Res Rev. 2000;31:371–384. doi: 10.1016/s0165-0173(99)00054-5. [DOI] [PubMed] [Google Scholar]
- 46.Yetkin FZ, et al. A comparison of functional MR activation patterns during silent and audible language tasks. AJNR Am J Neuroradiol. 1995;16:1087–1092. [PMC free article] [PubMed] [Google Scholar]
- 47.Lurito JT, et al. Comparison of rhyming and word generation with FMRI. Hum. Brain Mapp. 2000;10:99–106. doi: 10.1002/1097-0193(200007)10:3<99::AID-HBM10>3.0.CO;2-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hutchinson M, et al. Task-specific deactivation patterns in functional magnetic resonance imaging. Magn Reson Imaging. 1999;17:1427–1436. doi: 10.1016/s0730-725x(99)00093-4. [DOI] [PubMed] [Google Scholar]
- 49.Friedman L, et al. Brain activation during silent word generation evaluated with functional MRI. Brain Lang. 1998;64:231–256. doi: 10.1006/brln.1998.1953. [DOI] [PubMed] [Google Scholar]
- 50.Schlosser R, et al. Functional magnetic resonance imaging of human brain activity in a verbal fluency task. J Neurol Neurosurg Psychiatry. 1998;64:492–498. doi: 10.1136/jnnp.64.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Curtis VA, et al. Attenuated frontal activation during a verbal fluency task in patients with schizophrenia. Am J Psychiatry. 1998;155:1056–1063. doi: 10.1176/ajp.155.8.1056. [DOI] [PubMed] [Google Scholar]
- 52.Phelps EA, Hyder F, Blamire AM, Shulman RG. FMRI of the prefrontal cortex during overt verbal fluency. NeuroReport. 1997;8:561–565. doi: 10.1097/00001756-199701200-00036. [DOI] [PubMed] [Google Scholar]
- 53.Fu CH, et al. A functional magnetic resonance imaging study of overt letter verbal fluency using a clustered acquisition sequence: Greater anterior cingulate activation with increased task demand. NeuroImage. 2002;17:871–879. [PubMed] [Google Scholar]
- 54.Yurgelun-Todd DA, et al. Functional magnetic resonance imaging of schizophrenic patients and comparison subjects during word production. Am J Psychiatry. 1996;153:200–205. doi: 10.1176/ajp.153.2.200. [DOI] [PubMed] [Google Scholar]
- 55.Allen HA, Liddle PF, Frith CD. Negative features, retrieval processes, and verbal fluency in schizophrenia. Br J Psychiatry. 1993;163:769–775. doi: 10.1192/bjp.163.6.769. [DOI] [PubMed] [Google Scholar]
- 56.Howanitz E, Cicalese C, Harvey PD. Verbal fluency and psychiatric symptoms in geriatric schizophrenia. Schizophr Res. 2000;42:167–169. doi: 10.1016/s0920-9964(99)00137-1. [DOI] [PubMed] [Google Scholar]
- 57.Frith CD, et al. Regional brain activity in chronic schizophrenic patients during the performance of a verbal fluency task. Br J Psychiatry. 1995;167:343–349. doi: 10.1192/bjp.167.3.343. [DOI] [PubMed] [Google Scholar]
- 58.Fletcher PC, et al. Local and distributed effects of apomorphine on fronto-temporal function in acute unmedicated schizophrenia. J Neurosci. 1996;16:7055–7062. doi: 10.1523/JNEUROSCI.16-21-07055.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fu CH, et al. Effects of psychotic state and task demand on prefrontal function in schizophrenia: An fMRI study of overt verbal fluency. Am J Psychiatry. 2005;162:485–494. doi: 10.1176/appi.ajp.162.3.485. [DOI] [PubMed] [Google Scholar]
- 60.Artiges E, et al. Altered hemispheric functional dominance during word generation in negative schizophrenia. Schizophr Bull. 2000;26:709–721. doi: 10.1093/oxfordjournals.schbul.a033488. [DOI] [PubMed] [Google Scholar]
- 61.Clower DM, West RA, Lynch JC, Strick PL. The inferior parietal lobule Is the target of output from the superior colliculus, hippocampus, and cerebellum. J Neurosci. 2001;21:6283–6291. doi: 10.1523/JNEUROSCI.21-16-06283.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Celsis P, et al. Differential fMRI responses in the left posterior superior temporal gyrus and left supramarginal gyrus to habituation and change detection in syllables and tones. NeuroImage. 1999;9:135–144. doi: 10.1006/nimg.1998.0389. [DOI] [PubMed] [Google Scholar]
- 63.Hirshorn EA, Thompson-Schill SL. Role of the left inferior frontal gyrus in covert word retrieval: Neural correlates of switching during verbal fluency. Neuropsychologia. 2006;44:2547–2557. doi: 10.1016/j.neuropsychologia.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 64.Baldo JV, Schwartz S, Wilkins D, Dronkers NF. Role of frontal versus temporal cortex in verbal fluency as revealed by voxel-based lesion symptom mapping. J Int Neuropsychol Soc. 2006;12:896–900. doi: 10.1017/S1355617706061078. [DOI] [PubMed] [Google Scholar]
- 65.Xiao Z, et al. Differential activity in left inferior frontal gyrus for pseudowords and real words: An event-related fMRI study on auditory lexical decision. Hum Brain Mapp. 2005;25:212–221. doi: 10.1002/hbm.20105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vickery TJ, Jiang YV. Inferior parietal lobule supports decision making under uncertainty in humans. Cereb Cortex. 2009;19:916–925. doi: 10.1093/cercor/bhn140. [DOI] [PubMed] [Google Scholar]
- 67.Wang GJ, et al. Comparison of two PET radioligands for imaging extrastriatal dopamine transporters in human brain. Life Sci. 1995;57:L187–L191. doi: 10.1016/0024-3205(95)02099-5. [DOI] [PubMed] [Google Scholar]
- 68.Tunbridge E, Burnet PW, Sodhi MS, Harrison PJ. Catechol-O-methyltransferase (COMT) and proline dehydrogenase (PRODH) mRNAs in the dorsolateral prefrontal cortex in schizophrenia, bipolar disorder, and major depression. Synapse. 2004;51:112–118. doi: 10.1002/syn.10286. [DOI] [PubMed] [Google Scholar]
- 69.Goldman-Rakic PS, Muly EC, III, Williams GV. D(1) receptors in prefrontal cells and circuits. Brain Res Brain Res Rev. 2000;31:295–301. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- 70.Winterer G, Weinberger DR. Genes, dopamine, and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci. 2004;27:683–690. doi: 10.1016/j.tins.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 71.Gur RE, et al. Reduced dorsal and orbital prefrontal gray matter volumes in schizophrenia. Arch Gen Psychiatry. 2000;57:761–768. doi: 10.1001/archpsyc.57.8.761. [DOI] [PubMed] [Google Scholar]
- 72.Nakamura M, et al. Orbitofrontal volume deficit in schizophrenia and thought disorder. Brain. 2008;131:180–195. doi: 10.1093/brain/awm265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Convit A, et al. Volumetric analysis of the pre-frontal regions: Findings in aging and schizophrenia. Psychiatry Res. 2001;107:61–73. doi: 10.1016/s0925-4927(01)00097-x. [DOI] [PubMed] [Google Scholar]
- 74.Szeszko PR, et al. Investigation of frontal lobe subregions in first-episode schizophrenia. Psychiatry Res. 1999;90:1–15. doi: 10.1016/s0925-4927(99)00002-5. [DOI] [PubMed] [Google Scholar]
- 75.Lacerda ALT, et al. Morphology of the orbitofrontal cortex in first-episode schizophrenia: Relationship with negative symptomatology. Prog Neuro-Psychoph. 2007;31:510–516. doi: 10.1016/j.pnpbp.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 76.Duffau H, et al. New insights into the anatomo-functional connectivity of the semantic system: A study using cortico-subcortical electrostimulations. Brain. 2005;128:797–810. doi: 10.1093/brain/awh423. [DOI] [PubMed] [Google Scholar]
- 77.Mandonnet E, et al. Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain. 2007;130:623–629. doi: 10.1093/brain/awl361. [DOI] [PubMed] [Google Scholar]
- 78.Muramatsu T, Higuchi S. Dopamine transporter gene polymorphism and alcoholism. Biochem Biophys Res Commun. 1995;211:28–32. doi: 10.1006/bbrc.1995.1773. [DOI] [PubMed] [Google Scholar]
- 79.Vandenbergh DJ, et al. Smoking status and the human dopamine transporter variable number of tandem repeats (VNTR) polymorphism: Failure to replicate and finding that never-smokers may be different. Nicotine Tob Res. 2002;4:333–340. doi: 10.1080/14622200210142689. [DOI] [PubMed] [Google Scholar]
- 80.Moron JA, et al. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: Evidence from knockout mouse lines. J Neurosci. 2002;22:389–395. doi: 10.1523/JNEUROSCI.22-02-00389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gresch PJ, Sved AF, Zigmond MJ, Finlay JM. Local influence of endogenous norepinephrine on extracellular dopamine in rat medial prefrontal cortex. J Neurochem. 1995;65:111–116. doi: 10.1046/j.1471-4159.1995.65010111.x. [DOI] [PubMed] [Google Scholar]
- 82.Friston KJ. In: Human Brain Function. Frackowiak RS, et al., editors. New York: Academic; 2003. pp. 599–725. [Google Scholar]
- 83.Penny WD, Holmes AP, Friston KJ. In: Human Brain Function. Frackowiak RS, et al., editors. New York: Academic; 2003. pp. 725–911. [Google Scholar]
- 84.Prata DP, et al. Altered effect of DAT 3'UTR VNTR genotype on prefrontal and striatal function in schizophrenia. Arch Gen Psychiatry. 2009 doi: 10.1001/archgenpsychiatry.2009.147. in press. [DOI] [PubMed] [Google Scholar]
- 85.Mechelli A, et al. Genetic vulnerability to affective psychopathology in childhood: A combined voxel-based morphometry and functional magnetic resonance imaging study. Biol Psychiatry. 2009 Aug;66:231–237. doi: 10.1016/j.biopsych.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 86.Prata DP, et al. Effect of disrupted-in-schizophrenia-1 in pre-frontal cortical function. Mol Psychiatry. 2008;13:915–917. doi: 10.1038/mp.2008.76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.