Abstract
Diabetes is associated with poor outcomes following acute vascular occlusive events. This results in part from a failure to form adequate compensatory microvasculature in response to ischemia. Since vascular endothelial growth factor (VEGF) is an essential mediator of neovascularization, we examined whether hypoxic up-regulation of VEGF was impaired in diabetes. Both fibroblasts isolated from type 2 diabetic patients, and normal fibroblasts exposed chronically to high glucose, were defective in their capacity to up-regulate VEGF in response to hypoxia. In vivo, diabetic animals demonstrated an impaired ability to increase VEGF production in response to soft tissue ischemia. This resulted from a high glucose-induced decrease in transactivation by the transcription factor hypoxia-inducible factor-1α (HIF-1α), which mediates hypoxia-stimulated VEGF expression. Decreased HIF-1α functional activity was specifically caused by impaired HIF-1α binding to the coactivator p300. We identify covalent modification of p300 by the dicarbonyl metabolite methylglyoxal as being responsible for this decreased association. Administration of deferoxamine abrogated methylglyoxal conjugation, normalizing both HIF-1α/p300 interaction and transactivation by HIF-1α. In diabetic mice, deferoxamine promoted neovascularization and enhanced wound healing. These findings define molecular defects that underlie impaired VEGF production in diabetic tissues and offer a promising direction for therapeutic intervention.
Keywords: deferoxamine, HIF-1, hyperglycemia, ischemia
Vascular complications resulting from diabetes represent a significant public health burden (1). Over 40% of patients hospitalized with acute myocardial infarction have clinical diabetes, and another 35% exhibit impaired glucose tolerance (2). In the coronary circulation, impaired collateral vessel formation in diabetic hearts has been attributed to deficient ischemia-induced neovascularization (3, 4). Similarly, diabetic foot ulcers heal poorly as a result of impaired new vessel formation in response to tissue ischemia. At the cellular level, hypoxia induces expression of several angiogenic genes, most notably vascular endothelial growth factor (VEGF) (5). Existing evidence suggests that diabetes attenuates VEGF production, and diminished levels of VEGF have been observed in the myocardial tissue of diabetic patients (6, 7). VEGF levels are also reduced in diabetic animal models of wound repair (8). Despite its importance in blood vessel formation, the precise molecular mechanism responsible for VEGF insufficiency in diabetic tissue remains unknown.
In the setting of hypoxia, VEGF expression is directly controlled by the transcription factor hypoxia-inducible factor-1 (HIF-1), a master regulator of oxygen homeostasis that works to restore blood flow to ischemic regions (illustrated in Fig. S1) (9). Active HIF-1 is a heterodimer consisting of a hypoxia-stabilized α-subunit (HIF-1α) and a constitutively expressed β-subunit (HIF-1β). Under hypoxic conditions, stabilized HIF-1α translocates to the nucleus, dimerizes with HIF-1β, and binds to a conserved and defined hypoxia response element (5′-RCGTG-3′) present on a cascade of genes that work to enhance oxygen delivery (10). Recruitment of the coactivator protein p300 to the bound HIF-1 complex leads to transactivation of HIF-1-responsive genes (10).
In the present study, we investigated the molecular basis for impaired hypoxia-induced VEGF expression in diabetic tissues. Existing evidence suggests that hyperglycemia-mediated overproduction of superoxide by the mitochondrial electron transport system, with accumulation of glycolytic metabolites, is the common mechanism underlying the major pathways that produce diabetic complications (11). One of these metabolites is methylglyoxal, a degradation product of triose phosphates that forms stable adducts with intracellular proteins. We demonstrate that high glucose-induced methylglyoxal modifies the coactivator p300, which attenuates association of p300 with HIF-1α and prevents HIF-1-mediated gene transactivation. We also show that increased free iron (which has been associated with hyperglycemia) catalyzes the formation of reactive oxygen species (ROS) and methylglyoxal, with use of the iron chelator deferoxamine (DFO) attenuating ROS and methylglyoxal production in cells exposed to high glucose (12, 13). This results in decreased modification of p300 by methylglyoxal, correction of the high glucose-induced impairment in HIF-1α/p300 association, and normalization of the hypoxic response. In diabetic mice, topical application of DFO normalized healing of humanized wounds (14). Together, these data provide a molecular explanation for poor new blood vessel formation in diabetes, and offer a promising direction for the treatment of diabetic wounds.
Results
Cells from Diabetic Patients Do Not Up-Regulate VEGF During Hypoxia.
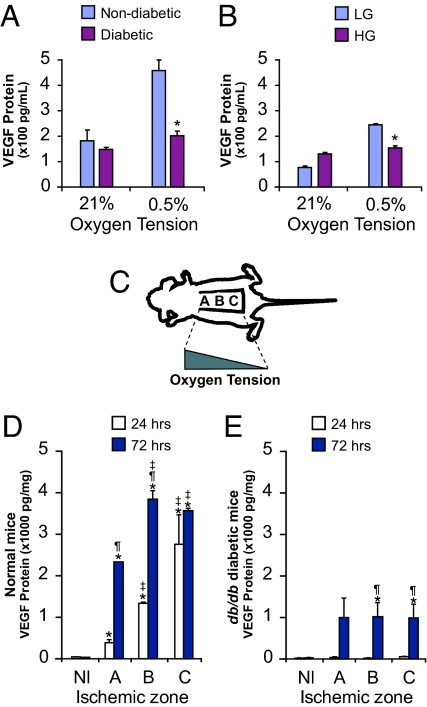
We evaluated the capacity of fibroblasts obtained from diabetic patients to up-regulate VEGF production in response to hypoxia. Dermal fibroblasts were isolated from type 2 diabetics and age-matched nondiabetic patients with vascular disease undergoing upper and lower extremity amputations. Fibroblasts from nondiabetic subjects up-regulated VEGF protein levels 2.2-fold in response to hypoxia (Fig. 1A). In contrast, fibroblasts isolated from diabetic patients were unable to demonstrate a significant increase in VEGF production (P = 0.09).
Fig. 1.
Diabetes and prolonged exposure to high glucose impair hypoxic induction of VEGF expression. Cells were placed in hypoxia (0.5% O2) or normoxia (21% O2), and levels of VEGF secretion were measured by ELISA. (A) Dermal fibroblasts isolated from diabetic patients produced less VEGF. (B) Fibroblasts from normal human foreskins were cultured chronically (4 weeks) in low glucose (LG) or high glucose (HG). VEGF protein expression was significantly attenuated in cells grown in HG and exposed to hypoxia. (C) Schematic depicting murine ischemic model used. Tissue oxygen tensions are highest in the most proximal (cranial) part of the tissue bed [A] and decrease in a gradient to the most distal (caudal) part [C]. (D) VEGF levels in ischemic skin segments of normal mice measured 24 and 72 h after ischemic induction. (E) VEGF protein levels in ischemic skin tissue from db/db mice. All values represent mean ± SEM. *, P < 0.05 vs. baseline. ‡, P < 0.05 vs. zone A. ¶, P < 0.05 vs. 24 h. n = 6 for all panels. Nl, normal skin.
On the basis of the above observations, we proceeded to evaluate whether cellular exposure to a high glucose environment was sufficient to induce the deficit in hypoxia-induced VEGF expression observed in diabetic fibroblasts. Normal human fibroblasts from nondiabetics without vascular disease were grown in low glucose (5 mM D-glucose) or high glucose (25 mM D-glucose) medium for 4 weeks to ensure chronic exposure. Cells were then placed in hypoxic conditions for 24 h, and VEGF protein levels were measured. Fibroblasts grown in low glucose up-regulated VEGF protein levels 3.2-fold, whereas cells cultured in high glucose increased VEGF expression by only 1.2-fold (P > 0.05 for the latter; Fig. 1B). A similar pattern of VEGF production was observed with murine cells grown chronically in high glucose culture (Fig. S2A). In contrast, cells acutely cultured in high glucose (<24 h) demonstrated no disparities in VEGF up-regulation compared to low glucose-treated cells, suggesting that extended exposure to a high glucose environment is required to compromise VEGF expression (Fig. S2B). This finding is consistent with the known time-course of methylglyoxal-modified proteins, which take longer than 24 h to accumulate (15).
Hypoxia-Induced VEGF Expression Is Impaired in Animal Models of Diabetes.
To exclude the possibility that the above findings were in vitro artifacts, analogous experiments were performed on wild-type and diabetic mice. Animals were subjected to a model of graded cutaneous ischemia developed in our laboratory (Fig. 1C) (16). In this model, tissue oxygen tensions are highest in the proximal (cranial) portion of a raised peninsular tissue bed and decrease in a gradient moving more distally. Although the gradient of oxygen tension was similar between wild-type and diabetic mice (Table S1), VEGF protein production in the setting of tissue ischemia differed significantly. Specifically, db/db diabetic mice demonstrated markedly lower levels of VEGF expression in comparison to wild-type mice at both 24 and 72 h post-injury (Fig. 1 D and E). Furthermore, VEGF up-regulation in db/db mice did not increase as an inverse function of tissue oxygen tension as was observed with wild-type mice at 24 h. Similar deficits were noted with streptozotocin (STZ)-induced diabetic mice (Fig. S2C). In aggregate, these data provide firm in vivo evidence to suggest a hyperglycemia-induced impairment in VEGF expression during tissue ischemia.
Chronic High Glucose Exposure Decreases HIF-1α Transactivation in Response to Hypoxia.
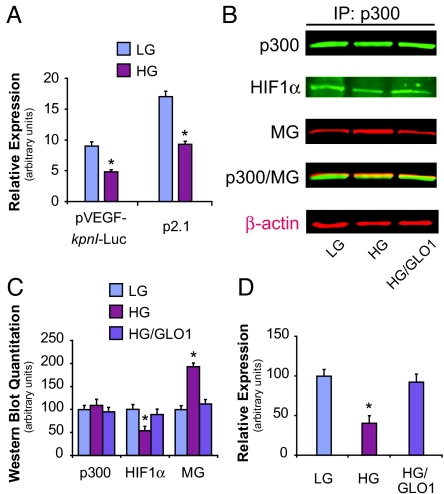
Given our observation of decreased VEGF expression in the high glucose hypoxic environment, we examined the effect of chronic hyperglycemic exposure on the capacity of HIF-1α to induce gene transcription. Cells were transiently transfected with a plasmid containing a luciferase reporter gene downstream of the full-length VEGF-A promoter (pVEGF-kpnI-luc). In addition to containing the hypoxia response element, the VEGF-A promoter also includes enhancer sequences and other transcription factor binding sites that influence VEGF transcription. Hypoxic induction of the full-length VEGF promoter construct was significantly impaired in high glucose compared to low glucose conditions (Fig. 2A). Analogous results were obtained with the use of a reporter construct containing only the hypoxia response element for the HIF-regulated enolase gene upstream of a luciferase reporter (plasmid p2.1) (17). Western blot analysis failed to demonstrate any decrease in HIF-1α stability in the setting of high glucose culture (Fig. S3). Taken together, these data suggest that the high glucose-induced impairment in HIF-mediated gene transcription results from a primary defect in HIF transactivation and not as a consequence of impaired HIF-1α stability or as a result of factors acting on other promoter elements.
Fig. 2.
Hyperglycemia-induced impairment in HIF-1 transactivation occurs as a result of decreased HIF-1α binding to p300. (A) Plasmids containing a full-length VEGF-A promoter (pVEGF-kpnI-luc) or the hypoxia response element for the enolase gene (p2.1), both linked to firefly luciferase, were transfected into cells and subsequently exposed to hypoxia. HG exposure decreased HIF-1 transactivation with use of either plasmid. (B) HAECs were treated for 5 days with LG, HG, or HG after infection of GLO1 adenovirus (HG/GLO1). After exposure to hypoxia for 18 h, cell lysates were collected for immunoprecipitation (IP) of p300 and subsequently immunoblotted (IB) for p300, methylglyoxal (MG), or HIF-1α. β-actin was used as an input control (shown in red typeface). (C) Western blot quantitation of panel B. (D) HAECs were transfected with plasmids containing the HIF-1α-CAD and the p300-CH1 domain together with a reporter plasmid containing a Gal4-binding motif upstream of a firefly luciferase reporter gene. Cells were grown in LG or HG and exposed to normoxia or hypoxia, and the level of luciferase reporter activity subsequently measured. Impaired HIF-1α/p300 binding in HG was completely reversed with GLO1 treatment. *, P < 0.05 vs. LG group. n = 3.
The Interaction Between HIF-1α and Its Coactivator p300 Is Decreased by High Glucose Exposure.
Once the HIF complex associates with the hypoxia response element of target genes, a central mechanism regulating HIF-1α transcriptional activity is modulation of its N- and C-terminal transactivation domains (NAD and CAD, respectively) by transcriptional coactivators including p300, src-1, and TIF-2 (18). The binding of the cysteine/histidine-rich (CH1) region of the coactivator p300 to the HIF-1α-CAD is especially critical for HIF-1 transcriptional activity (18–20). As described previously, covalent modification of proteins by methylglyoxal propagates diabetic complications at the cellular level. We consequently evaluated whether attenuated HIF-1 transcriptional activity in the setting of high glucose derives from a methylglyoxal-induced impairment in HIF-1α/p300 binding. Immunoprecipitation/Western blot (IP/WB) analysis demonstrated that chronic incubation of cells in high glucose decreased HIF-1α/p300 association by ≈50%, with a commensurate increase in methylglyoxal modification of p300 (Fig. 2 B and C). This was prevented by overexpression of glyoxalase 1 (GLO1), the rate-limiting enzyme of methylglyoxal catabolism. High glucose-induced impairment in HIF-1α/p300 binding was also noted using the mammalian 2-hybrid system and was similarly reversed with GLO1 overexpression (Fig. 2D). Analogous results were observed with dermal fibroblasts obtained from STZ-induced diabetic mice. Fibroblasts harvested from these mice and exposed to hypoxia were only able to up-regulate VEGF expression 1.23-fold, significantly lower than the 2.89-fold increase observed with cells obtained from wild-type mice (Fig. S4). Overexpression of GLO1 in diabetic fibroblasts normalized VEGF expression.
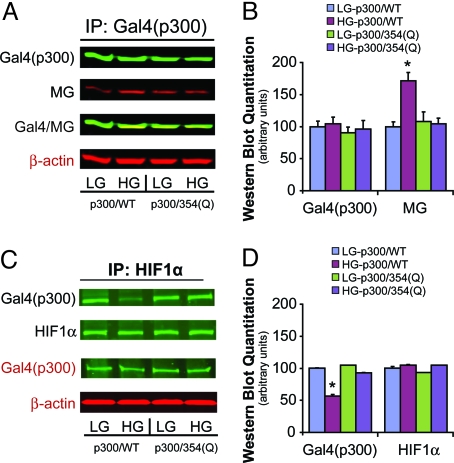
Methylglyoxal reacts with arginine (R) and lysine (K) residues in vivo to form the major methylglyoxal-derived epitope hydroimidazolone (21). Given the importance of the CH1 domain of p300 (amino acids 347–411) in the coactivator's interaction with HIF-1α, we evaluated the possible role of each R and K residue in this region as a potential site for methylglyoxal modification. Point mutants were created that converted each of these residues to glutamine (Q). IP/WB demonstrated that mutation of R354 completely prevented high glucose-induced methylglyoxal modification of p300 (Fig. 3 A and B). Mutation of other relevant residues within the CH1 domain had no effect. In addition to preventing formation of p300-methylglyoxal adducts, the R354Q mutant lost the high glucose-induced impairment in HIF-1α association observed with the wild-type p300 protein (Fig. 3 C and D).
Fig. 3.
High glucose-induced methylglyoxal modification of p300 at R354 decreases association of p300 with HIF-1α. (A) HAECs were transfected with either Gal4(AD) wild-type p300 or p300 single-mutant 354(Q), followed by treatment for 5 days in LG or HG, and subsequent exposure to 18 h of hypoxia. Cell lysates were collected for IP of Gal4(p300) and IB for Gal4(p300) and methylglyoxal (MG). (B) Western blot quantitation of panel A. (C) HAECs were treated as in panel A. Lysates were collected for IP of HIF-1α and IB for Gal4(p300) and HIF-1α. (D) Western blot quantitation of panel C. Immunoprecipitation inputs (shown in red typeface) were used as loading controls. *, P < 0.05 vs. LG group. n = 3. AD, activation domain.
We have previously demonstrated that high glucose culture can also result in the development of HIF-1α-methylglyoxal adducts (22). Formation of these adducts culminates in impaired HIF-1α-HIF-1β heterodimerization and a resultant decrease in HIF-1/DNA binding. Methylglyoxal modification of HIF-1α did not impair HIF-1α/p300 binding, but did produce a decrease in VEGF expression, suggesting that impairment of both HIF-1α-HIF1-β dimerization and HIF-1α-p300 association underlie the diabetes-induced defect in HIF-1 transcriptional activity (Fig. S5 and SI Text).
DFO Improves HIF-1α/p300 Binding and Augments HIF-1 Transactivation and VEGF Expression in High Glucose Culture.
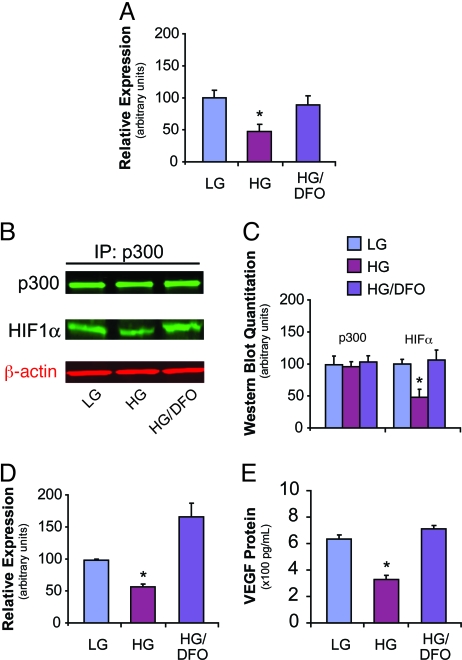
As stated previously, high glucose-induced superoxide formation increases intracellular hydroxyl radicals, in turn leading to excess methylglyoxal accumulation (11). In this context, DFO may improve HIF-1α/p300 interaction by scavenging hydroxyl radical-generating free iron (13). DFO has also been reported to increase HIF-1α stability and transactivation through sequestration of iron, a necessary cofactor for several enzymes involved in HIF-1 degradation (23, 24). We therefore proceeded to evaluate the effect of DFO administration on HIF-1α/p300 association using the mammalian 2-hybrid system. For cells cultured in high glucose, DFO treatment resulted in a 2-fold increase in HIF-1α/p300 binding under hypoxic conditions, approximating levels of association observed with cells exposed to low glucose (Fig. 4A). IP/WB assays corroborated this observation, demonstrating that administration of DFO to cells cultured in high glucose normalized HIF-1α/p300 association (Fig. 4 B and C).
Fig. 4.
DFO corrects HIF-1α/p300 binding and augments HIF-1 transactivation and VEGF expression in high glucose culture. (A) HAECs cultured with LG, HG, or HG with DFO (HG/DFO) were exposed to hypoxia and evaluated by mammalian 2-hybrid assay for HIF-1α/p300 binding. DFO treatment augmented HIF-1α/p300 binding in cells exposed to HG. (B) IP/WB of p300 and HIF-1α following treatment described in panel A. β-actin was used as an input control (shown in red typeface). (C) Western blot quantitation of panel B. (D) HIF-1 transactivation analyzed with the 5× hypoxia response element construct described in the Results section. DFO increased HIF-1-mediated luciferase activity in cells exposed to HG in mouse embryonic fibroblasts (MEFs). (E) VEGF ELISA demonstrating DFO's augmentation of VEGF expression by MEFs cultured in HG. *, P < 0.05 vs. HG/DFO group. n = 3.
As DFO treatment appeared to markedly improve the interaction between HIF-1α and p300, we evaluated if use of the compound would also correct the high glucose-induced impairment in HIF-1 transactivation. HIF-1 activity was evaluated using a construct consisting of 5 tandem repeats of the VEGF promoter hypoxia response element upstream of a luciferase gene (25). In cells exposed to high glucose, DFO administration reversed the blunted response to hypoxia and increased luciferase activity by nearly 3-fold (Fig. 4D). This was accompanied by a greater than 2-fold increase in VEGF protein expression, with levels of the cytokine equal to those expressed by cells exposed to low glucose (Fig. 4E).
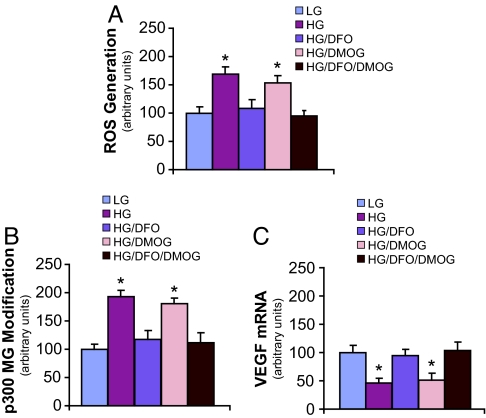
DFO Decreases ROS Generation, Prevents Methylglyoxal Modification of p300, and Restores Hypoxia-Induced VEGF Expression in High Glucose Culture.
Given our data demonstrating that methylglyoxal modification of p300 underlies its impaired interaction with HIF-1α in high glucose culture, we evaluated the potential role of DFO in mitigating the formation of methylglyoxal-protein adducts. As described previously, methylglyoxal accumulation in cells subjected to high glucose is the direct result of increased ROS levels (15). In the present study, administration of DFO to cells incubated in high glucose medium resulted in decreased ROS generation, with resultant ROS levels similar to those observed in low glucose culture (Fig. 5A). This effect was not observed with the addition of dimethyloxalylglycine (DMOG), a competitive inhibitor of several enzymes involved in HIF-1α degradation (26). DMOG administration is associated with increased HIF-1α stability. Simultaneous use of DMOG with DFO provided no further decrease in ROS generation over DFO addition alone.
Fig. 5.
DFO decreases ROS generation, prevents methylglyoxal modification of p300, and increases VEGF expression in high glucose culture. HAECs were treated for 5 days in LG, HG, HG with DFO (HG/DFO), HG with DMOG (HG/DMOG), or HG with DFO and DMOG (HG/DFO/DMOG). The following evaluations were then performed: (A) ROS generation, (B) IP/WB of p300 modification by methylglyoxal (MG), and (C) VEGF mRNA expression. *, P < 0.05 vs. LG group. n = 3.
High glucose-induced methylglyoxal modification of p300 was almost completely prevented in the presence of DFO (Fig. 5B). This was paralleled by an increase in VEGF expression in DFO-treated cells, with levels of the cytokine equivalent to those observed in low glucose culture (Fig. 5C). These findings were not observed with administration of DMOG, nor was there any further decrease in the development of methylglyoxal-protein adducts, or increase in VEGF expression, when DMOG was used in combination with DFO. These data are consistent with DFO's iron chelating effect and resultant attenuation of hydroxyl radical formation, rather than its potential influence on HIF-1α protein stability, as the mechanism by which it normalizes the high glucose-induced defect in HIF-1-mediated transactivation.
DFO Enhances Humanized Wound Healing and Neovascularization in Diabetic Mice.
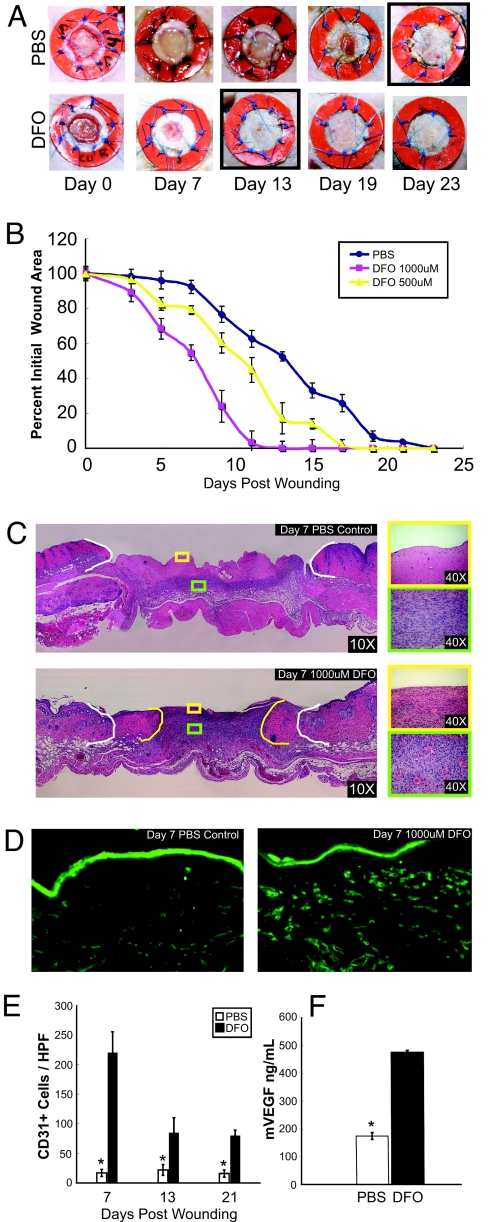
Decreased VEGF production within the ischemic environment of diabetic wounds markedly hinders the neovascularization necessary for proper wound healing, a finding documented in numerous studies (7, 8, 27, 28). Given DFO's demonstrated therapeutic benefit in the in vitro environment, we evaluated its potential efficacy in the in vivo setting. A model of wounding developed by our laboratory was used to examine the effect of DFO on the wound healing properties of db/db mice (14). Our model uses physical stenting to correct for the contracture that occurs in rodent wounds. Thus, wounds heal by granulation tissue deposition and subsequent reepithelialization as occurs in human beings, rather than by contraction, which occurs in conventional rodent wound models.
Serial topical application of 1,000 μM DFO on the wounds of diabetic mice significantly enhanced the rate of wound healing, with complete resurfacing occurring by 13 days in DFO-treated mice versus 23 days in untreated mice (Fig. 6 A and B). The wound healing rate with DFO administration was dose-dependent, with mice receiving 1,000 μM DFO showing faster rates of closure than those receiving 500 μM, and approaching those previously observed in nondiabetic mice. Both granulation tissue deposition (evaluated by H&E staining) and neovascularization (measured by the number of CD31-positive cells) were more abundant in wounds receiving topical DFO (Fig. 6 C and D). By day 7, DFO-treated wounds had 13-fold more CD31-positive cells than PBS-treated wounds (Fig. 6E). This increase in neovascularization was associated with 2.7-fold greater levels of VEGF expression in the wound beds of DFO-treated mice (Fig. 6F). In aggregate, these data illustrate DFO's potential therapeutic efficacy for the treatment of diabetic wounds, a phenomenon that is at least partly secondary to up-regulated VEGF expression and enhanced neovascularization.
Fig. 6.
DFO enhances wound healing and neovascularization in diabetic mice. Full-thickness skin wounds on diabetic mice were treated with every other day topical applications of 500 μM DFO, 1,000 μM DFO, or PBS. (A) Photographs of representative wounds for mice treated with PBS or 1,000 μM DFO. Wound resurfacing occurred at day 13 in the DFO-treated group versus day 23 in PBS-treated controls. (B) Graphical depiction of wound area as a function of time. DFO accelerated wound healing in a dose-dependent manner. (C) H&E stains of wound tissue at 7 days post-wounding showing increased granulation tissue in the wounds of DFO-treated animals. White borders delineate initial wound edges, and yellow borders indicate wound edges at day 7. (D) DFO- and PBS-treated mice were analyzed at 7 days post-wounding for vascularity (as assessed by CD31 immunohistochemistry). (E) Numbers of CD31-positive vessels per HPF in DFO-treated (1,000 μM) and PBS-treated mice at days 7, 13, and 21 post-wounding. (F) Wound tissue VEGF concentration at 7 days post-wounding for DFO-treated (1,000 μM) and PBS-treated mice. *, P < 0.05 vs. PBS group. n = 8.
Discussion
In this report, we show that VEGF production in response to hypoxia is decreased in cells obtained from diabetic patients and in ischemic tissue from diabetic animals. Although HIF-1α protein stability was unaffected by high glucose exposure, HIF-1-mediated transactivation was significantly impaired due to decreased association of HIF-1α and its coactivator p300, a consequence of ROS-induced modification of p300 by the GLO1 substrate methylglyoxal. The iron chelator DFO, used systemically in the treatment of chronic iron overload, corrected HIF-1 activity in high glucose culture by preventing iron-catalyzed ROS and methylglyoxal formation, and topical application of DFO-normalized healing of humanized diabetic wounds in mice.
Given the fundamental role of HIF-1 in the neovascular process, the mechanism described here may help explain how diabetes impairs new blood vessel formation across multiple organ systems. For example, in the process of vasculogenesis, endothelial progenitor cells (EPCs) from the bone marrow are mobilized to ischemic sites in the periphery to participate in new blood vessel development (29, 30). We have previously demonstrated that diabetes attenuates EPC mobilization, and more recent investigation suggests that this defect may be secondary to impaired endothelial nitric oxide synthase (eNOS) activation in bone marrow cells (31, 32). Diabetes also exerts an effect in peripheral tissue, where decreased expression of the chemokine stromal cell-derived factor-1 (SDF-1) results in diminished EPC recruitment (22, 32). As both eNOS and SDF-1 are HIF-1-regulated genes, our study findings provide a molecular explanation for how cells in such different tissue compartments may be similarly affected by a shared pathobiologic process (29, 30).
Development of foot ulcers in diabetics often takes decades and is promoted by late, time-dependent consequences of chronic hyperglycemia such as neuropathy. While the mouse lifespan may be too short to permit development of such sequelae, the dramatic impairment in wound healing observed with the diabetic mice used in our studies suggests that these late consequences of chronic hyperglycemia are not required for defective diabetic wound healing. It should be noted that native rodent wound healing differs significantly from human healing (14). In mice, most of the actual closure of the wound occurs as contraction (analogous to healing by primary intention). Humans, by contrast, heal wounds by secondary intention, which requires granulation tissue deposition and reepithelialization. Because muscular contraction in rodents is the primary factor driving wound healing, potential therapeutics that accelerate healing of rodent wounds have provided little translational success when used in humans. In addition, prior studies have often not used an end point based on wound closure, which is the only relevant outcome from a clinical perspective (33). Our studies used a wound healing model that replicates the unique aspects of human wound healing.
Presently, there is little to no evidence to support the use of topically applied therapeutics in the treatment of diabetic foot ulcers (34). Consequently, our finding that topical DFO completely normalizes healing of humanized diabetic wounds has significant clinical implications. Poor diabetic wound healing is a major health care burden, costing billions of dollars yearly in the United States alone. To the best of our knowledge, no published studies have evaluated the efficacy of either systemically or locally delivered DFO in the treatment of human diabetic wounds. Since systemic DFO has been in clinical use for decades, the potential of topical DFO to heal diabetic wounds in humans could be evaluated relatively quickly. Continued effort will be directed at identifying therapeutics that, like DFO, do not target hyperglycemia per se, but instead address defects in the HIF-mediated cellular cascade that culminate in diabetic complications in response to ischemia.
Methods
See SI Methods for further information.
Cell Culture.
Cells were maintained in DMEM (Gibco). DFO solution (100 μM; EMD Biosciences) was used in indicated experiments. Cultures were exposed to hypoxia in a custom-designed incubator (X Vivo Hypoxia Chamber; BioSpherix) set at 0.5% O2 and 5% CO2 or normoxia (21% O2 and 5% CO2); 1% O2 was used for experiments involving human aortic endothelial cells (HAECs).
Western Blotting.
Protein detection was performed with primary antibodies against p300, HIF-1α (Novus Biologicals), and β-actin (Thermo Fisher Scientific). Blots were simultaneously incubated with differentially labeled species-specific secondary antibodies after transfer to membranes [anti-rabbit IRDyeTM 800CW (green) and anti-mouse Alexa 680 (red); LI-COR Biosciences].
Reporter Assays.
Plasmid p2.1 and the 5× hypoxia response element were gifts from Drs. Gregg Semenza and Amato Giaccia, respectively. These reporter plasmids and pVEGF-kpnI-Luc (described in SI Methods) were transfected with Lipofectamine (Invitrogen), and activity was determined using the Dual Luciferase System (Promega).
Mammalian 2-Hybrid Assay.
Expression plasmids for experiments evaluating the interaction between the HIF-1α-CAD and the p300-CH1 domain were a kind gift from Dr. L. Eric Huang (35). Specific deletions and point mutations for mapping of the methylglyoxal-responsive sites in p300 were prepared using the Site-Directed Mutagenesis kit from Promega. All constructs were verified by DNA sequencing.
Mouse Models of Ischemia and Wounding.
Wild-type and diabetic mice were subjected to a previously described model of cutaneous ischemia (16). For experiments evaluating the effect of DFO on murine wound healing, paired 6-mm full-thickness cutaneous wounds were made on the dorsa of mice (14). A donut-shaped 12-mm silicone splint (Grace Bio-Labs) was placed around the wounds. Ten microliters 1,000 μM or 500 μM DFO, or PBS vehicle, was introduced into the wound bed. At indicated time points, wounds were excised and snap-frozen or, alternatively, processed for H&E staining. Vascular density was detected on frozen sections using a CD31 monoclonal antibody (BD Biosciences).
Statistical Analyses.
All data are expressed as mean ± the standard error of the mean (SEM). Statistical analysis of results from the hypoxic induction of VEGF was carried out using factorial ANOVA, and results from the peripheral wound healing model were analyzed using a paired Student's t-test. Analysis for the remainder of the experiments were evaluated by the analysis of variance and Tukey-Kramer statistical tests. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments.
This work was supported by grant no. RO1-DK-074095 from the National Institute of Diabetes and Digestive and Kidney Diseases (to G.C.G.), and grant no. RO1-DK074153 from the National Institute of Diabetes and Digestive and Kidney Diseases (to M.B.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906670106/DCSupplemental.
References
- 1.Caro JJ, Ward AJ, O'Brien JA. Lifetime costs of complications resulting from type 2 diabetes in the U.S. Diabetes Care. 2002;25:476–481. doi: 10.2337/diacare.25.3.476. [DOI] [PubMed] [Google Scholar]
- 2.Norhammar A, et al. Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: A prospective study. Lancet. 2002;359:2140–2144. doi: 10.1016/S0140-6736(02)09089-X. [DOI] [PubMed] [Google Scholar]
- 3.Abaci A, et al. Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation. 1999;99:2239–2242. doi: 10.1161/01.cir.99.17.2239. [DOI] [PubMed] [Google Scholar]
- 4.Wilson PW. Diabetes mellitus and coronary heart disease. Am J Kidney Dis. 1998;32(Suppl 3):S89–S100. doi: 10.1053/ajkd.1998.v32.pm9820468. [DOI] [PubMed] [Google Scholar]
- 5.Yla-Herttuala S, Rissanen TT, Vajanto I, Hartikainen J. Vascular endothelial growth factors: Biology and current status of clinical applications in cardiovascular medicine. J Am Coll Cardiol. 2007;49:1015–1026. doi: 10.1016/j.jacc.2006.09.053. [DOI] [PubMed] [Google Scholar]
- 6.Chou E, et al. Decreased cardiac expression of vascular endothelial growth factor and its receptors in insulin-resistant and diabetic States: A possible explanation for impaired collateral formation in cardiac tissue. Circulation. 2002;105:373–379. doi: 10.1161/hc0302.102143. [DOI] [PubMed] [Google Scholar]
- 7.Lerman OZ, Galiano RD, Armour M, Levine JP, Gurtner GC. Cellular dysfunction in the diabetic fibroblast: Impairment in migration, vascular endothelial growth factor production, and response to hypoxia. Am J Pathol. 2003;162:303–312. doi: 10.1016/S0002-9440(10)63821-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frank S, et al. Regulation of vascular endothelial growth factor expression in cultured keratinocytes. Implications for normal and impaired wound healing. J Biol Chem. 1995;270:12607–12613. doi: 10.1074/jbc.270.21.12607. [DOI] [PubMed] [Google Scholar]
- 9.Forsythe JA, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Covello KL, Simon MC. HIFs, hypoxia, and vascular development. Curr Top Dev Biol. 2004;62:37–54. doi: 10.1016/S0070-2153(04)62002-3. [DOI] [PubMed] [Google Scholar]
- 11.Brownlee M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 12.Strohmeyer G, et al. Abnormalities in estrogen, androgen, and insulin metabolism in idiopathic hemochromatosis. Ann NY Acad Sci. 1988;526:209–223. doi: 10.1111/j.1749-6632.1988.tb55507.x. [DOI] [PubMed] [Google Scholar]
- 13.Trivin F, Beaumont C, Emerit J. Iron metabolism, free radicals, and oxidative injury. Biomed Pharmacother. 2001;55:333–339. doi: 10.1016/s0753-3322(01)00068-3. [DOI] [PubMed] [Google Scholar]
- 14.Galiano RD, Michaels J, Dobryansky M, Levine JP, Gurtner GC. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen. 2004;12:485–492. doi: 10.1111/j.1067-1927.2004.12404.x. [DOI] [PubMed] [Google Scholar]
- 15.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 16.Tepper OM, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 17.Semenza GL, et al. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase a gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem. 1996;271:32529–32537. doi: 10.1074/jbc.271.51.32529. [DOI] [PubMed] [Google Scholar]
- 18.Arany Z, et al. An essential role for p300/CBP in the cellular response to hypoxia. Proc Natl Acad Sci USA. 1996;93:12969–12973. doi: 10.1073/pnas.93.23.12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ema M, et al. Molecular mechanisms of transcription activation by HLF and HIF1alpha in response to hypoxia: Their stabilization and redox signal-induced interaction with CBP/p300. EMBO J. 1999;18:1905–1914. doi: 10.1093/emboj/18.7.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kung AL, Wang S, Klco JM, Kaelin WG, Livingston DM. Suppression of tumor growth through disruption of hypoxia-inducible transcription. Nat Med. 2000;6:1335–1340. doi: 10.1038/82146. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed N, Thornalley PJ. Quantitative screening of protein biomarkers of early glycation, advanced glycation, oxidation and nitrosation in cellular and extracellular proteins by tandem mass spectrometry multiple reaction monitoring. Biochem Soc Trans. 2003;31:1417–1422. doi: 10.1042/bst0311417. [DOI] [PubMed] [Google Scholar]
- 22.Ceradini DJ, et al. Decreasing intracellular superoxide corrects defective ischemia-induced new vessel formation in diabetic mice. J Biol Chem. 2008;283:10930–10938. doi: 10.1074/jbc.M707451200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Appelhoff RJ, et al. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem. 2004;279:38458–38465. doi: 10.1074/jbc.M406026200. [DOI] [PubMed] [Google Scholar]
- 24.Hirsila M, et al. Effect of desferrioxamine and metals on the hydroxylases in the oxygen sensing pathway. Faseb J. 2005;19:1308–1310. doi: 10.1096/fj.04-3399fje. [DOI] [PubMed] [Google Scholar]
- 25.Shibata T, Giaccia AJ, Brown JM. Development of a hypoxia-responsive vector for tumor-specific gene therapy. Gene Ther. 2000;7:493–498. doi: 10.1038/sj.gt.3301124. [DOI] [PubMed] [Google Scholar]
- 26.Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5:343–354. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- 27.Martin A, Komada MR, Sane DC. Abnormal angiogenesis in diabetes mellitus. Med Res Rev. 2003;23:117–145. doi: 10.1002/med.10024. [DOI] [PubMed] [Google Scholar]
- 28.Walters DP, Gatling W, Mullee MA, Hill RD. The distribution and severity of diabetic foot disease: A community study with comparison to a non-diabetic group. Diabet Med. 1992;9:354–358. doi: 10.1111/j.1464-5491.1992.tb01796.x. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi T, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 30.Ceradini DJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 31.Capla JM, et al. Diabetes impairs endothelial progenitor cell-mediated blood vessel formation in response to hypoxia. Plast Reconstr Surg. 2007;119:59–70. doi: 10.1097/01.prs.0000244830.16906.3f. [DOI] [PubMed] [Google Scholar]
- 32.Gallagher KA, et al. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J Clin Invest. 2007;117:1249–1259. doi: 10.1172/JCI29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Botusan IR, et al. Stabilization of HIF-1alpha is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci USA. 2008;105:19426–19431. doi: 10.1073/pnas.0805230105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hinchliffe RJ, et al. A systematic review of the effectiveness of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Diabetes Metab Res Rev. 2008;24(Suppl 1):S119–S144. doi: 10.1002/dmrr.825. [DOI] [PubMed] [Google Scholar]
- 35.Gu J, Milligan J, Huang LE. Molecular mechanism of hypoxia-inducible factor 1alpha -p300 interaction. A leucine-rich interface regulated by a single cysteine. J Biol Chem. 2001;276:3550–3554. doi: 10.1074/jbc.M009522200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.