Abstract
Elevated intraocular pressure (IOP) in glaucoma causes loss of retinal ganglion cells (RGCs) and damage to the optic nerve. Although IOP is controlled pharmacologically, no treatment is available to restore retinal and optic nerve function. We evaluated the effects of NGF eye drops in a rat model of glaucoma. We also treated 3 patients with progressive visual field defects despite IOP control. Glaucoma was induced in rats through injection of hypertonic saline into the episcleral vein. Initially, 2 doses of NGF (100 and 200 μg/mL) were tested on 24 rats, and the higher dose was found to be more effective. Glaucoma was then induced in an additional 36 rats: half untreated and half treated with 200 μg/mL NGF QID for 7 weeks. Apoptosis/survival of RGCs was evaluated by histological, biochemical, and molecular analysis. Three patients with advanced glaucoma underwent psychofunctional and electrofunctional tests at baseline, after 3 months of NGF eye drops, and after 3 months of follow-up. Seven weeks of elevated IOP caused RGC degeneration resulting in 40% cell death. Significantly less RGC loss was observed with NGF treatment (2,530 ± 121 vs. 1,850 ± 156 RGCs/mm2) associated with inhibition of cell death by apoptosis. Patients treated with NGF demonstrated long lasting improvements in visual field, optic nerve function, contrast sensitivity, and visual acuity. NGF exerted neuroprotective effects, inhibiting apoptosis of RGCs in animals with glaucoma. In 3 patients with advanced glaucoma, treatment with topical NGF improved all parameters of visual function. These results may open therapeutic perspectives for glaucoma and other neurodegenerative diseases.
Keywords: NGF, optic nerve, retina
Glaucoma is the leading cause of irreversible blindness in the world (1). This chronic and progressive optic neuropathy is characterized by loss of axons of the retinal ganglion cells (RGC) that constitute the optic nerve (2). Elevated intraocular pressure (IOP) is the primary risk factor for glaucoma, responsible for long-term damage to the optic nerve (3). Patients with glaucoma typically lose their visual field and become blind if untreated. Reduction of IOP, the only modifiable causative factor, slows the onset and progression of the disease, yet no actual treatment is available to restore optic nerve damage (4).
Neuroprotection has gained substantial interest in recent years as a therapeutic approach to preventing neuronal degeneration and loss of function in glaucoma (4). Neuroprotective therapies currently under investigation to restore retinal/neural function include memantine, neurotrophins, erythropoietin, reactive oxygen species scavengers, and even vaccine therapies (4–6). Nevertheless, results of these randomized clinical trials have so far been inadequate.
Nerve growth factor (NGF) is an endogenous neurotrophin that exerts trophic and differentiating activity on neurons of the central and peripheral nervous systems with protective and/or regenerative effects observed in degenerative diseases or following injury (7–9). Intracerebral administration of NGF has been shown to be beneficial in Parkinson's and Alzheimer's patients (10–12), and intraocular administration of NGF in animal models has been shown to inhibit RGC degeneration after mechanical, ischemic or hypertensive injury (13–15). NGF applied topically to the eye has also been shown to restore sensory nerve function to the ocular surface of patients with neurotrophic keratitis (16). Interestingly, absorption studies have demonstrated that topical ocular NGF reaches the retina, optic nerve, and brain in animals (17, 18).
In the present study, we demonstrate that topical application of exogenous murine NGF to the eye prevents RGC degeneration in an experimental rat model of glaucoma. Based on these findings, we used the same dosage regimen to treat 3 patients with rapid and progressive visual field loss despite successful treatment of ocular hypertension.
Results
Effects of Episcleral Venous Injection of Hypertonic Saline.
At baseline time 0, the mean IOP in SD rats was 24.6 ± 2.1 mm Hg and 24.7 ± 2 mm Hg in the control and experimental eyes, respectively. Significant unilateral elevation of IOP was successfully induced in the glaucomatous eyes by saline injection into the episcleral veins (Fig. 1), as shown by mean IOP values measured weekly for 7 weeks starting 1 week after treatment (Fig. 2A). Mean IOP in the saline injected eyes was 35.8 ± 3.2 mm Hg, compared to 24.7 ± 2.2 mm Hg in the contralateral, sham operated eyes (P < 0.01).
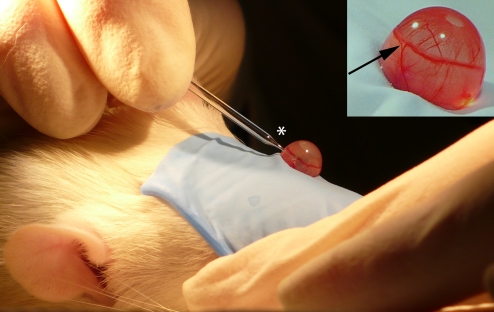
Fig. 1.
Glaucoma was induced in adult SD rats by single injection of 50 μL hypertonic saline solution (1.75M NaCl) into the superior episcleral vein. Once the rats were anesthetized, episcleral veins were isolated (arrow) under a led microscope and injections were performed using custom-made microneedle glass syringes (asterisk).
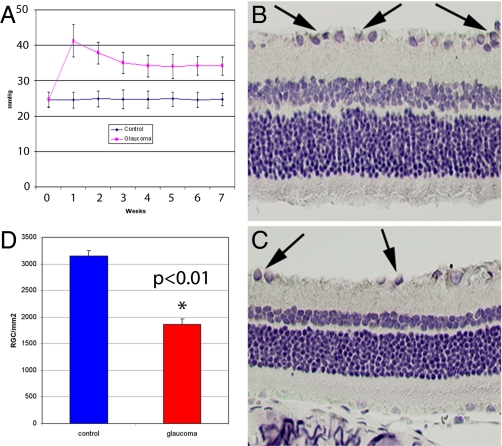
Fig. 2.
Measurement of intraocular pressure (IOP) demonstrated significant increases (P < 0.01) in rats that received episcleral injection with hypertonic saline solution (A). Hematoxylin/eosin staining of normal (B) and glaucomatous (C) retinas showed a significantly lower (P < 0.01) number of RGCs (arrows) in glaucomatous eyes (D).
Effects of Elevated IOP on RGC.
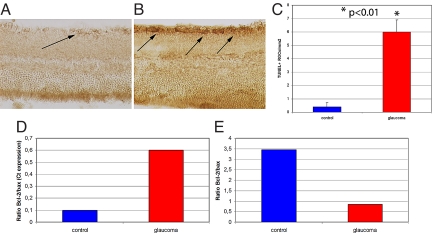
Histological evaluation indicated that compared to normal healthy retinas (Fig. 2B), 7 weeks of chronically elevated IOP induced approximately a 40% decrease in the number of RGCs (Fig. 2C; 1,861 ± 106 RGC/mm2 vs. 3,155 ± 98 RGC/mm2, respectively, P < 0.01, Fig. 2D). To assess whether the reduced RGC number was due to cell death through apoptosis, biomarkers involved in cell death and cell survival were studied. As shown in Fig. 3 A–C, anti-TUNEL staining, a biomarker for apoptotic cell death, was greater in RGCs of rats with elevated IOP compared to healthy controls (6 ± 0.9 vs. 0.4 ± 0.3 per mm2, P < 0.01). Moreover, molecular analysis of Bcl-2, a biomarker of cell survival, and Bax, a marker of cell death, demonstrated a lower mRNA Bcl-2/Bax ratio in the experimentally induced, untreated glaucomatous eyes (Fig. 3D, Ct values inversely correlated with mRNA expression values). The results of western blot analysis reported in Fig. 3E confirmed the molecular data indicating a lower Bcl-2/Bax protein ratio in glaucomatous eyes.
Fig. 3.
Anti-TUNEL immunostaining of RGCs (arrows) in normal (A) and glaucomatous (B) eyes showed a significantly greater (P < 0.01) number of apoptotic RGCs in glaucomatous eyes (C). Molecular analysis of glaucomatous compared to normal, healthy retinas showed greater mRNA expression of Bax (a biomarker of cell apoptosis) and lower expression of Bcl2 (a biomarker for cell survival), as illustrated by the Bcl-2/Bax ratio (D). The results of western blot protein analysis confirmed the significantly lower Bcl-2/Bax ratio in glaucomatous retinas (E).
Effects of Topical NGF in the Animal Model of Glaucoma.
Preliminary experiments comparing 100 and 200 μg/mL NGF eye drops showed a significantly higher biologic effect of 200 μg/mL NGF eye drops in protecting RGC loss in retinal sections from glaucomatous rat eyes, as demonstrated by E/E staining (2,145 ± 102 vs. 2,623 ± 138 RGCs/mm2, respectively, P < 0.05). Thus, in subsequent experiments, the 200-μg/mL concentration was used.
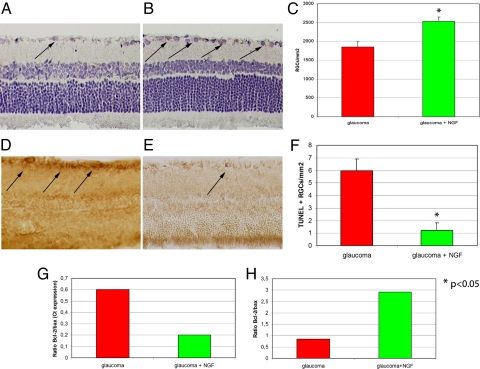
Histological analysis showed that topical ophthalmic administration 4 times daily with 200 μg/mL NGF for 7 consecutive weeks protected RGCs of rats with glaucoma (Fig. 4 A–H). Specifically, glaucomatous eyes treated with NGF had significantly more RCGs than a parallel group of glaucomatous eyes not treated with NGF (2,530 ± 121 vs. 1,850 ± 156 RGCs/mm2, P < 0.01). NGF-treated glaucomatous eyes also had significantly less anti-TUNEL staining of RGCs (1.2 ± 0.6 vs. 6 ± 0.9 per mm2, P < 0.01), and greater RGC survival, as shown by the significantly higher Bcl-2/Bax ratio (Fig. 4G, Ct values inversely correlated with mRNA expression values). The results of western blot protein analysis confirmed the significantly higher Bcl-2/Bax ratio in NGF-treated glaucomatous eyes, indicating greater RGC survival compared to the RGCs of untreated glaucomatous eyes.
Fig. 4.
Hematoxylin/eosin staining of retinas from untreated (A) and NGF-treated (B) glaucomatous eyes showed significantly less (P < 0.05) loss of RGCs (arrows) in animals that received 200 μg/mL NGF eye drops (C). Anti-TUNEL immunostaining of retinas from untreated (D) and NGF-treated (E) glaucomatous eyes showed significantly less (P < 0.05) apoptotic RGCs (arrows) in animals that received NGF (F). Molecular analysis showed significantly lower mRNA expression of Bax (a biomarker of cell apoptosis) associated with greater expression of Bcl2 (a biomarker for cell survival), as illustrated by the Bcl-2/Bax ratio (G), in glaucomatous eyes treated with NGF compared to untreated glaucomatous eyes. Western blot analysis of Bcl and 2/Bax (H) confirmed this protective effect of NGF.
No statistically significant differences were observed between NGF-treated glaucomatous eyes and control eyes in TUNEL staining (1.2 ± 0.6 vs. 0.4 ± 0.3 per mm2) and Bcl-2/Bax ratio. Although RGC cell count showed a protective effect of NGF in glaucomatous eyes, a significantly higher number of RGCs was still observed in control eyes as compared to NGF-treated glaucomatous eyes (3,155 ± 98 vs. 2,530 ± 121, P < 0.05).
Effects of Topical NGF in Patients with Glaucoma.
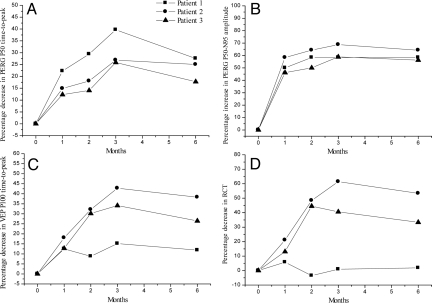
All 3 patients treated with 200 μg/mL NGF showed improvements in psychofunctional and electrofunctional parameters after 3 months of treatment. This effect was sustained even after a subsequent 3 months without NGF therapy. The patients with glaucoma had severe dysfunction of the innermost retinal layers and a delay of neural conduction along the postretinal visual pathways, as indicated by PERG P50 and VEP P100 values with longer time-to-peaks and longer RCT and PERG P50-N95 with reduced amplitudes with respect to control data (19). A progressive improvement of inner retinal layer function and postretinal neural conduction was observed during NGF treatment. This enhanced neuronal function was then maintained even 3 months after discontinuation of NGF treatment (Table 1 and Fig. 5).
Table 1.
Effects of NGF treatment in 3 patients affected by advanced glaucoma
| Age, Sex | IOP, mmHg | Visual field, MD | PERG P50 latency, ms | PERG P50-N95 amplitude, μV | VEP P100 latency, ms | VEP N75–100 amplitude, μV | Retinocortical time, ms | CSV 3° | CSV 6° | CSV 12° | CSV 18° | Visual | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | |||||||||||||
| Patient 1 | 74, M | 15 | −32.90 | 63 | 1.0 | 167 | 1.3 | 104 | 1.085 | 1.210 | 1.080 | 0.470 | 0.40 |
| Patient 2 | 59, M | 15 | −33.90 | 69 | 0.8 | 145 | 0.7 | 76 | 1.085 | 1.290 | 0.910 | 0.640 | 0.40 |
| Patient 3 | 82, F | 18 | −34.27 | 60 | 0.8 | 179 | 1.4 | 119 | 1.160 | 1.210 | 0.910 | 0.640 | 0.50 |
| At 1 month of NGF treatment | |||||||||||||
| Patient 1 | 17 | −33.14 | 57 | 1.5 | 150 | 2.9 | 93 | 1.085 | 1.290 | 0.910 | 0.640 | 0.40 | |
| Patient 2 | 15 | −33.15 | 60 | 1.2 | 122 | 2.4 | 62 | 1.160 | 1.210 | 0.910 | 0.640 | 0.40 | |
| Patient 3 | 15 | −34.40 | 63 | 1.1 | 168 | 1.5 | 105 | 1.085 | 1.210 | 1.080 | 0.470 | 0.50 | |
| At 3 months of NGF treatment | |||||||||||||
| Patient 1 | 15 | −31.50 | 52 | 1.6 | 145 | 5.2 | 93 | 1.010 | 1.290 | 0.910 | 0.640 | 0.40 | |
| Patient 2 | 12 | −32.10 | 67 | 1.5 | 109 | 2.8 | 42 | 1.850 | 1.540 | 0.910 | 0.710 | 0.70 | |
| Patient 3 | 17 | −34.30 | 59 | 1.4 | 174 | 2.6 | 115 | 1.010 | 1.210 | 0.910 | 0.470 | 0.60 | |
| At 3 months of NGF treatment discontinuation | |||||||||||||
| Patient 1 | 14 | −27.70 | 52 | 1.6 | 145 | 5.2 | 93 | 1.630 | 1.850 | 1.080 | 0.640 | 0.70 | |
| Patient 2 | 12 | −29.20 | 67 | 1.5 | 109 | 2.8 | 42 | 2.000 | 1.530 | 1.080 | 0.800 | 0.80 | |
| Patient 3 | 16 | −33.90 | 59 | 1.4 | 174 | 2.6 | 115 | 1.010 | 1.210 | 1.250 | 0.470 | 0.80 | |
Fig. 5.
Results of NGF eye drop treatment in 3 glaucomatous patients. Presented are relative changes in the electrophysiological parameters that reflected function of the innermost retinal layer (Panels A and B: pattern electroretinogram: PERG P50 time-to-peak and P50-N95 amplitude), the bioelectric visual cortical response (Panel C: visual evoked potential: VEP P100 time-to-peak) and neural conduction along the postretinal visual pathways (Panel D: retinocortical time: RCT) observed after 30, 60, and 90 days of NGF treatment and after another 90 days of follow-up (time 1, 2, 3, and 6) with respect to the baseline condition (time 0). The relative changes are expressed as percentage increase in amplitude or percentage decrease in time-to-peak from baseline. Percentage increases in PERG P50-N95 amplitude and percentage decreases in PERG P50 time-to-peak indicated a reduction in ganglion cell dysfunction after NFG treatment. Percentage decreases in VEP P100 time-to-peak and percentage decreases in RCT indicated a reduction of the neural conduction delay along visual pathways after NFG treatment.
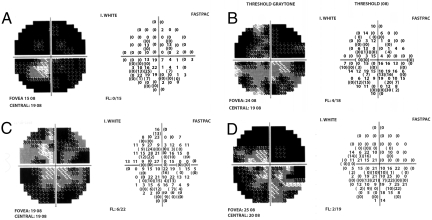
These electrophysiological changes were accompanied by improvements in clinical parameters as well. Visual field mean defects (MD) improved from 0% to 5% in all patients by the end of NGF treatment (Table 1 and Fig. 6) and a further 1% to 15.8% 3 months after NGF discontinuation.
Fig. 6.
A representative visual field illustrate changes from baseline (A) to 1 month of NGF treatment (B), to 3 months of NGF treatment (C) and to 3 months after discontinuation of NGF (D) in a patient affected by advanced glaucoma.
Contrast sensitivity at 12 cyc/deg in Patient 1 improved from 0.91 (baseline) to 1.080 (15.7%) (end of NGF treatment); in Patient 2, from 0.91 to 1.080 (15.7%), and in Patient 3 from 1.080 to 1.250 (13.6%). These values remained unchanged 3 months after discontinuation of NGF treatment (Table 1).
The best corrected visual acuity in Patient 1 improved from 0.4 to 0.7 (42.8%), in Patient 2, from 0.4 to 0.8 (50%), and in Patient 3 from 0.5 to 0.8 (37.5%). These visual acuity values remained unchanged 3 months after discontinuation of NGF treatment (Table 1).
No side effects were observed during NGF treatment and during the follow-up period, with the exception of a transient (1 week) burning sensation in 1 patient.
Discussion
This study demonstrated that murine NGF administered topically to the eye rescued RGCs from apoptosis in rats. We used a well-characterized experimental model of glaucoma, in which a single injection of hypertonic saline into the episcleral veins of rat eyes induced chronic elevation of IOP, optic-nerve degeneration, and selective RGC loss by apoptosis, the sum effects of which resemble human glaucoma (20–22). The beneficial effect of NGF on RGC survival was demonstrated to be due to inhibition of apoptosis, as shown by the reduction in TUNEL RGC immunostaining and the greater retinal Bcl-2/Bax ratio.
It is known that RGCs express NGF receptor (TrkA) and that NGF binding to TrkA up-regulates Bcl-2 protein, which protects cells from apoptosis by preventing caspase activation (21, 23). Furthermore, intravitreal NGF delivery to the retina and optic nerve is crucial to the survival of RGCs and NGF is known to be responsible for functional recovery of the retina following ocular ischemia and hypertension in animal models (13–15). Lastly, an ophthalmic solution of NGF administered topically to the ocular surface has been shown to reach the retina and optic nerve where it is biologically active (17).
Three months of topical, ocular NGF treatment in 3 patients with advanced glaucoma at risk of vision loss resulted in long-lasting improvement in visual field, contrast sensitivity, and best corrected visual acuity. Despite successful IOP control by medical therapy, these patients had progressive visual field defects and severe abnormalities in PERG and VEP responses that indicated dysfunction of the innermost retinal layers, delay in visual cortical responses, and delay in neural conduction along postretinal visual pathways (24, 25). In glaucoma, up to 20% of patients show progression of visual field defects with RGC and optic nerve degeneration despite successful management of ocular hypertension (26). In fact, elevated IOP is thought to be only the primum movens that triggers a cascade of events leading to optic nerve damage (4). An approach that would vastly improve the treatment of this challenging disease would involve neuroprotection with exogenous neurotrophic factors (6, 27).
PERG and VEP amplitudes and times-to-peak were the first electrofunctional parameters improved in our patients, suggesting a functional recovery of RGCs and an improvement of neural conduction along the postretinal visual pathways. The observed increase in CSV at 12 cyc/deg further supports the efficacy of NGF treatment on RGC (28). These effects are in line with the crucial role of neurotrophins in modulating RGC function and visual cortical neuronal activity reflected by receptive field size, orientation selectivity, visual acuity, response latency, and habituation (29–31).
NGF treatment also improved mean visual field defects in 2 patients (patient 1 and 2), and stabilized the defect in the third patient. Improvement of visual field persisted 90 days after discontinuation of treatment, indicating that changes induced by NGF had a prolonged duration. Two patients were actually followed up after 18 months, at which time improvements in visual field were still stable. This “long-term” NGF effect may be related not only to a protective activity against neural apoptosis, but also to the formation of new neural pathways, since it is known that NGF promotes neural plasticity and axonal regeneration (8, 32–34). In fact, NGF acts on numerous levels to promote neuronal recovery following ischemic and chemical injuries: through a neosynaptogenetic mechanism, by directly affecting precursor cells and/or by induction of other growth factors, including BDNF (8, 35–37). These multiple activities may cause the progressive improvement in visual acuity observed in our 3 patients during and after NGF treatment (Table 1).
The neuroprotective effects of NGF in glaucoma demonstrated in this study, together with the recently gained knowledge of NGF's ability to reach the brain when topically administered to the eye, allude to exciting possibilities for the treatment of neurodegenerative diseases of the central nervous system (18, 38). A major challenge in treating neurodegenerative disorders such as Alzheimer's disease has been the difficulty of delivering neurotrophic factors across the blood-brain barrier (8). This obstacle might be overcome by ophthalmic topical NGF treatment, and absorption and diffusion studies following this premise should be undertaken.
Many similarities between glaucoma and Alzheimer's disease go far beyond the challenges encountered in their treatment: (i) RGCs die by apoptosis in glaucoma through activation of specific caspases, which are also activated in Alzheimer's; (ii) caspase activation with cleavage of APP has been shown to up-regulate amyloid-beta production in Alzheimer's and in animal models of glaucoma; (iii) age-related mitochondrial dysfunction plays a key role in the etiology of both neurodegenerative disorders; (iv) elevated glutamate and nitric oxide synthase up-regulation with reactive oxygen species formation have been implicated in both glaucoma and Alzheimer's neurotoxicity; and (v) glutamate toxicity is involved in both glaucoma and Alzheimer's synaptic dysfunction (39, 40). All of these similarities have led glaucoma to be dubbed the “ocular Alzheimer's disease” (39). The obvious benefit to this likeness is the combining of forces in identifying new strategies to treat either disease.
In summary, this study indicates that topical NGF treatment may be an effective adjunct therapy for glaucoma, reducing neuron death and nerve loss. These encouraging results merit further investigation of topical NGF in controlled clinical trials in glaucoma and other forms of neurodegenerative disorders.
Materials and Methods
For this study, we used pathogen-free, adult male Sprague-Dawley (SD) rats (n = 78) maintained on a 12-h light-dark cycle and provided with food and water ad libitum. All procedures regarding housing, care and experimental procedures were carried out following the guidelines of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, International law (EEC council directive 86/609, OJ L 358, 1, December 12, 1987), and the Italian National Research Council's Ethical Commission on Animal Experimentation (1992).
Preliminary histological studies aimed to identify the best NGF concentration were performed on 24 rats: 6 untreated normal rats (control group), 6 untreated rats with glaucoma, 6 100 μg/mL NGF-treated rats with glaucoma, and 6 200 μg/mL NGF-treated rats with glaucoma. NGF was administered 4 times daily for 7 consecutive weeks. Based on these results, a second set of experiments was performed on an additional 54 rats: 18 normal controls, 18 NGF-treated (200 μg/mL) rats with glaucoma and 18 untreated rats with glaucoma. NGF was administered with the same dosage regimen, 4 times daily for 7 weeks.
Animal Model of Glaucoma.
Glaucoma was induced as described by Morrison et al. (20) Briefly, was injected once into the superior episcleral vein of 1 eye (Fig. 1), indicated as the ipsilateral glaucomatous eye, while the contralateral eye served as a sham, non-glaucomatous control eye. Glaucoma was defined as a significant loss of RGCs by apoptosis (20, 21). Rats were housed in single cages in a constant low-light environment (40–90 lux) to minimize IOP circadian oscillations and treated as indicated below.
IOP was measured weekly with a TonoPen XL tonometer (Mentor) under topical anesthesia and the values recorded were the mean of 10 valid measurements, expressed as TonPen readings. Mean changes were then calculated for each eye ± the standard deviation of the mean (SD). NGF treatment was initiated immediately at time 0, and results were compared among the 3 parallel groups: healthy eyes, glaucomatous eyes, and glaucomatous eyes treated with NGF.
Histological Evaluation.
Rats were euthanized with an overdose of Nembutal after 7 weeks. Eyes were removed and fixed with 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4, for 24 h. For light histological analysis of the retina, eyes were fixed in Bouin's fluid for 1 week, and then immersed for 3 days in phosphate buffer containing 20% sucrose, pH 7.4. Retinal sections 20 μm in width were cut with a cryostat at 4 °C, and stained with haematoxylin-eosin. Using ImageJ image processing and analysis software, RGCs were counted in a masked fashion in 4 quadrants of the retinal sections approximately 2 mm from the center of the optic disc. Counts were taken from comparable areas under a Zeiss Microscope at 400× magnification. The results were averaged and converted to cells per mm2.
Analysis of Cell Death.
For the determination of RGC death and survival, sections of the retina were immunostained with TUNEL (TdT-dUTP Terminal Nick-End), a marker of apoptotic cell death, combined with western blot and molecular analyses for Bcl-2 and Bax. Briefly, retinal sections were first incubated in a blocking solution (3% H2O2 in methanol) for 10 min at 15–25 °C, incubated in a permeabilization solution containing 1× PBS 0.1% Triton X-100 for 2 min at 4 °C, and then labeled with an in situ cell death detection kit (Roche Diagnostic, Boheringer) according to the manufacturer's instructions. For TUNEL-positive cells, DNA strand breaks were labeled and visualized with 0.4% DAB-H202. TUNEL-positive cells with nuclear condensation or fragmentation were considered as apoptotic cells.
For Bcl-2 and Bax western blot experiments, protein concentrations were determined using the Micro BCA protein assay kit. After determination of protein concentrations, equivalent amounts of retinal lysates (50 μg) were denatured in sample buffer (final concentration of 2% SDS, 10% glycerol, and 2% 2-mercaptoethanol, pH 6.8) and electrophoresed through 10% denaturing polyacrylamide gels. Following SDS polyacrylamide gel electrophoresis, samples were transferred electrophoretically to nitrocellulose membranes in transfer buffer. Membranes were blocked for 1 h in 1× TBS/0.1% Tween-20 with 5% defatted milk powder. Anti-Bcl-2 and anti-Bax primary antibodies (Santa Cruz Biotechnology) were incubated with the appropriate membranes at a dilution of 1:500 overnight. The GAPDH primary antibody (Sigma) was used at a dilution of 1:5,000. Appropriate HRP-conjugated secondary antibodies, all diluted to 1:2,500, were incubated with the membranes for 1 h. After incubation with secondary antibody, membranes were washed 3 times in 1× TBS (pH 7.4) with 1% Tween-20, and then developed using chemiluminescence. Images were digitalized in a Kodak Imager Station and bands were subjected to densitometric analysis using 1D Kodak software.
Molecular Analysis/Real-time PCR.
Bcl-2 and Bax mRNA were measured in rat retinas (average 0.010 mg wet weight for each sample). Tissues were pretreated with proteinase K (20 mg/mL; Finnzyme) in HIRT buffer at 56 °C/3 h, and total RNA was extracted from samples using the Puregene RNA purification kit (Gentra Systems). The resulting total RNA was re-suspended in 25 μL diethyl pyrocarbonate-treated water (ICN) and treated with RNase-Free DNase I to eliminate any genomic DNA contamination according to the supplier's protocol (2 U/μL Turbo DNA free kit AM-1907; Ambion Ltd.). Total RNA samples were checked for both RNA quantity (Nanodrop; Celbio), purity (>1.6) and absence of any RNA degradation (RIN ≈8). Equivalent amounts of RNA (3 μg) per sample were used as a template in normalized cDNA synthesis. Reverse transcription was performed according to the standardized Mu-MLV protocol (final volume reaction of 20 μL using 50 pM oligo dT-primer, 1 mM dNTP mix, and 200 U reverse transcriptase; Mu-MLV, F-605L; Finnzyme) in a PTC-100 programmable thermocycler (MJ Research). The resulting cDNA was amplified using the SYBR Green PCR core reagent kit (Applied Biosystems) and an Opticon2 MJ Research system (MJ Research). The reaction contained 10 μL SYBR reagent, 3 μL cDNA (for the target) or 1 μL cDNA (for the referring gene), and 20 nM primers in a 20-μL final volume. The temperature profile included initial denaturation at 95 °C/15 min, followed by 35–47 cycles of denaturation at 95 °C/30 s, annealing at 55–60 °C/25 s (the annealing time depended on the primer's Tm), elongation at 72 °C/30 s, fluorescence monitoring at 60–90 °C, and further incubation at 72 °C/5 m. Specific previously published primers for Bcl2 were used for this study (21). Primer specificities were further confirmed by the single melting curves obtained during each amplification. Negative controls (without template) were produced for each run. Experiments were performed in duplicate for each data point. Quantitative values were obtained from the threshold cycle value (Ct), which is the point where a significant increase of fluorescence is first detected. According to the REST© software, results are expressed as N-fold difference (increase or decrease) in target gene expression. Lastly, ratios between Bcl-2/Bax were calculated according to the single Ct values.
NGF Eye Drop Preparation and Treatment.
NGF was obtained from murine salivary glands as previously described, following the Bocchini and Angeletti method (41). Briefly, gel filtration at pH 7.5 was performed on the aqueous gland extract of adult mice, followed by dialysis at pH 5.0 and fractioning by cellulose-chromatography. In the present study, the biologically active form of highly purified murine NGF weighing 26 kDa was used, dissolved in a sterile 0.9% NaCl solution at 2 different concentrations (100 and 200 μg/mL) (17).
Selection of Patients.
Three patients (69 ± 6 years old, 2 males and 1 female) affected by advanced and progressive glaucoma (disease duration 21 ± 9 years), with impending risk of vision loss, despite good pharmacological control (timolol 0.5% and pilocarpine 2% in a fixed combination BID and latanoprost QID) of intraocular pressure (measured by applanation tonometry) were included in the study. Advanced glaucoma was defined by the following functional criteria: a mean deviation less than −24 dB, the presence of only a central or temporal island remaining in the visual field gray scale (42); and optic disk rim deterioration as an additional morphological criterion (43).
Treatment Regimen in Patients with Glaucoma.
Based on the dosage regimen used in the animal model, the 3 patients were treated topically with 1 drop (≈50 μL) of highly purified murine NGF, 200 μg/mL, instilled into the conjunctival fornix of 1 eye only 4 times daily for 3 months.
The tenets of the Declaration of Helsinki were followed in this study. Informed consent was obtained from the subjects after explanation of the nature and possible consequences of the study. All patients were at imminent risk of irreversible and complete vision loss for uncontrolled progression of glaucoma despite adequate IOP control and were, therefore, treated on a compassionate basis.
Electrofunctional and Psychosensorial Evaluation of Patients.
Patients were evaluated at baseline, every month during treatment and 3 months post-therapy by complete ocular examination including visual acuity, tonometry, optic disk photography, contrast sensitivity (CSV-1000, Vector Vision), visual field (Humphrey, program 10/2), and and electrofunctional tests (Pattern Electroretinigram, PERG, Visual Evoked Potentials, VEPs). Static perimetry was also performed and repeated 3 times using a Humphrey field analyzer (model 740, central 10–2 achromatic full threshold strategy, showing fixation losses, false positive rate, and false negative rates each less than 20%, and numeric loss of sensitivity). The mean defect (MD) defined the mean obtained in all tested points, considering the increasing scatter of sensitivity values with respect to the data obtained in normal subjects according to eccentricity, and therefore indicating the severity of global damage (44). We used the central 10° with a finer grid pattern to improve resolution of the remaining visual field and to reduce testing time.
Foveal contrast sensitivity was tested using a commercially available chart (CSV1000; Vector Vision). At the testing distance of 8 feet, the translucent chart presents 4 spatial frequencies, each on a separate row: 3, 6, 12, and 18 cyc/deg. According to the Pomerance and Evans procedure (28), the sensitivity threshold was measured twice, allowing only a few seconds between measurements. The second measurements were considered for analysis. The test-retest variability was consistent with that previously reported.
Simultaneous recordings of VEPs and PERGs were assessed using a previously published method (19). Transient VEP was characterized by 3 peaks that appeared after 75, 100, and 145 ms and had negative (N75), positive (P100), and negative (N145) polarity, respectively. The transient PERG was characterized by 3 peaks that appeared after 35, 50, and 95 ms and had negative (N35), positive (P50), and negative (N95) polarity, respectively. Amplitudes (in mV) and time-to-peaks (in ms) were measured. Simultaneous recordings of VEPs and PERGs identified an index of neural conduction along the postretinal visual pathways, defined as retinocortical time (RCT, the difference between the VEP P100 and the PERG P50 time-to-peak).
Data Analysis.
Statistical analysis was performed using the SuperANOVA package for Macintosh (Abacus Concepts Inc.) and the Tukey-Kramer comparison; a P value of less than 0.05 was considered statistically significant. Animal data of parallel control groups were evaluated and compared at endpoint. While the group of 3 patients was too small to elaborate statistically, the data presented are individual changes over time from baseline.
Acknowledgments.
This work was supported by Italian Ministery of Health Grant RF-FGB-2005-625679, Italian Ministery of University and Scientific Research Grant 2007AF3XH4, and an unrestricted research grant from Fondazione Rome.
Footnotes
The authors declare no conflict of interest.
References
- 1.Kingman S. Glaucoma is second leading cause of blindness globally. Bull World Health Organ. 2004;82:887–888. [PMC free article] [PubMed] [Google Scholar]
- 2.Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80:389–393. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwon YH, Fingert JH, Kuehn MH, Alward WL. Primary open-angle glaucoma. N Engl J Med. 2009;360:1113–1124. doi: 10.1056/NEJMra0804630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 5.Whitcup SM. Clinical trials in neuroprotection. Prog Brain Res. 2008;173:323–335. doi: 10.1016/S0079-6123(08)01123-0. [DOI] [PubMed] [Google Scholar]
- 6.Levin LA. Retinal ganglion cells and neuroprotection for glaucoma. Surv Ophthalmol. 2003;48(Suppl 1):S21–S24. doi: 10.1016/s0039-6257(03)00007-9. [DOI] [PubMed] [Google Scholar]
- 7.Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- 8.Hefti F. Neurotrophic factor therapy for nervous system degenerative diseases. J Neurobiol. 1994;25:1418–1435. doi: 10.1002/neu.480251109. [DOI] [PubMed] [Google Scholar]
- 9.Verge VM, et al. Colocalization of NGF binding sites, trk mRNA, and low-affinity NGF receptor mRNA in primary sensory neurons: Responses to injury and infusion of NGF. J Neurosci. 1992;12:4011–4022. doi: 10.1523/JNEUROSCI.12-10-04011.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olson L, et al. Intraputaminal infusion of nerve growth factor to support adrenal medullary autografts in Parkinson's disease. One-year follow-up of first clinical trial. Arch Neurol. 1991;48:373–381. doi: 10.1001/archneur.1991.00530160037011. [DOI] [PubMed] [Google Scholar]
- 11.Seiger A, et al. Intracranial infusion of purified nerve growth factor to an Alzheimer patient: The first attempt of a possible future treatment strategy. Behav Brain Res. 1993;57:255–261. doi: 10.1016/0166-4328(93)90141-c. [DOI] [PubMed] [Google Scholar]
- 12.Tuszynski MH, et al. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med. 2005;11:551–555. doi: 10.1038/nm1239. [DOI] [PubMed] [Google Scholar]
- 13.Carmignoto G, Maffei L, Candeo P, Canella R, Comelli C. Effect of NGF on the survival of rat retinal ganglion cells following optic nerve section. J Neurosci. 1989;9:1263–1272. doi: 10.1523/JNEUROSCI.09-04-01263.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siliprandi R, Canella R, Carmignoto G. Nerve growth factor promotes functional recovery of retinal ganglion cells after ischemia. Invest Ophthalmol Vis Sci. 1993;34:3232–3453. [PubMed] [Google Scholar]
- 15.Lambiase A, et al. Nerve growth factor (NGF) reduces and NGF antibody exacerbates retinal damage induced in rabbit by experimental ocular hypertension. Graefes Arch Clin Exp Ophthalmol. 1997;235:780–785. doi: 10.1007/BF02332863. [DOI] [PubMed] [Google Scholar]
- 16.Lambiase A, Rama P, Bonini S, Caprioglio G, Aloe L. Topical treatment with nerve growth factor for corneal neurotrophic ulcers. N Engl J Med. 1998;338:1174–1180. doi: 10.1056/NEJM199804233381702. [DOI] [PubMed] [Google Scholar]
- 17.Lambiase A, Tirassa P, Micera A, Aloe L, Bonini S. Pharmacokinetics of conjunctivally applied nerve growth factor in the retina and optic nerve of adult rats. Invest Ophthalmol Vis Sci. 2005;46:3800–3806. doi: 10.1167/iovs.05-0301. [DOI] [PubMed] [Google Scholar]
- 18.Lambiase A, et al. Nerve growth factor eye drop administrated on the ocular surface of rodents affects the nucleus basalis and septum: Biochemical and structural evidence. Brain Res. 2007;1127:45–51. doi: 10.1016/j.brainres.2006.09.102. [DOI] [PubMed] [Google Scholar]
- 19.Parisi V, et al. Correlation between optical coherence tomography, pattern electroretinogram, and visual evoked potentials in open-angle glaucoma patients. Ophthalmology. 2001;108:905–912. doi: 10.1016/s0161-6420(00)00644-8. [DOI] [PubMed] [Google Scholar]
- 20.Morrison JC, et al. A rat model of chronic pressure-induced optic nerve damage. Exp Eye Res. 1997;64:85–96. doi: 10.1006/exer.1996.0184. [DOI] [PubMed] [Google Scholar]
- 21.Coassin M, et al. Retinal p75 and bax overexpression is associated with retinal ganglion cells apoptosis in a rat model of glaucoma. Graefes Arch Clin Exp Ophthalmol. 2008;246:1743–1749. doi: 10.1007/s00417-008-0913-5. [DOI] [PubMed] [Google Scholar]
- 22.Fortune B, et al. Selective ganglion cell functional loss in rat with experimental glaucoma. Invest Ophthalmol Vis Sci. 2004;45:1854–1862. doi: 10.1167/iovs.03-1411. [DOI] [PubMed] [Google Scholar]
- 23.la Sala A, Corinti S, Federici M, Saragovi HU, Girolomoni G. Ligand activation of nerve growth factor receptor TrkA protects monocytes from apoptosis. J Leukoc Biol. 2000;68:104–110. [PubMed] [Google Scholar]
- 24.Parisi V. Impaired visual function in glaucoma. Clin Neurophysiol. 2001;112:351–358. doi: 10.1016/s1388-2457(00)00525-3. [DOI] [PubMed] [Google Scholar]
- 25.Parisi V, Miglior S, Manni G, Centofanti M, Bucci MG. Clinical ability of pattern electroretinograms and visual evoked potentials in detecting visual dysfunction in ocular hypertension and glaucoma. Ophthalmology. 2006;113:216–228. doi: 10.1016/j.ophtha.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 26.Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998;126:487–497. doi: 10.1016/s0002-9394(98)00223-2. [DOI] [PubMed] [Google Scholar]
- 27.Tatton W, et al. Hypothesis for a common basis for neuroprotection in glaucoma and Alzheimer's disease: anti-apoptosis by alpha-2-adrenergic receptor activation. Surv Ophthalmol. 2003;48(suppl 1):S25–S37. doi: 10.1016/s0039-6257(03)00005-5. [DOI] [PubMed] [Google Scholar]
- 28.Pomerance GN, Evans DW. Test-retest reliability of the CSV-1000 contrast test and its relationship to glaucoma therapy. Invest Ophthalmol Vis Sci. 1994;35:3357–3361. [PubMed] [Google Scholar]
- 29.Pizzorusso T, Porciatti V, Tseng J-L, Aebischer P, Maffei L. Transplant of polymer-encapsulated cells genetically engineered to release nerve growth factor allows a normal functional development of the visual cortex in dark-reared rats. Neuroscience. 1997;80:307–311. doi: 10.1016/s0306-4522(97)00182-6. [DOI] [PubMed] [Google Scholar]
- 30.Lodovichi C, Berardi N, Pizzorusso T, Maffei L. Effects of neurotrophins on cortical plasticity: Same or different? J Neurosci. 2000;20:2155–2165. doi: 10.1523/JNEUROSCI.20-06-02155.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rohrer B, Korenbrot JI, LaVail MM, Reichardt LF, Xu B. Role of neurotrophin receptor TrkB in the maturation of rod photoreceptors and establishment of synaptic transmission to the inner retina. J Neurosci. 1999;19:8919–8930. doi: 10.1523/JNEUROSCI.19-20-08919.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimohama S, et al. Protective effect of nerve growth factor against glutamate-induced neurotoxicity in cultured cortical neurons. Brain Res. 1993;632:296–302. doi: 10.1016/0006-8993(93)91164-n. [DOI] [PubMed] [Google Scholar]
- 33.Wiesmann C, de Vos AM. Nerve growth factor: Structure and function. Cell Mol Life Sci. 2001;58:748–759. doi: 10.1007/PL00000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu B. Acute and long-term synaptic modulation by neurotrophins. Prog Brain Res. 2004;146:137–150. doi: 10.1016/s0079-6123(03)46010-x. [DOI] [PubMed] [Google Scholar]
- 35.Tirassa P, Triaca V, Amendola T, Fiore M, Aloe L. EGF and NGF injected into the brain of old mice enhance BDNF and ChAT in proliferating subventricular zone. J Neurosci Res. 2003;72:557–564. doi: 10.1002/jnr.10614. [DOI] [PubMed] [Google Scholar]
- 36.Liberini P, Cuello A-C. Effects of nerve growth factor in primate models of neurodegeneration: Potential relevance in clinical neurology. Rev Neurosci. 1994;5:89–104. doi: 10.1515/revneuro.1994.5.2.89. [DOI] [PubMed] [Google Scholar]
- 37.Bregman BS, et al. Transplants and neurotrophic factors increase regeneration and recovery of function after spinal cord injury. Prog Brain Res. 2002;137:257–273. doi: 10.1016/s0079-6123(02)37020-1. [DOI] [PubMed] [Google Scholar]
- 38.Di Fausto V, Fiore M, Tirassa P, Lambiase A, Aloe L. Eye drop NGF administration promotes the recovery of chemically injured cholinergic neurons of adult mouse forebrain. Eur J Neurosci. 2007;26:2473–2480. doi: 10.1111/j.1460-9568.2007.05883.x. [DOI] [PubMed] [Google Scholar]
- 39.McKinnon SJ. Glaucoma: Ocular Alzheimer's disease? Front Biosci. 2003;8:s1140–1156. doi: 10.2741/1172. [DOI] [PubMed] [Google Scholar]
- 40.Kong GY, Van Bergen NJ, Trounce IA, Crowston JG. Mitochondrial dysfunction and glaucoma. J Glaucoma. 2009;18:93–100. doi: 10.1097/IJG.0b013e318181284f. [DOI] [PubMed] [Google Scholar]
- 41.Bocchini V, Angeletti PU. The nerve growth factor: Purification as a 30,000-molecular-weight protein. Proc Natl Acad Sci USA. 1969;64:787–794. doi: 10.1073/pnas.64.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blumenthal E-Z, Sapir-Pichhadze R. Misleading statistical calculations in far-advanced glaucomatous visual field loss. Ophthalmology. 2003;110:196–200. doi: 10.1016/s0161-6420(02)01297-6. [DOI] [PubMed] [Google Scholar]
- 43.The Advanced Glaucoma Intervention Study (AGIS): 1. Study design and methods and baseline characteristics of study patients. Control Clin Trials. 1994;15:299–325. doi: 10.1016/0197-2456(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 44.Lachenmayr BJ, Vivell PMO. Perimetry and its clinical correlation. New York: Thieme Medical Publisher Inc; 1993. pp. 12–13. [Google Scholar]