Abstract
The molecules that define human regulatory T cells (Tregs) phenotypically and functionally remain to be fully characterized. We recently showed that activated human Tregs express mRNA for a transmembrane protein called glycoprotein A repetitions predominant (GARP, or LRRC32). Here, using a GARP-specific mAb, we demonstrate that expression of GARP on activated Tregs correlates with their suppressive capacity. However, GARP was not induced on T cells activated in the presence of TGFβ, which expressed high levels of FOXP3 and lacked suppressive function. Ectopic expression of FOXP3 in conventional T cells was also insufficient for induction of GARP expression in most donors. Functionally, silencing GARP in Tregs only moderately attenuated their suppressive activity. CD25+ T cells sorted for high GARP expression displayed more potent suppressive activity compared with CD25+GARP− cells. Remarkably, CD25+GARP− T cells expanded in culture contained 3–5 fold higher IL-17-secreting cells compared with either CD25+GARP+ or CD25−GARP− cells, suggesting that high GARP expression can potentially discriminate Tregs from those that have switched to Th17 lineage. We also determined whether GARP expression correlates with FOXP3-expressing T cells in human immunodeficiency virus (HIV) −infected subjects. A subset of HIV+ individuals with high percentages of FOXP3+ T cells did not show proportionate increase in GARP+ T cells. This finding suggests that higher FOXP3 levels observed in these HIV+ individuals is possibly due to immune activation rather than to an increase in Tregs. Our findings highlight the significance of GARP both in dissecting duality of Treg/Th17 cell differentiation and as a marker to identify bona fide Tregs during diseases with chronic immune activation.
Keywords: Foxp3, HIV, human, TGFbeta, Tregs
Regulatory T cells (Tregs) play a key role in maintaining peripheral tolerance, preventing autoimmune diseases, and limiting chronic inflammatory diseases. Naturally occurring Tregs, among different subsets of suppressor T cells, are currently defined by expression of the transcription factor FOXP3 and a variety of T-cell activation markers (1, 2). Functionally, Tregs respond poorly to TCR activation in terms of cytokine secretion and proliferation, and possess the ability to suppress the immune responses of effector cells (1, 2). In recent years, a myriad of mechanisms of Treg-mediated regulation have been identified (2). However, it is still unclear whether there are additional, yet-to-be-identified molecular pathways that can mediate Treg inhibitory activity.
Naturally occurring Tregs express high levels of FOXP3, a master transcription factor known to be necessary for Treg development and function (1, 2). Ectopic overexpression of FOXP3 in naive T cells is able to partially endow these cells with Treg characteristics (3−5). TGFβ-treated naive T cells in both the human and murine systems can also up-regulate FOXP3 expression (6−8). Furthermore, TGFβ was shown to induce contact-dependent suppressive activity in human naive T cells (9). However, in contrast to murine T cells, TGFβ-induced FOXP3 appears to be insufficient to confer a regulatory phenotype in human T cells (8). This finding renders FOXP3 an unreliable marker to define human T cells with suppressive ability. Other molecules that have been shown to be differentially expressed on Tregs, including CD25, CD62L, CTLA-4, and CD127, are modified during T-cell activation or differentiation, and under chronic immune activation conditions these may not adequately discriminate Tregs from recently activated T cells (1, 2).
We recently identified a leucine rich repeat (LRR)−containing molecule called LRRC32 or GARP, which is highly expressed at the mRNA level in activated naturally occurring Tregs (10). Here we show that GARP identifies a subset of activated FOXP3+ T cells with high suppressive capacity and discriminates FOXP3+ non-suppressor TGFβ-treated T cells from bona fide Tregs. We also found that sorting for GARP expression excludes most IL-17–secreting cells within the CD25+ T-cell subset. In addition, we show the major discordance between FOXP3 and GARP expression in HIV-infected individuals, where a portion of the FOXP3+ T cells are possibly recently activated cells masquerading as Tregs. These findings reveal GARP as a highly specific molecule for activated Tregs, which could play an important role in their identification and function.
Results
Expression of GARP on Treg Cells.
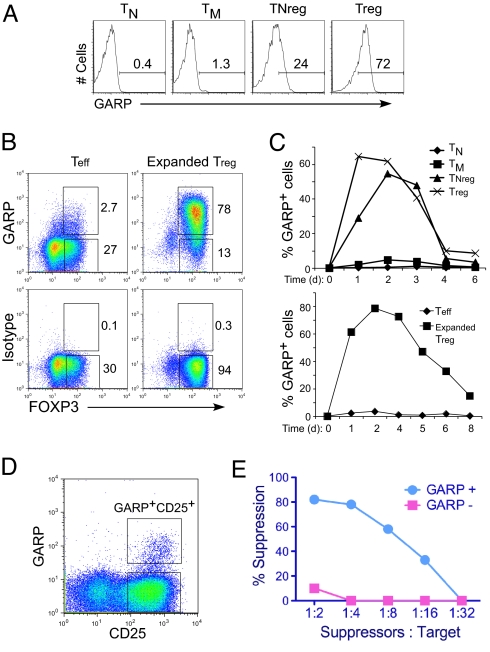
In the periphery, tolerance to self-antigens is partly mediated by naturally occurring Tregs. However, effector molecules expressed on the cell surface of activated Tregs are not fully determined. To search for novel molecules expressed on activated Tregs, we performed a microarray analysis and identified an mRNA encoding a cell surface protein named GARP that is specifically expressed on Tregs post–TCR activation (10). Recently, a specific mAb against GARP was made available, which we positively confirmed on GARP-transduced Jurkat T cells [supporting information (SI) Fig. S1A]. Using this antibody, we confirmed our previous finding (10) that GARP is expressed at high levels specifically on human Tregs but not conventional T-cell subsets (Fig. 1A). Furthermore, GARP expression was restricted to the FOXP3+ population (Fig. 1B).
Fig. 1.
GARP is specifically expressed on activated Tregs and defines suppressor T cells. (A) Surface expression of GARP on TN (naive), TM (memory), TNreg, and Tregs. Different T-cell subsets were isolated based on CD25 and CD45RO expression as previously described (10). Cells were stimulated with anti-CD3 and anti-CD28 beads (TCR stimulation) overnight and stained for surface GARP. (B) GARP expression restricted to FOXP3+ cells. TN and TNregs were activated and expanded in vitro for 2 weeks to obtain Teff and expanded Tregs. Cells were activated through TCR and stained for GARP/isotype and FOXP3. (C) Kinetics of GARP expression on T-cell subset postactivation. Data represent percentage of GARP+ T cells at different time points postactivation. (D) Induction and sorting of GARP+ T cells. CD4+ T cells were stimulated through TCR for 2 days and stained for GARP and CD25. (E) Suppressive function of GARP+ T cells. GARP+CD25+ and GARP-CD25+ populations were sorted 2 days post–TCR activation, rested in culture for 8–10 days, and assayed for suppressive activity using CFSE-labeled resting target T cells and different ratios of GARP+ suppressor or GARP− effector T cells. The cells were then stimulated with anti-CD3 antibody (50 ng/ml) in the presence of dendritic cells for 4 days, and proliferating cells were determined based on decrease in CFSE expression as shown in Fig. S1D. Percent suppression was calculated based on CFSE dilution of target T cells in suppression culture as described before (10). Briefly, the percentage of target cells that undergo division in response to the stimuli alone, in the presence of expanded Tregs, or control Teff cells, was determined. The percent suppression was then calculated by percent reduction in proliferation of the target cells with suppressors as compared with target cells alone. All data shown are representative of at least three experiments performed with T cells from different healthy donors.
We next explored the kinetics of GARP expression in different T-cell subsets. In Tregs, GARP was up-regulated quickly after TCR stimulation and maintained on most Tregs for 2 days before the expression levels declined (Fig. 1C). In naive Tregs, defined as CD45RO-CD45RA+CD25+FOXP3+ T cells (TNregs), GARP expression was induced 1 day postactivation, and reached a level similar to mature Tregs on day 2 (Fig. 1C). We and others have also shown that after in vitro expansion, the TNregs differentiate into mature Tregs (11–14). GARP expression was up-regulated on in vitro expanded Tregs upon reactivation (Fig. 1C Lower), comparable to ex vivo analyzed Tregs (Fig. 1C Upper).
Together, these findings suggested that GARP is preferentially induced on T cells with Treg phenotype. Therefore, we studied the possibility of using GARP as a marker for activated T cells with suppressor function. We first activated CD4+ T cells through their TCR and stained for GARP expression. As expected, most cells also up-regulated CD25 (Fig. 1D). A portion of CD25+ T cells also expressed GARP after TCR-activation (Fig. 1D). We then sorted these cells based on GARP and CD25 expression (Fig. 1D). More than 90% of the GARP+CD25+ sorted T cells also expressed FOXP3 (Fig. S1B). The sorted cells were rested for 1 week in IL-2–containing media and then reactivated to determine their suppressive activity. We found that the GARP+CD25+ cells very strongly inhibited the proliferation of TCR-stimulated target cells at different target to suppressor ratios (Fig. 1E). The GARP+CD25+ cells also secreted significantly less IL-2 and IFNγ as compared with the GARP-CD25+ population, a characteristic of Tregs (Fig. S1C). Together, our data show that GARP identifies suppressor T cells within the activated CD4+ T-cell subset.
GARP Plays a Role in Treg-Mediated Suppression.
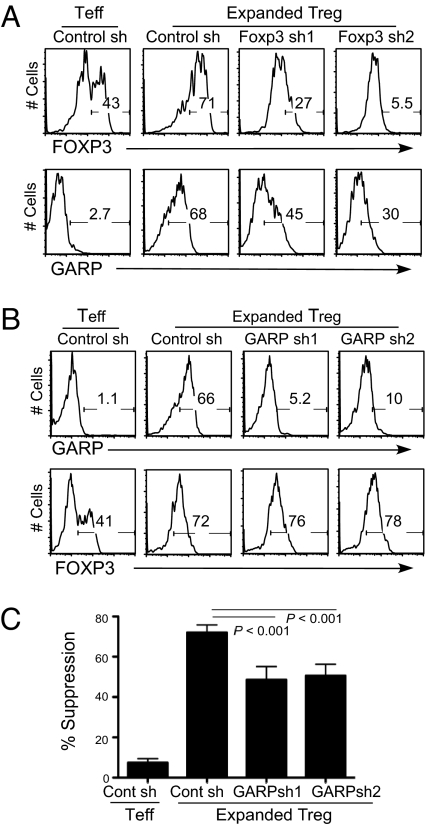
Because almost all GARP+ T cells also express FOXP3, we hypothesized that GARP is an effector molecule downstream of FOXP3 expression and investigated whether FOXP3 is required for GARP induction in Tregs. We expressed two different shRNAs against FOXP3 in expanded Tregs, which have been shown to knock down FOXP3 efficiently (Fig. 2A) (11). The FOXP3 shRNA-expressing expanded Tregs showed a reduction of GARP up-regulation in comparison to control shRNA-expressing Tregs (Fig. 2A). In contrast, knockdown of GARP did not change FOXP3 expression in expanded Tregs (Fig. 2B).
Fig. 2.
Silencing FOXP3 and GARP expression in Tregs. (A) Silencing FOXP3 in Tregs through shRNAs. Purified TN or TNreg cells were stimulated and transduced with control or FOXP3 shRNA encoding lentiviruses. Cells were expanded in vitro for 2 weeks, sorted based on GFP expression (expressed by vectors) and FOXP3 levels, and induction of GARP expression was determined 2 days post–TCR stimulation. (B) Knockdown of GARP by shRNAs in expanded Tregs. Cells were transduced with viruses encoding control or GARP shRNA, expanded for 2 weeks in vitro, and sorted for GFP expression encoded by the same lentiviral vector. Cells were then stained for FOXP3. GARP was stained 24 hours post–TCR reactivation. Data shown are representative of three healthy donors. (C) Suppressive activity of control or shRNA-expressing Teff and Tregs were assessed as described in Fig. 1. Representative data using a 1-to-4 ratio of Treg/Teff to target T cells are shown.
We have shown before that overexpression of GARP in naive human T cells endows them with partial Treg phenotype (10). In a reverse experiment, we found that silencing GARP induction by shRNA expression in expanded Tregs resulted in modest but significant impairment in suppressive function (Fig. 2C). Thus, GARP could contribute to Treg-mediated suppression but does not account for the majority of their regulatory activity under our in vitro conditions. Silencing GARP in Tregs did not affect their low cytokine secretion characteristic (Fig. S2).
TGFβ-Induced FOXP3+ Human T Cells Do Not Express GARP.
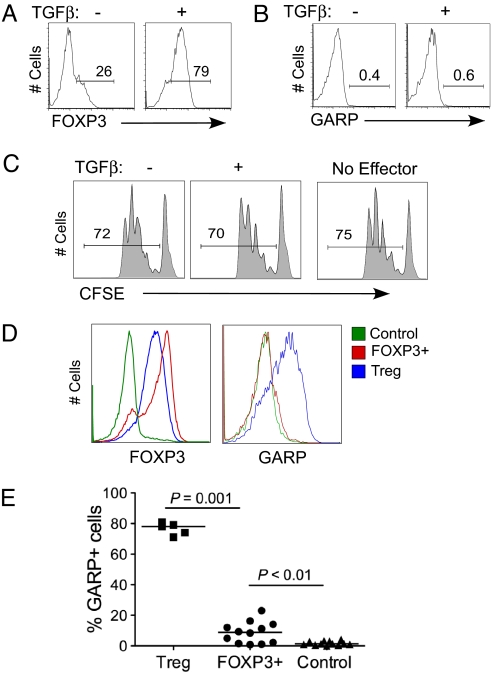
TGFβ has been shown to induce FOXP3 expression in both human and murine T cells (6–8). Although TGFβ is shown to differentiate naive T cells into functional Tregs in mice, in the human system TGFβ-induced FOXP3+ cells do not display suppressive activity (8, 15). We showed that addition of TGFβ during activation of CD25− naive human T cells induces FOXP3 expression in a majority of these cells (Fig. 3A), which was maintained at high levels for at least 1 week (Fig. S3). However, despite expressing FOXP3 at levels comparable to those of natural Tregs, the TGFβ-treated cells did not express GARP protein on the cell surface upon TCR activation (Fig. 3B) or GARP mRNA as determined by real-time polymerase chain reaction (PCR) analysis. TGFβ-treated human T cells also did not show suppressive activity in vitro (Fig. 3C), consistent with a previous report (8). We conclude that TGFβ-induced FOXP3 is not sufficient for GARP expression. Consistent with this finding, we found that in T cells overexpressing FOXP3, at levels higher than those of natural Tregs (Fig. 3D), induction of GARP post–TCR activation was either completely absent (Fig. 3D) or was present at much lower levels compared with Tregs (Fig. 3E). Together, these findings suggest that although FOXP3 is important, clearly it is not sufficient to program the cells to induce GARP expression.
Fig. 3.
Expression of GARP in TGFβ-treated or FOXP3-overexpressing T cells. (A) Induction of FOXP3 by TGFβ in TCR-stimulated T cells. TN cells were activated through TCR in the presence or absence of 20 ng/ml TGFβ. FOXP3 expression was determined by intracellular staining at different time points postactivation. Data shown are representative of day 6 postactivation. (B) Expression of GARP on TGFβ-treated cells. TGFβ-treated or untreated cells were restimulated through TCR at different time points between 6 and 12 days of culture post–original activation, and GARP expression was determined after 2 days. A representative GARP staining performed post–day 6 of first activation is shown. (C) Suppressive activity of TGFβ-treated T cells. Activated T cells treated with or without TGFβ were expanded in vitro, and their suppressive activity was assayed at different days postactivation. Representative CFSE data are shown using a 1-to-4 suppressor-to-target ratio. (D) Expression of FOXP3 on overexpressing T cells. Purified CD4+ T cells were activated and transduced with FOXP3 lentiviruses that also express RFP as a marker. After 12–14 days postculture, cells were sorted based on RFP expression, restimulated through TCR for 2 days, and expression of FOXP3 and GARP determined on FOXP3-overexpressing cells (FOXP3+), vector control-transduced cells (Control), or Tregs. GARP expression was determined for FOXP3+ and Tregs after gating on FOXP3 expression. (E) Expression of GARP on FOXP3-overexpressing T cells analyzed from multiple donors.
GARP Expression Defines Suppressive Activated Tregs and Excludes IL-17–Secreting CD25+ T Cells.
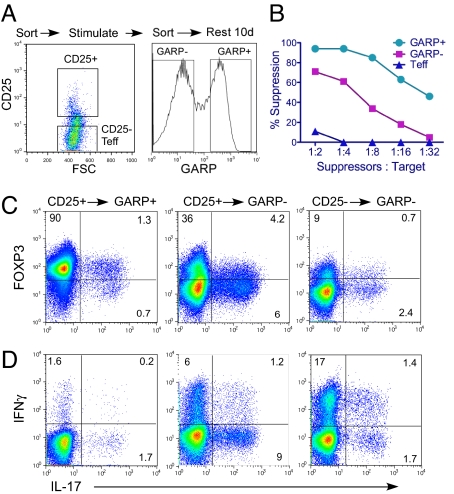
An important implication of the above results is that TGFβ-induced FOXP3 greatly confounds analysis of suppressive natural Tregs during disease conditions or infections in humans, where T cells could be activated to induce FOXP3 (in the presence of TGFβ) without differentiating into Tregs. We therefore further explored whether GARP can be a more reliable marker for determining Tregs after immune activation. To address this question, we first isolated CD4+CD25− (Teff) and CD4+CD25+ (canonical Tregs) T cells, which were stimulated through TCR for 48 hours, and then further sorted based on GARP expression (GARP+ and GARP− respectively) (Fig. 4A). The CD25− Teff cells, which do not express GARP, were also mock sorted and used as control cells. Because GARP-sorted T cells were activated to induce GARP expression, they were cultured and rested in IL-2–containing media for 10 days and then activated through the TCR to assess their suppressive capacity. CD25+GARP+ were more potent in suppressing the proliferation of the target T cells as compared with the CD25+GARP− subset (Fig. 4B and Fig. S4). Teff control cells did not show any suppressive activity at different suppressor-to-target ratios (Fig. 4B and Fig. S4)
Fig. 4.
Suppressive activity and IL-17 secretion by CD25+ T cells based on GARP expression. (A) Experimental setup was as follows: CD4+ T cells were first sorted into CD25+ and CD25− subsets. The cells were then activated with anti-CD3/28 beads for 2 days, stained with GARP antibody, and sorted into GARP− and GARP+ cells. These cells were further expanded and rested in culture for an additional 10 days. (B) Suppressive activity of CD25+GARP+ and GARP− cells. Suppressive activity was performed and calculated as described in Fig. 1. Representative CFSE data are shown in Fig. S4. (C) Cells activated and sorted as shown in Fig. 4A were cultured in IL-2 for 8–10 days and then reactivated with PMA and ionomycin for 5 hours, followed by intracellular staining with IL-17–FITC, FOXP3.APC, and IFNγ-PE.Cy7 antibodies. (D) Expression of IFNγ versus IL-17 from the same staining shown in (C). Results are representative of three separate experiments from different donors.
In two recent reports, it was shown that a portion of human CD25+ or FOXP3+ T cells contain high levels of IL-17–secreting Th17 cells (16, 17). Therefore we determined the percentages of IL-17 and IFNγ-secreting cells in GARP+ and GARP− subsets, which were sorted from CD4+CD25+ T cells (Fig. 4A). These sorted cells were activated with phorbol myristate acetate and ionomycin and stained with FOXP3, IL-17, and IFNγ. We found that most CD25+GARP+ cells remained FOXP3+ and that a small portion (≈1–2%) expressed IL-17 at levels lower or comparable to those in CD25− Teff cells (Fig. 4C). Remarkably, CD25+GARP− sorted cells contained much greater percentage of IL-17–secreting cells, which was 3–4-fold higher compared with CD25− Teff cells (Fig. 4C). CD25+GARP+ cells also expressed much lower levels of IFNγ+ cells compared with CD25+GARP− cells (Fig. 4D). In contrast to IL-17 expression, CD25+GARP− T cells also did not display higher IFNγ+ cells compared with CD25−GARP− Teff cells (Fig. 4D). Together, these findings suggest that GARP not only consistently defines T cells with the highest suppressive activity but can also discriminate CD25+ T cells that contain high levels of IL-17–secreting cells.
Can GARP Expression Better Identify Tregs During HIV Infection?
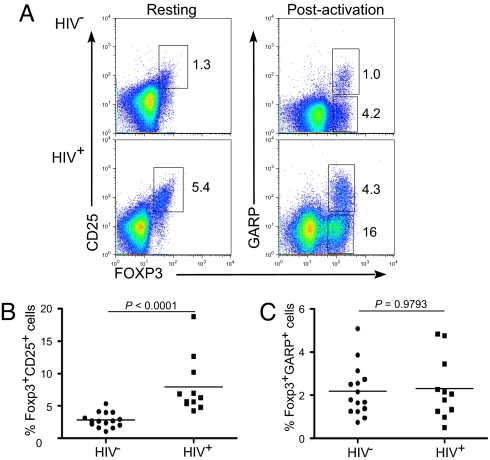
We had observed that some chronically HIV-infected individuals express a higher proportion of FOXP3+ T cells compared with healthy donors (Figs. 5A, 5B). It is not clear whether FOXP3+ cells in HIV+ individuals represent a suppressor subset or whether they are induced as a consequence of chronic immune activation, which is a hallmark of HIV infection. Reliably identifying suppressor Tregs from HIV patients based on functional in vitro assays is also challenging because of the limited number of cells that are typically obtained in the clinical setting. Therefore, we examined whether GARP expression could be a better defining tool to identify true Tregs in these clinical instances. Accordingly, CD4+ T cells from HIV+ and HIV− individuals were stained for GARP expression post–TCR activation as shown in Fig. 5A. Interestingly, although this subset of HIV+ patients had a much higher percentage of FOXP3+ T cells than healthy donors (Fig. 5B), the proportion of T cells expressing GARP within the two groups was similar (Fig. 5C). In corollary analysis, there was a striking discordance between the percent of FOXP3+CD25+ T cells to FOXP3+GARP+ T cells within this HIV+ population as compared with HIV− healthy subjects (Fig. S5). This finding suggests that FOXP3 expression in the T cells of HIV+ subjects may reflect chronic T-cell activation resulting from HIV infection (18, 19), rather than true Tregs.
Fig. 5.
GARP expression is not increased proportionate to FOXP3+ T cells in HIV+ individuals. (A) Representative phenotypic staining of HIV− and HIV+ sorted CD4+ T cells for GARP, CD25, and FOXP3 expression. CD4+ T cells were sorted from both HIV+ and HIV− subjects and were stained both immediately as resting T cells for CD25 and FOXP3 and after 1 day of TCR activation for GARP and FOXP3 expression. (B) Analysis of FOXP3+CD25+ T cells from multiple HIV− and HIV+ donors. (C) Analysis of percentage of T cells that express GARP and FOXP3 postactivation from same donors shown in Fig. 5B.
Discussion
Tregs play pivotal roles in maintaining peripheral tolerance. A variety of cell surface markers have been suggested to identify the naturally developed human Treg subset, which are most often defined as CD4+ T cells expressing high levels of CD25 and FOXP3. However, CD25, as well as many other human Treg–attributed cell surface markers (such as CTLA-4, GITR, and CD127), are either up-regulated or down-regulated on non-Tregs after TCR stimulation, rendering these unsuitable to be used as a marker for Tregs during T-cell activation. FOXP3 is also not completely restricted to human Tregs, as TGFβ can induce FOXP3 expression on most conventional T cells during activation, which do not acquire suppressive function (15) (Fig. 3). Recently, we identified a surface molecule GARP which is specifically up-regulated on Tregs post–TCR activation (10). Here we show that GARP is a useful marker to identify activated bona fide human Tregs. Using a monoclonal antibody, we showed that expression of GARP postactivation was maintained for 2–3 days before gradually declining over time, suggesting that functionally GARP is important only for brief period post–TCR stimulation. GARP expression was slightly delayed on activated naive Tregs, possibly reflecting their less mature differentiation state (11).
Implications for Human Treg Function and Differentiation.
We found that reduced GARP expression, via shRNA expression in Tregs, attenuates their suppressive activity moderately but significantly, suggesting that GARP could have a relatively small contribution to Treg-mediated suppression. Indeed, we had previously shown that overexpression of GARP in non-Tregs endowed them with modest suppressive capacity, consistent with these findings (10). Reducing GARP induction in Tregs did not affect their FOXP3 expression or their low response to TCR stimulation as measured by IL-2 and IFNγ secretion. This finding is in agreement with our prior results that silencing FOXP3 in Tregs, which reduces GARP expression, also does not significantly affect their response to TCR signals (11). How GARP could be contributing to suppressive function of Tregs, and why these cells selectively express GARP upon activation remains unclear. An important advance in understanding GARP function was provided by Tran et al. (25), who discovered that GARP is a receptor for latency-associated peptide (LAP), which requires TGFβ for binding to GARP. It is conceivable that expression of the LAP-TGFβ complex on the cell surface of Tregs or GARP-overexpressing cells deliver a suppressive signal to target T cells that express TGFβ receptors. The association of LAP-TGFβ with GARP would also provide a mechanistic explanation for our previous finding that ectopic expression of GARP induces FOXP3 in conventional T cells (10). Thus, we postulate that LAP-TGFβ bound to GARP could induce expression of FOXP3 through the signaling of TGFβ receptors, as has been previously suggested (20).
We have shown that TCR stimulation is required for rapid induction of GARP on resting human Tregs. Silencing FOXP3 in Tregs reduced the level of GARP expression postactivation. Interestingly, GARP was only minimally up-regulated on cells ectopically expressing FOXP3. This finding, together with the result that TGFβ-induced FOXP3 is not sufficient for GARP induction, suggests that there are other unknown factors required for specific GARP expression. Because TGFβ was shown recently to contribute to Th17 differentiation in conjugation with IL-6 or IL-1β (21, 22), perhaps other cytokines or soluble factors could also program GARP expression in conjunction with TGFβ. It is also possible that the level and duration of FOXP3 expression is important in determining whether a cell can express GARP upon activation (23). However, we show that even when ectopic expression of FOXP3 is at higher levels than natural Tregs, GARP is not induced (Fig. 3D), indicating that the level of FOXP3 is not a limiting factor for GARP induction. Alternatively, FOXP3 expression during development of Tregs results in epigenetic modifications that may allow induction of GARP from these cells. It will also be of interest in future to determine whether GARP expression in murine T cells is regulated similarly to that in humans, as TGFβ signals are sufficient to confer Treg function in naive murine T cells (6, 7).
Implications for Human Th17 Cell Differentiation.
Recently it has been shown that CD25+ Treg-like cells also secrete IL-17 (16, 17). These could be either common Tregs that have switched sides to Th17 lineage or cells with dual functionality. We found that CD25+ T cells that are induced to express high GARP levels contain few IL-17+ cells, whereas the GARP-negative portion is greatly enriched in IL-17-secreting cells, regardless of FOXP3 expression (Fig. 4C). Our findings are consistent with recent reports that a sizeable portion of human CD25+FOXP3+ cells contain IL-17–secreting cells (16, 17). Similar to these reports, we found that although CD25+GARP− cells that contain high IL-17 secretor also display suppressive function, this was significantly lower compared with the suppressive capacity of CD25+GARP+ cells. Because it is difficult to separate IL-17+–secreting cells within this subset, it is not yet clear whether CD25+GARP−IL-17+ cells have suppressive capacity, as has recently been suggested (16). It also remains to be determined whether these CD25+ T cells with dual Treg/Th17 cell characteristic are derived from naive T cells, or alternatively, whether they differentiate from Tregs that switch sides to Th17 lineage while retaining some Treg functionality. Voo et al. provided evidence for the latter model (17), which we also favor. In this regard, a small percentage of IL-17+ cells present in the GARP+ cells could be transitional Th17-lineage committed T cells, which eventually down-regulate FOXP3 expression and have lost the capacity to up-regulate GARP expression. Indeed, CD25+GARP− cells expressed relatively lower FOXP3 as compared with CD25+GARP+ cells after expansion in culture. Thus, expression of GARP could be an invaluable tool to further dissect the original duality or cross-differentiation pathways of Treg and Th17 lineage T cells.
Implications for HIV Pathogenesis.
Our results led us to explore the possibility of using GARP as a marker to isolate Tregs from the activated T-cell population. We showed that all GARP+ T cells possess Treg characteristics including FOXP3 expression, hyporesponsiveness, and suppression of T-cell activation. These findings predict that GARP expression would reliably identify suppressive Tregs in ex vivo analysis of human T-cell subsets. As a proof-of-principle experiment, we show that there is a very large discordance between GARP+ and FOXP3+ T cells in some HIV+ individuals. This group of HIV+ subjects had much greater levels of FOXP3+ cells compared with GARP+ cells. Based on our findings that GARP is preferentially expressed on highly suppressor bona fide Tregs, we suggest that FOXP3 expression in these patients is mostly caused by chronic immune activation, which can potentially result in FOXP3 induction through TGFβ (8). Thus, future studies using GARP as a marker to identify Tregs during HIV infection could resolve some of the contradictory findings on the level of Tregs at various stages of HIV disease (24). It will also be interesting to determine whether, at mucosal tissues or sites of inflammation, where there is continuous T-cell activation, GARP-expressing T cells can be directly identified, which would suggest ongoing activation of Tregs in these locations.
In summary, we demonstrated that surface expression of GARP identifies both activated FOXP3+ human T cells with high suppressive activity and excludes IL-17–secreting Treg-like cells. Thus, expression of GARP can be of great use in expanding bona fide human Tregs in vitro, both to dissect their biology and for clinical in vivo use, for example to establish transplantation tolerance. Our findings also implicate additional factors other than FOXP3 or an epigenetic program for development or lineage commitment of true Tregs that express GARP. Expression of GARP will also be useful in defining whether T cells with Treg phenotype that secrete IL-17 are derived from bona fide Tregs that have switched to the Th17 side or whether these cells represent a separate effector lineage. In addition, expression of GARP can be a highly relevant biomarker to better identify Tregs in human diseases with high immune activation, such as HIV infection, or at sites of inflammation.
Materials and Methods
Cell Purification and Culture.
Human peripheral blood mononuclear cells (PBMCs) were isolated with Ficoll-Hypaque (Amersham Pharmacia), from peripheral blood from normal donors and CD4+ T cells were then separated using magnetic cell sorting (Dynabeads; Invitrogen). Purified CD4+ T cells were then stained with anti-CD25 and CD45RO antibodies (BD Biosciences), and the different T-cell subsets, including naive and Tregs, were sorted using FACS Aria flow cytometer (BD Biosciences) (10). Cells were cultured in RPMI (Life Technologies), media containing FCS (Atlanta Biologicals), as described before (3). Monocyte-derived dendritic cells (DCs) were generated from CD14+ cells from normal donors as previously described (3). The superantigen, staphylococcal enterotoxin B (SEB), was purchased from Sigma.
FACS Analysis.
Cells were stained with relevant antibodies on ice for 30 minutes in PBS buffer containing 2% FCS and 0.1% sodium azide. Cells were washed twice before being analyzed by BD LSRII flow cytometer. Live cells were gated based on forward and side scatter profiles or based on exclusion with propidium iodide (PI) staining, and analysis was performed using FlowJo software (Tree Star). To stain for cytokines intracellularly, cells were activated by PMA and ionomycin (Sigma) for 5 hours, followed by fixation and permeabilization using a commercial kit (BD Biosciences), according to the manufacturer's instructions. The following antibodies were used for staining: IFNγ and IL-17 (eBioscience), CD45RO, CD25 (both from BD Biosciences). GARP staining was performed 1–2 days post–TCR activation by first staining with mouse anti-GARP (Alexis Biochemicals), followed by anti-mIgG2b bio (BD Biosciences), and finally with strep-APC (eBioscience). FOXP3 expression was performed via intracellular staining. Briefly, cells were washed and resuspended in 1X Fixation/Permeabilization buffer (eBioscience) and incubated at 4 °C for 30–60 minutes. Cells were then washed twice with 1X Permeabilization buffer and stained with anti-FOXP3 antibody (clone 259D, Biolegend), for 30 minutes at 4 °C. Cells were washed twice with 1X Permeabilization buffer and analyzed by LSR II.
Cell Proliferation and Cytokine Assays.
Cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) (Molecular Probes), and activated by DC and either anti-CD3 antibody (ATCC clone OKT3) or SEB (Sigma) at different concentrations as previously described (3). For GARP induction or cytokine secretion, cells were activated with CD3/CD28 beads (Invitrogen). Supernatants from activated T-cell cultures were collected 16 hours later; cytokine production was measured using cytometric bead array (CBA; BD Biosciences) and analyzed by FlowJo (Tree Star). Proliferation of the cells was assessed on day 4 or day 5 post–TCR activation by monitoring dilution of CFSE in the cells. Statistical analysis was performed with a paired t test for comparison between two groups and one-way analysis of variance (ANOVA) for comparison within three or more data groups using Prism (GraphPad Software).
Suppression Assay.
Resting CD4+ T cells were sorted as described above, labeled with CFSE and used as targets as described (3). The CFS-labeled resting T cells and non-labeled “suppressor or effector” cells were mixed together at different suppressor-to-target ratios. The cells were stimulated by soluble anti-CD3 antibody (OKT-3, 10–100 ng/ml) in the presence of DCs (1:10) or alternatively DCs pulsed with suboptimal concentrations of SEB (range, 0.1–0.001 ng/ml). Cells were harvested and analyzed by FACS (LSR-II, BD Biosciences) on day 4 or day 5 postactivation. The percentage of suppression was calculated as previously described (10); data represent mean ± SEM of duplicates or similar experiments from at least three different donors. Statistical analysis was performed with a paired t test for comparison between two groups and one-way ANOVA for comparison within three or more data groups.
Study Subjects and Statistical Analysis.
Buffy coats from healthy adult subjects (n = 15) who were HIV negative and with no documentation of chronic viral infections such as hepatitis B or C were obtained from NY Blood bank. Blood samples from adults with HIV infection (n = 11) were obtained during routine patient care visits in Virology Clinic at Bellevue Hospital (New York, NY). No selection criteria based on race or sex was included. All subjects provided written consent and the study was approved by the NYU Institutional Review Board. Variables within healthy and HIV+ donors were compared using a paired t test. Variables between healthy and infected individuals were compared by a Mann–Whitney test. Categorical variables were determined by a χ2 test. All significance levels were based on two-tailed tests. Statistical analyses were performed using Prism (GraphPad).
Quantitative PCR.
Total RNA from cells was isolated and cDNA was synthesized as previously described (3). Quantitative PCR was performed with the synthesized cDNA using Applied Biosystems 7300 apparatus. TaqMan primer and probe mixes were purchased from Applied Biosystems; their IDs are as follows: β-actin (used as a reference gene): Hs99999903_ml; GARP: Hs00194136_ml; FOXP3 primer mix ID: Hs00203958_m1.
Supplementary Material
Acknowledgments.
We thank Dat Tran, Ethan Shevach, Aimee ElHed, and Qi Wan for critical reading and suggestions for the manuscript. This study was supported by National Institutes of Health (NIH) Grant R01 AI065303 (to D.U.), Centers for AIDS Research NIH Grant P30AI027742 (to A.K. and D.U.), R01 AI059315 (to H.F.), and NIH Training Grant 2T32 AI007180–26A2 (to F.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901965106/DCSupplemental.
References
- 1.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oswald-Richter K, et al. HIV infection of naturally occurring and genetically reprogrammed human regulatory T-cells. PLoS Biol. 2004;4:E198. doi: 10.1371/journal.pbio.0020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yagi H, et al. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:1643–1656. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- 5.Allan SE, et al. Generation of potent and stable human CD4+ T regulatory cells by activation-independent expression of FOXP3. Mol Ther. 2008;16:194–202. doi: 10.1038/sj.mt.6300341. [DOI] [PubMed] [Google Scholar]
- 6.Weber SE, et al. Adaptive islet-specific regulatory CD4 T cells control autoimmune diabetes and mediate the disappearance of pathogenic Th1 cells in vivo. J Immunol. 2006;176:4730–4739. doi: 10.4049/jimmunol.176.8.4730. [DOI] [PubMed] [Google Scholar]
- 7.Fantini MC, et al. Transforming growth factor beta induced FoxP3+ regulatory T cells suppress Th1 mediated experimental colitis. Gut. 2006;55:671–680. doi: 10.1136/gut.2005.072801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamagiwa S, Gray JD, Hashimoto S, Horwitz DA. A role for TGF-beta in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. J Immunol. 2001;166:7282–7289. doi: 10.4049/jimmunol.166.12.7282. [DOI] [PubMed] [Google Scholar]
- 10.Wang R, Wan Q, Kozhaya L, Fujii H, Unutmaz D. Identification of a regulatory T cell specific cell surface molecule that mediates suppressive signals and induces Foxp3 expression. PLoS ONE. 2008;3:e2705. doi: 10.1371/journal.pone.0002705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antons AK, et al. Naive precursors of human regulatory T cells require FoxP3 for suppression and are susceptible to HIV infection. J Immunol. 2008;180:764–773. doi: 10.4049/jimmunol.180.2.764. [DOI] [PubMed] [Google Scholar]
- 12.Valmori D, Merlo A, Souleimanian NE, Hesdorffer CS, Ayyoub M. A peripheral circulating compartment of natural naive CD4 Tregs. J Clin Invest. 2005;115:1953–1962. doi: 10.1172/JCI23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seddiki N, et al. Persistence of naive CD45RA+ regulatory T cells in adult life. Blood. 2006;107:2830–2838. doi: 10.1182/blood-2005-06-2403. [DOI] [PubMed] [Google Scholar]
- 14.Sereti I, et al. In vivo expansion of CD4CD45RO-CD25 T cells expressing foxP3 in IL-2-treated HIV-infected patients. J Clin Invest. 2005;115:1839–1847. doi: 10.1172/JCI24307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shevach EM, Tran DQ, Davidson TS, Andersson J. The critical contribution of TGF-beta to the induction of Foxp3 expression and regulatory T cell function. Eur J Immunol. 2008;38:915–917. doi: 10.1002/eji.200738111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beriou G, et al. IL-17-producing human peripheral regulatory T cells retain suppressive function. Blood. 2009;113:4240–4249. doi: 10.1182/blood-2008-10-183251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voo KS, et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci USA. 2009;106:4793–4798. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Appay V, Sauce D. Immune activation and inflammation in HIV-1 infection: Causes and consequences. J Pathol. 2008;214:231–241. doi: 10.1002/path.2276. [DOI] [PubMed] [Google Scholar]
- 19.Grossman Z, Meier-Schellersheim M, Paul WE, Picker LJ. Pathogenesis of HIV infection: What the virus spares is as important as what it destroys. Nat Med. 2006;12:289–295. doi: 10.1038/nm1380. [DOI] [PubMed] [Google Scholar]
- 20.Andersson J, et al. CD4+ FoxP3+ regulatory T cells confer infectious tolerance in a TGF-beta-dependent manner. J Exp Med. 2008;205:1975–1981. doi: 10.1084/jem.20080308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Awasthi A, Murugaiyan G, Kuchroo VK. Interplay between effector th17 and regulatory T cells. J Clin Immunol. 2008;28:660–670. doi: 10.1007/s10875-008-9239-7. [DOI] [PubMed] [Google Scholar]
- 22.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allan SE, Song-Zhao GX, Abraham T, McMurchy AN, Levings MK. Inducible reprogramming of human T cells into Treg cells by a conditionally active form of FOXP3. Eur J Immunol. 2008;38:3282–3289. doi: 10.1002/eji.200838373. [DOI] [PubMed] [Google Scholar]
- 24.Holmes D, Jiang Q, Zhang L, Su L. Foxp3 and Treg cells in HIV-1 infection and immuno-pathogenesis. Immuol Res. 2008;41:248–266. doi: 10.1007/s12026-008-8037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tran, et al. GARP (LRRC32) is essential for the surface expression of latent TGFβ on platelets and activated FOXP3+ regulating cells. Proc Natl Acad Sci USA. 2009 doi: 10.1073/PNAS.0901944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.