Abstract
A signature of eclosion hormone (EH) action in insect ecdysis is elevation of cGMP in Inka cells, leading to massive release of ecdysis triggering hormone (ETH) and ecdysis initiation. Although this aspect of EH-induced signal transduction is well known, the receptor mediating this process has not been identified. Here, we describe a receptor guanylyl cyclase BdmGC-1 and its isoform BdmGC-1B in the Oriental fruit fly Bactrocera dorsalis that are activated by EH. The B form exhibits the conserved domains and putative N-glycosylation sites found in BdmGC-1, but possesses an additional 46-amino acid insertion in the extracellular domain and lacks the C-terminal tail of BdmGC-1. Combined immunolabeling and in situ hybridization reveal that BdmGC-1 is expressed in Inka cells. Heterologous expression of BdmGC-1 in HEK cells leads to robust increases in cGMP following exposure to low picomolar concentrations of EH. The B-isoform responds only to higher EH concentrations, suggesting different physiological roles of these cyclases. We propose that BdmGC-1 and BdmGC-1Β are high- and low-affinity EH receptors, respectively.
Keywords: Bactrocera dorsalis, ecdysis, oriental fruit fly, cyclic GMP, ecdysis triggering hormone
Eclosion hormone (EH), an insect neuropeptide involved in shedding of old cuticle during ecdysis, was first described in the giant silk moth (1), and subsequently identified in Manduca sexta, Bombyx mori, and Drosophila melanogaster (2–5). In Manduca, EH is produced by ventromedial (VM) cells in the brain and released into the hemolymph (6) in response to circulating ecdysis-triggering hormone (ETH) from Inka cells in epitracheal glands (7, 8). EH in turn acts on Inka cells to cause massive secretion of ETH, leading to ecdysis (7, 9, 10). A similar scenario for EH action has been described in Drosophila (11, 12). The signature of EH action in target cells, such as the Inka cell, is an elevation in cGMP.
Guanylyl cyclases (GCs), which convert GTP to cyclic 3′, 5′-guanosine monophosphate (cGMP) and pyrophosphate, have 2 forms: “membrane-bound forms” and “soluble forms” (13). The soluble form, a heterodimer consisting of α and β subunits, binds gas ligands such as NO or CO with an associated heme group. The receptor form of GC, a homodimer, is activated by peptide ligands such as atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), C-type natriuretic peptide (CNP), or bacterial heat-stable enterotoxins (STs) corresponding to GC-A, -B, and -C, respectively (13). In insects cGMP, which is involved in learning and memory, adaptation of olfactory receptor cells, and control of ecdysis behavior, was initially considered to be regulated by soluble GCs (14–16). Nevertheless, in Manduca assays, the NO donor sodium nitroprusside (SNP) failed to stimulate increased cGMP production in Inka cells (16, 17). These data suggest other types of guanylyl cyclase are involved in EH signaling. Subsequently, an atypical GC MsGC-β3 was identified in Inka cells and subtransverse nerve region (STNR) cells by RT-PCR and immunohistochemistry (16, 17), which might represent another type of modulator of ecdysis through its interaction with EH.
We reported previously that BdmGC-1, a guanylyl cyclase from Bactrocera dorsalis, exhibits all of the characteristics of a typical receptor form (18). In this study, we describe an alternatively spliced variant BdmGC-1B that encodes an isoform of this enzyme, BdmGC-1B. Heterologous expression of these receptor GCs confers robust cGMP responses to EH. The significance of this phenomenon, that is, receptor guanylyl cyclases with a peptide ligand, with respect to the mechanisms of ecdysis behavior in insects, is discussed.
Results
BdmGC-1B and BdmGC-1 Have Comparable Structures.
Tissue-specific RT-PCR revealed the existence of a transcript of the receptor guanylyl cyclase BdmGC-1. In addition, the BdmGC-1 ortholog CG10738 in Drosophila was also found to have 2 alternatively spliced variants from sequence analysis of the Drosophila database (NP_729905.1 and NP_648653.1). Differences between the 2 variants revealed by alignment analysis were used to design specific primers flanking the predicted diverse regions of BdmGC-1. After amplification of cDNA by PCR, a DNA fragment of approximately 850 bp was confirmed as an isoform sequence of BdmGC-1. Its full-length sequence was obtained subsequently by RACE.
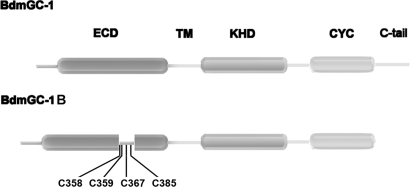
Sequence analysis showed that this B-isoform of BdmGC-1 possesses all of the functional domains of a receptor guanylyl cyclase, that is, a signal sequence, an extracellular ligand-binding domain (ECD), a hydrophobic transmembrane region (TM), a regulatory kinase-homology domain (KHD), and an activity core cyclase catalytic region (CYC) (Fig. 1). Comparisons with BdmGC-1 indicate that this isoform possesses a longer ECD containing 4 additional cysteines, but lacks the C-terminal tail (Fig. 1).
Fig. 1.
Diagrammatic comparison of BdmGC-1 and -1B. Both BdmGC-1 isoforms exhibit all of the characteristics of receptor GCs, including (from left to right) an extracellular domain (ECD; 82–443 of BdmGC-1 and 82–489 of BdmGC-1B), a transmembrane region (TM; 504–524 of BdmGC-1 and 550–570 of BdmGC-1B), a kinase-homology domain (KHD; 584–845 of BdmGC-1 and 630–891 of BdmGC-1B), and a cyclase catalytic region (CYC; 911-1097 of BdmGC-1 957-1143 of BdmGC-1B). An extra insertion (347–392) with 4 additional cysteines in the ECD and absence of a C-terminal sequence are distinctive characteristics of BdmGC-1B.
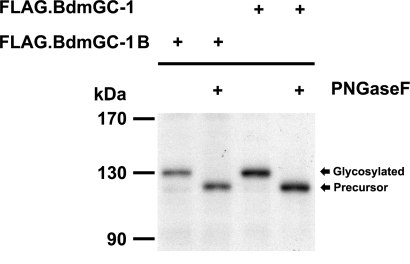
FLAG·BdmGC-1B in membranes of cells transiently expressing this protein had a molecular mass of approximately 130 kDa as visualized by Western blotting analysis, similar to that of FLAG·BdmGC-1 (Fig. 2). Incubation of FLAG·BdmGC-1B with N-glycosidase resulted in ≈10% loss of molecular mass (Fig. 2), consistent with the result observed for BdmGC-1. In addition, at least 4 putative glycosylation sites were detected in the ligand-binding domain of this enzyme form.
Fig. 2.
BdmGC-1s are N-glycosylated in HEK-293T cells. Cells transiently expressing FLAG·BdmGC-1 or FLAG·BdmGC-1Β were lysed in buffer containing protease inhibitors and incubated in G7 reaction buffer in the presence or absence of PNgaseF for 30 min at 37 °C. Western blot analysis using an anti-FLAG M2 antibody was performed.
BdmGC-1 Is Located in Inka Cells.
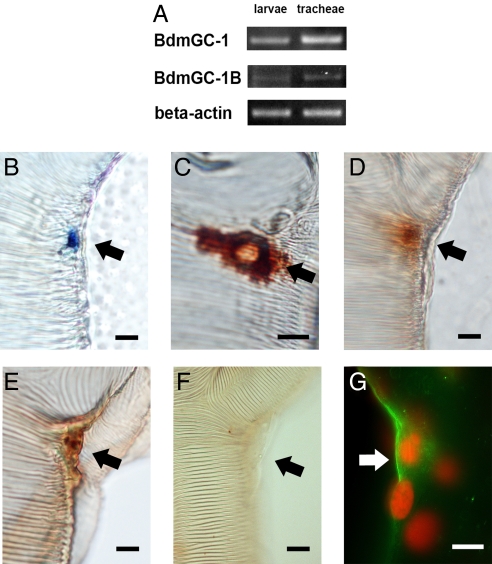
We previously reported that BdmGC-1 is expressed during embryonic, larval, and pupal stages, but is absent in B. dorsalis adults (18). Tissue-specific RT-PCR indicated that both BdmGC-1 and BdmGC-1B are associated with tracheae, with BdmGC-1 being expressed at substantially higher levels (Fig. 3A). Therefore, the main tracheal trunks from late third instar larvae were dissected and examined by in situ hybridization using a specific probe against the ECD region of BdmGC-1. The results showed that the BdmGC-1 transcripts are present in Inka cells along the trunks of tracheae at the main segmental branches (Fig. 3B). While RT-PCR results indicate low level expression of BdmGC-1B in tracheal extracts, attempts to visualize transcripts from this isoform in Inka cells by in situ hybridization have been unsuccessful, perhaps because of low transcript number.
Fig. 3.
BdmGC-1 is expressed at main tracheal junctions. (A) Transcripts of BdmGC-1 and -1B expressed in third instar larvae and trachea. (B) RNA labeling with anti-sense RNA probes showing that BdmGC-1 transcripts are located at sites coincident with Inka cells at junctions along the main tracheal trunks. Specific Dig-labeled probes derived from the ECD region of BdmGC-1 were used for mRNA detection. Visualization is via alkaline phosphatase. (C) Inka cells exhibit strong ETH1-immunoreactivity, visualized with HRP-DAB. (D and E) BdmGC-1 signals visualized at junctions of tracheae using BdmGC-1 C-terminal-specific antibodies and BdmGC-1 N-terminal-specific antibodies, respectively, visualized by HRP/DAB. (F) N-terminal-specific antibodies neutralized by peptide antigen as control. (G) Merged view of fluorescent labeling of BdmGC-1 and propidium iodide counterstaining. (Scale bar, 20 μm.)
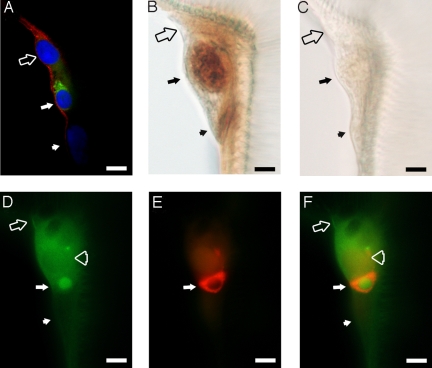
Immunohistochemistry was carried out to confirm the cellular localization of BdmGC-1. Examination of the main tracheal trunks of third instar larvae in whole mount preparations revealed BdmGC-1-like immunoreactivity (BdmGC-1-IR) in Inka cells when either C-terminal-specific or N-terminal-specific antisera were used (Fig. 3 D, E, and G). When epitracheal gland preparations were exposed to Drosophila ETH-1 specific antiserum, Inka cells of B. dorsalis were labeled as in Drosophila (19) and this labeling pattern coincided with the pattern of BdmGC-1-IR (Fig. 3C). In double-stained preparations, we confirmed the colocalization of BdmGC-1-IR and ETH1-IR at the main tracheae junctions (Fig. 4A and D–F). Using confocal microscopy, we demonstrated BdmGC-1 possesses the property of cell surface expression and again revealed that BdmGC-1 is coexpressed in cells with ETH1-IR (Fig. 4A). Moreover, we observed significant BdmGC-1-IR in adjacent cells, including a cell lying atop the epitracheal gland, a cell adjacent to the Inka cell, and a long shaped cell with mild BdmGC-1 signal beneath the epitracheal gland (Fig. 4 A–F). These cells are likely the small endocrine, large exocrine and transducing canal cell as described in a study of epitracheal glands in gypsy moth (20). These data indicate that BdmGC-1 and ETH1 are coexpressed in the epitracheal gland of B. dorsalis.
Fig. 4.
BdmGC-1 is colocalized with ETH1 and cGMP in epitracheal glands. Epitracheal glands from third instar larvae before pupal ecdysis were used for immuno-visualization. (A) Immuno-visualization of epitracheal gland by confocal microscopy. BdmGC-1 (red color; labeled by N-terminal-specific antibodies) shows cell surface expression and ETH-1 (green color) is localized in the cytoplasm of the Inka cell. The tissue is counterstained with DAPI (blue color). (B) To verify that Inka cells of B. dorsalis respond to EH by producing cGMP, tracheae of larvae were dissected and treated with 200 pM EH, then labeled with cGMP-specific antibodies and visualized by HRP/DAB. (C) Tracheae were treated with PBS instead of EH, followed by cGMP antibody labeling. (D and E) Epitracheal gland labeled by BdmGC-1 C-terminal specific (green color) antibodies and ETH1-(red color) antibodies, respectively. (F) Merged image of (E and F). Filled arrow: Inka cell, filled arrowhead: canal cell, open arrow: cell lying atop of epitracheal gland, and open arrowhead: cell adjacent to Inka cell. (Scale bars, 10 μm.)
Exposure of tracheal preparations to EH led to an increase in cGMP along the trunks of tracheae at locations corresponding to Inka cells (Fig. 4B). Control experiments in which Inka cells were exposed to PBS only resulted in no detectable elevation of cGMP (Fig. 4C).
BdmGC-1 and its B Form Are Activated by EH.
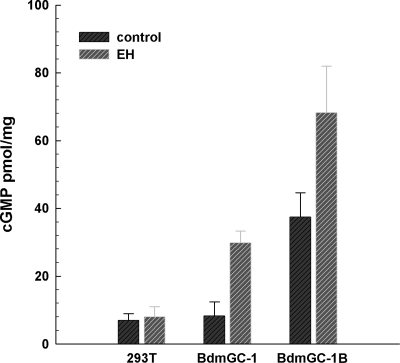
Localization of BdmGC-1 to Inka cells led us to hypothesize that it and the related isoform BdmGC-1B are direct receptors of EH. Indeed, transient expression of BdmGC-1s in HEK-293T cells conferred EH sensitivity as measured by cGMP production. As shown in Fig. 5, treatment with 200 pM EH caused a 3.6-fold increase in cGMP in cells expressing BdmGC-1 and a 1.8-fold increase in cells expressing BdmGC-1Β. Cells expressing the B isoform exhibit a higher basal level of cGMP than cells expressing BdmGC-1.
Fig. 5.
HEK-293T cells transiently expressing BdmGC-1 or -1B generate cGMP in response to EH exposure. Cells transiently expressing FLAG·BdmGC-1 or FLAG·BdmGC-1B were incubated with serum-free medium for 1 h. Before assay, cells were washed twice with PBS and then incubated in PBS supplemented with IBMX for 10 min at room temperature. Thereafter, 200 pM EH was added and incubated for 30 min at 37 °C. Total cGMP content was measured as described in the Materials and Methods. GC activity was measured in HEK cells transiently transfected with empty vector as control. Results are expressed as means ± SEM (n = 3). Cell lysates were immunoblotted with anti-FLAG antibodies to confirm similar expression levels of BdmGC-1 and -1B form.
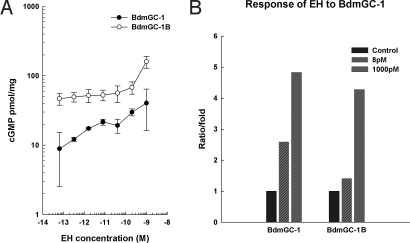
The BdmGC-1 isoforms showed clearly different sensitivities to EH. BdmGC-1 appears to be a high affinity EH receptor. While no apparent effect was observed at 64 fM, the activity of BdmGC-1 was increased approximately 2-fold at 1.6 pM EH (Fig. 6A). Increasing EH concentration from 10 pM to 1 nM led to progressive elevation of cGMP levels. Interestingly, a plateau appeared between 10 and 40 pM EH. Further increases in EH concentration to 100 pM and 1 nM EH yielded the highest levels of cGMP production (Fig. 6 A and B). Cells expressing BdmGC-1Β displayed relatively high basal cGMP levels (≈40–50 pmol/mg). Exposure to EH in the concentration range 0.1–100 pM elicited no significant increase in cGMP, but a robust 4.3-fold increase was observed at 1 nM (Fig. 6 A and B). These results show that BdmGC-1 and -1Β produce different basal levels of cGMP and have strikingly different sensitivities to EH.
Fig. 6.
BdmGC-1 and -1B exhibit different sensitivities to EH. (A) HEK-293T cells transiently expressing FLAG·BdmGC-1 or FLAG·BdmGC-1B were incubated with increasing concentrations of EH, i.e., 64 fM, 320 fM, 1.6 pM, 8 pM, 40 pM, 200 pM, and 1 nM, for 30 min at 37 °C. Total cGMP mobilized was measured as described in the Materials and Methods and results are expressed as mean ± SEM (n = 3). Cell lysates were immunoblotted with anti-FLAG antibodies to confirm similar expression levels of BdmGC-1 and -1B. (B) Relative increase of cGMP production in response to physiological (8 pM) or pharmacological (1,000 pM) concentrations of EH for BdmGC-1 and -1B.
Discussion
A receptor guanylyl cyclase BdmGC-1 was identified recently from the Oriental fruit fly B. dorsalis (18). In the present study, we identified BdmGC-1B, an alternatively spliced variant of BdmGC-1. While the B form has all functional domains of a receptor guanylyl cyclase, its ECD is 50 aa longer and contains 4 additional cysteine residues.
Receptor guanylyl cyclases of mammals are known to possess cysteine residues in their ECD, which form intrachain disulphide bonds (13). Disruption of these disulphide bonds by DTT affects ligand-binding and thus enzymatic activity (13). Mutagenesis experiments demonstrated that deletion or insertion of cysteines in the ECD of mouse guanylyl cyclase GC-A resulted in dimerization via interchain disulfide bonds, which affected the binding affinity of atrial natriuretic peptide (21). The cg10738PA possesses one additional unpaired cysteine in the ECD insertion, and is proposed to form an interchain disulphide bridge. In the BdmGC-1B insertion, C358 and C359 are too close to form a disulphide bridge, thus the two free cysteines may contribute to formation of interchain disulphide bridges between the two subunits.
BdmGC-1 possesses a long and unique C-terminal tail whose functions have not been identified (18). In mammals, the carboxyl terminal extension observed in sensory GCs and GC-C (13) is believed to be involved in interactions with cytoplasmic proteins, such as the cytoskeleton (13). For example, the C-terminal tail of GC-E binds with the tubulin complex in photoreceptors, and that of GC-C seems to interact with intestinal and kidney-enriched PDZ domains to desensitize the activation of STs (22, 23). Since BdmGC-1B lacks the long C-terminal extension of BdmGC-1, we expect that these 2 cyclases have distinct physiological roles, at least in part because of differences in coupling to intracellular signal transduction pathways.
The release of ETH from Inka cells is mediated by brain neuropeptides, which mobilize cytoplasmic calcium and cGMP (11, 24). In Manduca, corazonin mediates low rates of ETH release during pre-ecdysis before EH-induced cGMP elevation. The elevation of cGMP by EH appears to be a key step in the induction of massive ETH release, leading its depletion in Inka cells (10). In Drosophila, corazonin appears not to be involved in ETH release, but elevation of cGMP in Inka cells before ecdysis initiation lends credence to a role for EH in ETH release in this organism as well (11).
Epitracheal glands of Lepidoptera are comprised of an ETH-secreting Inka cell, a transducing canal cell, a small endocrine cell, and a large exocrine cell, with the functions of the 2 latter ones to be identified [reviewed in (20, 25, 26)]. Herein, based on their relative localization and morphology, 4 cells were observed in the epitracheal gland. The cell showing ETH1-IR appears to be the Inka cell and a long shaped cell beneath the epitracheal gland could be the canal cell. Functions of the cell lying atop the epitracheal gland and the cell adjacent to Inka cell are uncertain. Nevertheless, it is reasonable to propose that these 2 cells, in which BdmGC-1 is expressed, may be involved along with the Inka cell in signal transduction following release of EH into the hemolymph.
Our immunolabeling and in situ hybridization results indicate that the receptor guanylyl cyclase BdmGC-1 is located in Inka cells, which is positioned at segmental junctions along the main tracheal trunks in Drosophila (19, 27). This observation prompted us to hypothesize that BdmGC-1 could be the potential receptor of neuropeptide EH.
We found that EH (200 pM) elicited a 2-fold larger cGMP increase in BdmGC-1 expressing cells compared with cells expressing BdmGC-1Β. The sensitivity of BdmGC-1 to low nanomolar concentrations of EH (threshold of 1.6 pM) is in good agreement with data reported by Kingan et al., who examined EH-induced elevation of cGMP and ETH secretion in Inka cells of Manduca (9). Furthermore, a plateau in the concentration-response relationship occurring in the range 10–40 pM EH is remarkably similar to those published for Manduca Inka cells with respect to both cGMP mobilization and ETH secretion (9). We conclude that BdmGC-1 is a plausible candidate for a high affinity EH receptor.
In contrast, BdmGC-1B expressing cells require >200 nM EH to elicit significant increases in cGMP (Figs. 5 and 6B). Given that hemolymph EH concentrations are not expected to reach such high levels under physiological conditions, this raises questions regarding the function of BdmGC-1Β. Several reports indicate that some receptor GCs such as GC-E and GC-F, when expressed heterologously, generate high basal levels of cGMP (28, 29). However, these cyclases are tightly regulated in the presence of GC-activating proteins (GCAPs) (30–34). Since EH is known to elevate Ca2+ in Inka cells (24, 35), the possibility that EH action on this cyclase could operate via Ca2+ regulation must be seriously considered. Finally, it is also possible that BdmGC-1Β is involved with signal transduction within the CNS, where EH levels are expected to be higher (7). Investigations into the expression of EH-sensitive GCs in the CNS are underway.
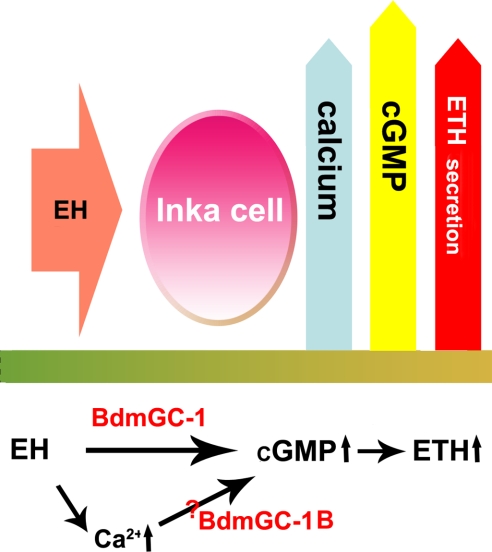
In conclusion, we have identified 2 forms of receptor guanylyl cyclase in B. dorsalis, BdmGC-1 and BdmGC-1B, which share similar structural characteristics and are localized in Inka cells of epitracheal glands. Functional analysis of these cyclases shows that both BdmGC-1 and its B form could serve as receptors of EH, although it is likely that they operate with different ligand-binding affinities and signal transduction properties in vivo. A model for ETH secretion by Inka cells in response to EH is proposed in Fig. 7. Based on the accumulated evidence, we propose that BdmGC-1s function as EH receptors, providing further insights into the machinery of insect ecdysis and eclosion behavior.
Fig. 7.
Model for EH-mediated ETH secretion by Inka cells. EH evokes cGMP synthesis by binding to its receptor, BdmGC-1. EH may act via 2 distinct pathways: A direct action on BdmGC-1 elevates cGMP content; a second signal transduction pathway functions via calcium mobilization, which regulates BdmGC-1B activity, a common regulation phenomenon in sensory GCs of mammals.
Materials and Methods
Animal Rearing and RNA Extraction.
Oriental fruit flies (B. dorsalis) were maintained in the laboratory as described elsewhere (18).
Total RNA from B. dorsalis was prepared using TRI Reagent (Molecular Research Center) following instructions provided by the manufacturer.
Full-Length cDNA Cloning.
Total RNAs (5 μg each sample) isolated from pupae were used for complementary DNA synthesis by SuperScript III (Invitrogen), and RT-PCR was carried out with Platinum TaqDNA polymerase (Invitrogen). Based on the sequence of the Drosophila ortholog CG10738 from the National Center for Biotechnology Information, specific primers for isolation of isoform BdmGC-1 were designed. The expected fragment was cloned into the pGEM-T easy vector (Promega) and sequenced using the Prism kit (Applied Biosystems) on an ABI 3100 DNA sequencer (Applied Biosystems).
Full-length cDNA was obtained from the isolated sequence by RACE using 5′ and 3′ cDNA amplification kits (Invitrogen). The 5′ end cDNA was amplified with 5′-GGC TGG TGC GTA TAA GCG GGT GAG T-3′ as primary primer and 5′-CGT CCA CAC GCT TAG CAC CGC CCT CAC TTC TCG A-3′as nested PCR primer. The 3′ end cDNA was amplified with 5′-CTC ATT CGC TAA GTT TGC CAA AGA A-3′ as primary primer and 5′-GGG GCT TTC CTC CGC TTT AAC AAA GTG CTG TCT CC-3′ as nested PCR primers. The PCR products were subcloned into pGEM-T EASY (Promega) and sequenced, and the final cDNA is designated as BdmGC-1B.
Expression Plasmid.
The cDNA coding for mature BdmGC-1B (amino acids 51–1251 or -1214) was amplified by Platinum TaqDNA polymerase (Invitrogen) with primers (sense 5′-CCC AAG CTT ATC GGC CAA GCA GCA GCA GA-3′ and antisense 5′-GCT CTA GAA TGT AAA AAA CTC GAG GCT GGA GG-3′) and cloned into pFLAG-CMV1 (Sigma). This expression plasmid produced an amino-terminal FLAG-tagged recombinant protein (FLAG·BdmGC-1B).
Reverse Transcription (RT)-PCR Analysis.
To observe BdmGC-1 and -1B expression, a cDNA mixture derived from total RNA of B. dorsalis larvae served as a template for the PCR amplification. The specific PCR primers for BdmGC-1s were 5′-CTC ATT CGC TAA GTT TGC CAA AGA A-3′ (for BdmGC-1, sense), 5′-GGG GCT TTC CTC CGC TTT AAC AAA GT-3′ (for BdmGC-1B, sense) and 5′-GGC TGG TGC GTA TAA GCG GGT GAG T-3′ (for BdmGC-1 and -1B, antisense). These primers span 3 introns to avoid PCR amplification from genomic DNA, and the amplified fragment of BdmGC-1B was 100 bases longer than that of BdmGC-1. For BdmGC-1, the reactions were 94 °C for 2 min, followed by 30 cycles of 30 s at 94 °C, 20 s at 58 °C, and 60 s at 72 °C and a final incubation at 72 °C for 7 min; for BdmGC-1B, the PCR parameters were the same as those used for BdmGC-1, except 35 amplification cycles were used. The amplified products were then analyzed on a 1% agarose gel and stained with ethidium bromide. These experiments were independently repeated 3 times.
Cell Culture, Transfection, and N-Glycosylation Assay.
Human embryonic kidney 293T cells were maintained in Dulbecco modified Eagle's medium (GIBCO), supplemented with 10% FBS, 100 units/mL penicillin, and 100 μg/mL streptomycin. One day before transfection, cells were seeded in 6-well plates and transfected by calcium phosphate-precipitation with 2 μg expression plasmid DNA.
To detect N-glycosylation in BdmGC-1B, cells transiently expressing FLAG·BdmGC-1B were lysed in lysis buffer (20 mM Tris·HCl, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 25 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, and 1 μg/mL leupeptin), and then treated with PNGaseF (New England BioLabs) in G7 reaction buffer with 1% Nonidet P-40. Samples were incubated for 1 h at 37 °C for subsequent Western blot analysis.
Immunoprecipitation and Western Blot Analysis.
Transfected cells were collected, lysed in lysis buffer, and centrifuged at 10,000 × g for 15 min at 4 °C. Supernatants were incubated with M2-agarose (Sigma) for 2 h, washed with lysis buffer, and the precipitated complexes were dissolved by boiling in Laemmli sample buffer. Soluble samples were fractionated by SDS/PAGE, and transferred to polyvinylidine difluoride membranes. Membranes were then blocked with 0.1% gelatin in PBST and incubated with appropriate antibodies. Proteins were detected with the enhanced chemiluminescence system (Amersham Biosciences).
Effect of EH on HEK-293T Cells Transiently Expressing BdmGC-1.
The effect of EH on cGMP production was assayed in HEK-293T cells transiently expressing BdmGC-1s. In brief, BdmGC-1- or BdmGC-1B-transfected HEK-293T cells and cells transfected with vector only were incubated in wells with serum-free medium 1 h before assay. After washing twice with PBS and incubating in 0.4 mL PBS with IBMX (0.8 mM) for 10 min, cells were treated with different concentrations of EH at 37 °C for 20 min. After incubation, cells were lysed, and total cGMP contents were determined following the instructions of the CatchPoint cGMP Fluorescent Assay Kit (Molecular Devices). Synthetic eclosion hormone was prepared as described in ref. 9.
Antibodies, Immunohistochemistry and Whole Mount In Situ Hybridization.
All antisera specific for the N or C terminus of BdmGC-1 or Drosophila ETH1 were produced in rabbits. BdmGC-1 antigens were synthesized by coupling with multiple antigen peptide (MAP) containing 20 N- or 18 C-terminal amino acids of BdmGC-1 (GYLTGSQRSPGNLDYQRSGI for the N terminus and EDEDARNKRNYERSQRRG corresponding to the C terminus). Both N- and C-terminal-specific antibodies cross-reacted with BdmGC-1 and BdmGC-1B.
Preabsorption of BdmGC-1 specific antibodies by antigen peptide, aliquots of BdmGC-1 antibodies and peptide antigen (2 μL antibodies + 1 mg BdmGC-1 N-terminal peptide) were coincubated in PBS with 1% BSA overnight at 4 °C. Precipitated complexes were removed by sedimentation, and the supernatants were used as primary antibodies.
For immunohistochemistry, epitracheal glands were dissected from late third instar larvae before pupal ecdysis and then fixed in 2% paraformaldehyde for 40 min at room temperature (RT), followed by blocking nonspecific epitopes with 10% goat serum. Tissues were processed with BdmGC-1- or ETH-specific antibodies (1:500 or 1:100, respectively) for 24 h at 4 °C, followed by washing 3 times in PBS + 0.2% saponin. Samples were then incubated with peroxidase-conjugated secondary antibodies (1:200; A2074, Sigma) for 4 h at 4 °C. After washing, target complexes were visualized using Vector Impact DAB substrate (Vector Laboratories), dehydrated, and mounted on gelatin-coated slides with Vector Mount (Vector Laboratories). For control tests of BdmGC-1 or ETH staining, tissues were incubated with secondary antibodies only; subsequent procedures were followed as described above.
For cGMP staining, tracheal trunks were dissected from third instar larvae before pupal ecdysis and incubated in PBS with the phosphodiesterase inhibitor IBMX (0.8 mM) for 10 min at 4 °C. Tissues were then transferred to PBS containing 200 pM EH and incubated for 30 min at 37 °C. After fixation in 4% paraformaldehyde, tissues were processed with cGMP-specific antibodies (1:100; ab12416, Abcam) for 24 h at 4 °C followed by washing 3 times in PBS + 0.1% Triton X-100. Tissues were incubated with peroxidase-conjugated secondary antibodies (1:200; A2074, Sigma) for 4 h at 4 °C. After washing, target complexes were visualized using Vector Impact DAB substrate (Vector Laboratories), dehydrated, and mounted on gelatin-coated slides with Vector Mount (Vector Laboratories). In cGMP-staining control tests, IBMX-treated tissues were incubated in PBS without EH for 30 min at 37 °C, and the following treatments were the same as described above.
Double Staining of BdmGC-1 and ETH1.
For double staining of BdmGC-1 and ETH1, tissues were dissected and fixed in 4% paraformaldehyde. Immunostaining procedures were the same as described above, but induction of GC activity by EH was omitted. In brief, dissected tissues were blocked in Image-iT signal enhancer (Invitrogen, I36933), incubated with ETH-1 specific antibodies (1:200) for 24 h at 4 °C, and were subsequently labeled with Alexa Fluor 594 goat anti-rabbit secondary antibodies (1:500) (Invitrogen, A31631) for 6 h at RT. Epitracheal glands were then incubated with Alexa Fluor 488 labeled (Invitrogen, Z25302) BdmGC-1 specific antibodies (1:100) for 2 h at RT. Precipitated signals were visualized by Leica microscope systems, and collected images were adjusted and merged using Photoshop CS (Adobe).
For confocal visualization, tracheae were incubated with BdmGC-1 specific antibodies overnight at 4 °C and before incubation with Alexa Fluor 594 goat anti-rabbit secondary antibodies (1:500) (Invitrogen, A31631). To localize ETH-1, the same tissues were incubated with Alexa Fluor 488 labeled (Invitrogen, Z25302) ETH-1 specific antibodies (1:100) for 2 h at RT. Finally, the precipitated complexes were visualized by confocal microscopy.
For in situ hybridization, the unique region ECD (nt 565–779) of BdmGC-1 was selected as template for digoxigenin-labeled RNA probe preparation. Epitracheal glands were fixed in 4% paraformaldehyde at 4 °C overnight, permeabilized by protease K digestion, and hybridized with digoxigenin-labeled specific antisense probes or control sense probes for 24 h at 55 °C. After incubation, tissues were washed twice in washing buffer, and incubated with anti-Dig alkaline phosphatase-conjugated antibodies (11093274910, Roche Diagnostics GmbH) for 4 h at 4 °C. Target expression sites were chemically developed with Vector Blue (Vector Laboratories), dehydrated, and mounted on gelatin-coated slides with Vector Mount.
Acknowledgments.
We thank the anonymous referees' detailed reviews of this report, which improved the quality of the paper and Dr. Chih-Ning Sun for critical reading and useful discussions of the article. This work supported by Bureau of Animal and Plant Health Inspection and Quarantine, Council of Agriculture, Republic of China Grants 94AS-13.4.3-BQ-B1 and 95AS-13.3.1-BQ-B2 (1) (to K.H.L.); National Science Council, Republic of China Grant NSC97–2320-B-001–009-MY3 (to R.B.Y.); National Institutes of Health Grant GM67310 (to M.E.A.); and ATU plan of the Ministry of Education, Republic of China.
Footnotes
References
- 1.Truman JW, Riddiford LM. Neuroendocrine control of ecdysis in silk moths. Science. 1970;167:1624–1626. doi: 10.1126/science.167.3925.1624. [DOI] [PubMed] [Google Scholar]
- 2.Kataoka H, Troetschler RG, Kramer SJ, Cesarin BJ, Schooley DA. Isolation and primary structure of the eclosion hormone of the tobacco hornworm, Manduca sexta. Biochem Biophys Res Commun. 1987;146:746–750. doi: 10.1016/0006-291x(87)90592-4. [DOI] [PubMed] [Google Scholar]
- 3.Kono T, Nagasawa H, Isogai A, Fugo H, Suzuki A. Amino acid sequence of eclosion hormone of the silkworm Bombyx mori. Agr Biol Chem. 1987;51:2307–2308. [Google Scholar]
- 4.Marti T, Takio K, Walsh KA, Terzi G, Truman JW. Microanalysis of the amino acid sequence of the eclosion hormone from the tobacco hornworm Manduca sexta. FEBS Lett. 1987;219:415–418. doi: 10.1016/0014-5793(87)80263-6. [DOI] [PubMed] [Google Scholar]
- 5.Horodyski FM, Ewer J, Riddiford LM, Truman JW. Isolation, characterization and expression of the eclosion hormone gene of Drosophila melanogaster. Eur J Biochem. 1993;215:221–228. doi: 10.1111/j.1432-1033.1993.tb18026.x. [DOI] [PubMed] [Google Scholar]
- 6.Truman JW, Copenhaver PF. The larval eclosion hormone neurons in Manduca sexta: Identification of the brain-proctodeal neurosecretory system. J Exp Biol. 1989;147:457–470. [Google Scholar]
- 7.Ewer J, Gammie SC, Truman JW. Control of insect ecdysis by a positive-feedback endocrine system: Roles of eclosion hormone and ecdysis triggering hormone. J Exp Biol. 1997;200:869–881. doi: 10.1242/jeb.200.5.869. [DOI] [PubMed] [Google Scholar]
- 8.Zitnan D, et al. Steroid induction of a peptide hormone gene leads to orchestration of a defined behavioral sequence. Neuron. 1999;23:523–535. doi: 10.1016/s0896-6273(00)80805-3. [DOI] [PubMed] [Google Scholar]
- 9.Kingan TG, Gray W, Zitnan D, Adams ME. Regulation of ecdysis-triggering hormone release by eclosion hormone. J Exp Biol. 1997;200:3245–3256. doi: 10.1242/jeb.200.24.3245. [DOI] [PubMed] [Google Scholar]
- 10.Kim YJ, et al. Corazonin receptor signaling in ecdysis initiation. Proc Natl Acad Sci USA. 2004;101:6704–6709. doi: 10.1073/pnas.0305291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark AC, del Campo ML, Ewer J. Neuroendocrine control of larval ecdysis behavior in Drosophila: Complex regulation by partially redundant neuropeptides. J Neurosci. 2004;24:4283–4292. doi: 10.1523/JNEUROSCI.4938-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim YJ, Zitnan D, Galizia CG, Cho KH, Adams ME. A command chemical triggers an innate behavior by sequential activation of multiple peptidergic ensembles. Curr Biol. 2006;16:1395–1407. doi: 10.1016/j.cub.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 13.Lucas KA, et al. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev. 2000;52:375–413. [PubMed] [Google Scholar]
- 14.Stengl M, Hatt H, Breer H. Peripheral processes of in insect olfaction. Ann Rev Physiol. 1992;54:665–681. doi: 10.1146/annurev.ph.54.030192.003313. [DOI] [PubMed] [Google Scholar]
- 15.Bicker G. Sources and targets of nitric oxide signalling in insect nervous systems. Cell Tissue Res. 2001;303:137–146. doi: 10.1007/s004410000321. [DOI] [PubMed] [Google Scholar]
- 16.Morton DB, Simpson PJ. Cellular signaling in eclosion hormone action. J Insect Physiol. 2002;48:1–13. doi: 10.1016/s0022-1910(01)00157-3. [DOI] [PubMed] [Google Scholar]
- 17.Morton DB, Giunta MA. Eclosion hormone stimulates cyclic GMP levels in Manduca sexta nervous tissue via arachidonic acid metabolism with little or no contribution from the production of nitric oxide. J Neurochem. 1992;59:1522–1530. doi: 10.1111/j.1471-4159.1992.tb08469.x. [DOI] [PubMed] [Google Scholar]
- 18.Chang JC, Yang RB, Chen YH, Lu KH. A novel guanylyl cyclase receptor, BdmGC-1, is highly expressed during the development of the oriental fruit fly Bactrocera dorsalis (Hendel) Insect Mol Biol. 2006;15:69–77. doi: 10.1111/j.1365-2583.2006.00609.x. [DOI] [PubMed] [Google Scholar]
- 19.Park Y, Filippov V, Gill SS, Adams ME. Deletion of the ecdysis-triggering hormone gene leads to lethal ecdysis deficiency. Development. 2002;129:493–503. doi: 10.1242/dev.129.2.493. [DOI] [PubMed] [Google Scholar]
- 20.Klein C, Kallenborn HG, Radlicki C. The ‘Inka cell’ and its associated cells: Ultrastructure of the epitracheal glands in the gypsy moth, Lymantria dispar. J Insect Physiol. 1999;45:65–73. doi: 10.1016/s0022-1910(98)00085-7. [DOI] [PubMed] [Google Scholar]
- 21.De Léan A, McNicoll N, Labrecque J. Natriuretic peptide receptor A activation stabilizes a membrane-distal dimer interface. J Biol Chem. 2003;278:11159–11166. doi: 10.1074/jbc.M212862200. [DOI] [PubMed] [Google Scholar]
- 22.Schrem A, Lange C, Beyermann M, Koch KW. Identification of a domain in guanylyl cyclase-activating protein 1 that interacts with a complex of guanylyl cyclase and tubulin in photoreceptors. J Biol Chem. 1999;274:6244–6249. doi: 10.1074/jbc.274.10.6244. [DOI] [PubMed] [Google Scholar]
- 23.Scott RO, Thelin WR, Milgram SL. A novel PDZ protein regulates the activity of guanylyl cyclase C, the heat-stable enterotoxin receptor. J Biol Chem. 2002;277:22934–22941. doi: 10.1074/jbc.M202434200. [DOI] [PubMed] [Google Scholar]
- 24.Kingan TG, Cardullo RA, Adams ME. Signal transduction in eclosion hormone-induced secretion of ecdysis-triggering hormone. J Biol Chem. 2001;276:25136–25142. doi: 10.1074/jbc.M102421200. [DOI] [PubMed] [Google Scholar]
- 25.Zitnan D, Kingan TG, Hermesman J, Adams ME. Identification of ecdysis-triggering hormone from an epitracheal endocrine system. Science. 1996;271:88–91. doi: 10.1126/science.271.5245.88. [DOI] [PubMed] [Google Scholar]
- 26.Weiss BL, Merritt DJ. Demonstration of an ecdysis-triggering compound in the epitracheal gland of the cotton bollworm, Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) Australian J Entomol. 1998;37:366–368. [Google Scholar]
- 27.Zitnan D, et al. Conservation of ecdysis-triggering hormone signalling in insects. J Exp Biol. 2003;206:1275–1289. doi: 10.1242/jeb.00261. [DOI] [PubMed] [Google Scholar]
- 28.Shyjan AW, de Sauvage FJ, Gillett NA, Goeddel DV, Lowe DG. Molecular cloning of a retina-specific membrane guanylyl cyclase. Neuron. 1992;9:727–737. doi: 10.1016/0896-6273(92)90035-c. [DOI] [PubMed] [Google Scholar]
- 29.Yang RB, Foster DC, Garbers DL, Fulle HJ. Two membrane forms of guanylyl cyclase found in the eye. Proc Natl Acad Sci USA. 1995;92:602–606. doi: 10.1073/pnas.92.2.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dizhoor AM, Lowe DG, Olshevskaya EV, Laura RP, Hurley JB. The human photoreceptor membrane guanylyl cyclase, RetGC, is present in outer segments and is regulated by calcium and a soluble activator. Neuron. 1994;12:1345–1352. doi: 10.1016/0896-6273(94)90449-9. [DOI] [PubMed] [Google Scholar]
- 31.Palczewski K, et al. Molecular cloning and characterization of retinal photoreceptor guanylyl cyclase-activating protein. Neuron. 1994;13:395–404. doi: 10.1016/0896-6273(94)90355-7. [DOI] [PubMed] [Google Scholar]
- 32.Dizhoor AM, et al. Cloning, sequencing, and expression of a 24-kDa Ca2+-binding protein activating photoreceptor guanylyl cyclase. J Biol Chem. 1995;270:25200–25206. doi: 10.1074/jbc.270.42.25200. [DOI] [PubMed] [Google Scholar]
- 33.Lowe DG, et al. Cloning and expression of a second photoreceptor-specific membrane retina guanylyl cyclase (RetGC), RetGC-2. Proc Natl Acad Sci USA. 1995;92:5535–5539. doi: 10.1073/pnas.92.12.5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haeseleer F, et al. Molecular characterization of a third member of the guanylyl cyclase-activating protein subfamily. J Biol Chem. 1999;274:6526–6535. doi: 10.1074/jbc.274.10.6526. [DOI] [PubMed] [Google Scholar]
- 35.Zitnan D, Kim YJ, Zitnanová I, Roller L, Adams ME. Complex steroid-peptide-receptor cascade controls insect ecdysis. Gen Comp Endocrinol. 2007;153:88–96. doi: 10.1016/j.ygcen.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]