Abstract
Intestinal cancer is one of the most common human cancers. Aberrant activation of the canonical Wnt signaling cascade, for example, caused by adenomatous polyposis coli (APC) gene mutations, leads to increased stabilization and accumulation of β-catenin, resulting in initiation of intestinal carcinogenesis. The aryl hydrocarbon receptor (AhR) has dual roles in regulating intracellular protein levels both as a ligand-activated transcription factor and as a ligand-dependent E3 ubiquitin ligase. Here, we show that the AhR E3 ubiquitin ligase has a role in suppression of intestinal carcinogenesis by a previously undescribed ligand-dependent β-catenin degradation pathway that is independent of and parallel to the APC system. This function of AhR is activated by both xenobiotics and natural AhR ligands, such as indole derivatives that are converted from dietary tryptophan and glucosinolates by intestinal microbes, and suppresses intestinal tumor development in ApcMin/+ mice. These findings suggest that chemoprevention with naturally-occurring and chemically-designed AhR ligands can be used to successfully prevent intestinal cancers.
Keywords: cecal cancer, ubiquitin ligase, β-catenin, tumor chemoprevention
The aryl hydrocarbon receptor (AhR, also known as dioxin receptor) is a member of a transcription factor superfamily that is characterized by structural motifs of basic helix–loop–helix (bHLH)/Per-AhR nuclear translocator (Arnt)-Sim (PAS) domains, and also includes hypoxia-inducible factors (HIFs). Over the past decade, many studies have been focused on elucidating the functions of AhR as a mediator of multiple pharmacological and toxicological effects such as the induction of drug-metabolizing enzymes, teratogenesis, tumor promotion, and immunosuppression caused by environmental contaminants such as 3-methylcholanthrene (MC) and 2,3,7,8-tetrachloro dibenzo-p-dioxin (TCDD) (1, 2). On ligand binding, AhR translocates from the cytoplasm into the nucleus where it heterodimerizes with the Arnt and activates the transcription of target genes such as Cyp1a1. Induction of the Cyp1a1 gene leads to the biotransformation of polycyclic aromatic hydrocarbons into active genotoxic metabolites, resulting in the initiation of chemical carcinogenesis (3). AhR-deficient (AhR−/−) mice are resistant to most, if not all, of these toxicological adverse effects, indicating that AhR is a key factor in the development of these chemical-induced diseases (4, 5). Also, we recently found that AhR functions as a ligand-dependent E3 ubiquitin ligase of certain nuclear receptors (6), such as the estrogen (ER) and androgen receptors (AR). Most recently, AhR has been reported to have a crucial role in the differentiation of regulatory T cells (7–9).
AhR is a nucleocytoplasmic shuttling protein, the intracellular localization of which is changed depending on cell density in the absence of exogenous ligands (10). Such cell density-dependent movements between the cytoplasm and nucleus have also been reported for some tumor suppressor gene products, such as VHL (11) and adenomatous polyposis coli (APC) (12). Also, the natural AhR ligands of indole derivatives (13, 14), such as indole-3-acetic acid (IAA, so-called plant auxin), indole-3-carbinol (I3C) and 3,3′-diindolylmethane (DIM), are natural AhR ligands and generated through conversion from dietary tryptophan (Trp) and glucosinolates, respectively, by commensal intestinal microbes (15). Notably, glucosinolates have been reported to exert the chemopreventive effects on colorectal cancers in humans by cruciferous vegetables (16–18). Together, these lines of evidence suggest that AhR has some functional association with intestinal carcinogenesis.
Results
Cecal Tumor Development in AhR−/− Mice.
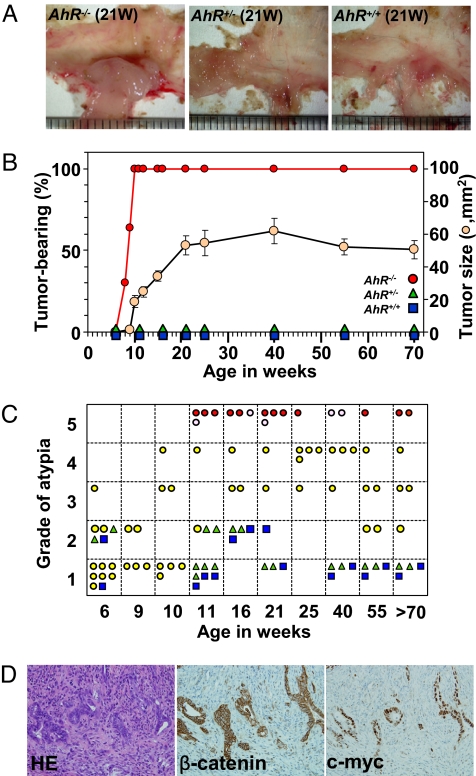
After thoroughly examining the digestive tracts of AhR−/− mice, we found that AhR−/− mice, but not heterozygous AhR+/− or wild-type AhR+/+ mice, frequently developed colonic tumors, mostly in the cecum near the ileocecal junction (Fig. 1 A and B). AhR−/− mice bred at 2 independent animal houses showed a similar time course of macroscopic tumor incidence (Fig. S1B), and the tumor size increased gradually by age, reached a plateau at ≈30 to 40 weeks (Fig. 1B). To date, 3 independent AhR−/− mice lines have been reported (4, 19, 20). Although one report described frequent rectal prolapse (Fig. S1A) and marked colonic hyperplasia with severe inflammation in AhR−/− mice (19), there have been no systematic studies on intestinal carcinogenesis, which may explain why the tumor suppressor function of AhR has been unreported to date. Colorectal cancer is one of the most common human cancers, 5–10% of which originates in the cecum. Therefore, we were interested in investigating how AhR−/− mice develop spontaneous cecal tumors.
Fig. 1.
Cecal tumor development in AhR−/− mice. (A) Representative profiles of colon tumors at the cecum in AhR−/− mice. (B) Relationship between the time course of macroscopic tumor incidence and tumor growth by age. Tumor size was estimated based on NIH images as shown by beige circles. Error bars, means ± SD. (C) Summary of histological atypia grades of tumors in AhR−/− mice by age. AhR+/+ (blue squares), AhR+/− (green triangles), and AhR−/− (yellow circles) are shown. AhR−/− mice with adenocarcinomas (Grade 5) that had invaded the submucosal region or beyond (red circles) and within the intramucosal region (pink circles) are shown separately. (D) Representative H&E staining profile of a moderately differentiated adenocarcinoma and immunohistochemical staining with an antibody against β-catenin or c-myc.
Randomly selected mice were examined histologically for atypia classified according to the standards as shown in Fig. S2. Although AhR+/+ and AhR+/− mice of all ages had normal (Grade 1) to mild hyperplasia (Grade 2) at worst, AhR−/− mice older than 11 weeks had abnormal histology with atypia ranging from mild malignancy of polyps to severe carcinomas that were exacerbated with age (Fig. 1C). Close microscopic examination revealed that the AhR−/− mice bore cecal lesions with a moderate (Grade 3: 9/42) or a high grade of atypia, adenoma (Grade 4: 12/42), and adenocarcinoma (Grade 5: 17/42). Among the 17 diagnosed adenocarcinomas, 12 tumors (71%) invaded the submucosal region or beyond, and the remainder were located within the intramucosal region. Overall survival rates estimated by the Kaplan-Meyer method (Fig. S1C) revealed that AhR−/− mice had a significantly shorter lifespan than wild-type or heterozygous mice (log-rank test; P = 4.4 × 10−9), although this shorter longevity might not be only due to cecal tumors in the AhR−/− mice (19).
The detected cecal cancers were predominantly tubular adenocarcinomas with various degrees of malignancy (Fig. S3). A representative profile of moderately differentiated adenocarcinomas with irregularly shaped and fused tubular structures that sometimes invaded the submucosal regions is presented in Fig. 1D. In these cells, immunohistochemical staining showed concomitant overexpression of β-catenin and c-myc, a target gene of β-catenin/TCF4 (21). It remains uninvestigated whether there should occur any further genetic alterations in AhR−/− mice leading to carcinogenesis. In human cecal cancers, markedly reduced expression of AhR was also found concomitantly with an abnormal accumulation of β-catenin in all of 12 cancer specimens from our hospital (Fig. S4).
The β-Catenin Accumulation in AhR−/− Mice.
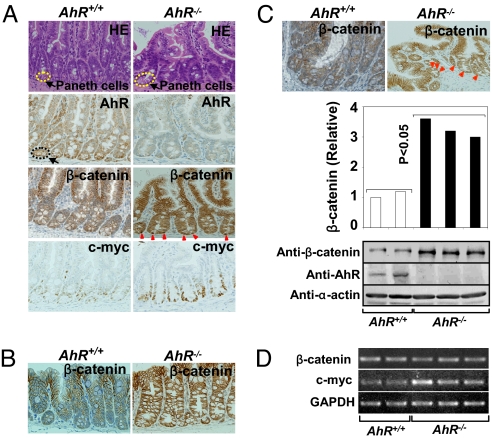
To examine the molecular mechanism underlying tumor development in AhR−/− mice, we analyzed the expression of both AhR and β-catenin in the intestines of 6-week-old AhR+/+ and AhR−/− mice, which had a morphologically normal epithelium. AhR expression was relatively abundant in Paneth cells (22), which have a host-defensive role against microbes in the small intestine and the cecum in AhR+/+ mice, but was undetectable in AhR−/− mice (Fig. 2A). Significant AhR expression was also observed in Paneth cells of the small intestine and the cecum in humans (Fig. S5). Notably, β-catenin expression was abnormally high in epithelial cells of the ileum (Fig. 2A), colon (Fig. 2B), and cecum (Fig. 2C) in AhR−/− mice, suggesting that the intestines of AhR−/− mice may be in a “cancer-prone” or “precancerous” state (23). In particular, these elevated levels of β-catenin were observed in the nuclei of Paneth cells compared with the corresponding regions in wild-type mice (Fig. 2A).
Fig. 2.
Abnormal β-catenin accumulation in the intestines of AhR−/− mice. (A) H&E staining and immunohistochemical staining of mouse small intestines. Paneth cells were observed at the bottom of the crypts in the small intestine in both genotypes. Expression of AhR, β-catenin, and c-myc are shown. Nuclear accumulation of β-catenin in Paneth cells of the small intestine and cecum is noted by red arrowheads. Immunohistochemical staining of β-catenin in the colons (B) or cecum (C) of AhR+/+ or AhR−/− mice. (C) Levels of β-catenin, AhR and α-actin in the cecum were detected by Western blotting. The amount of β-catenin was quantified using the ImageJ software (NIH). (P < 0.05; AhR+/+ versus AhR−/− group). (D) RT-PCR was performed to detect mRNA levels for β-catenin, c-myc (P < 0.05; AhR+/+ versus AhR−/− group), and GAPDH in the cecal epithelium of AhR+/+ or AhR−/− mice. Data are representative of 3 independent experiments.
Using Western blotting (Fig. 2C), we confirmed that AhR−/− mice had significantly higher levels of β-catenin in the cecum than wild-type mice (P < 0.05), whereas β-catenin mRNA expression levels were unchanged (Fig. 2D), suggesting that the stabilization, but not enhanced synthesis of the β-catenin protein in the AhR−/− intestine leads to β-catenin accumulation. Consistent with the abnormal accumulation of β-catenin, expression of the downstream target, c-myc, showed ≈2-fold induction (Fig. 2 A and D).
Ligand-Dependent Degradation of β-Catenin.
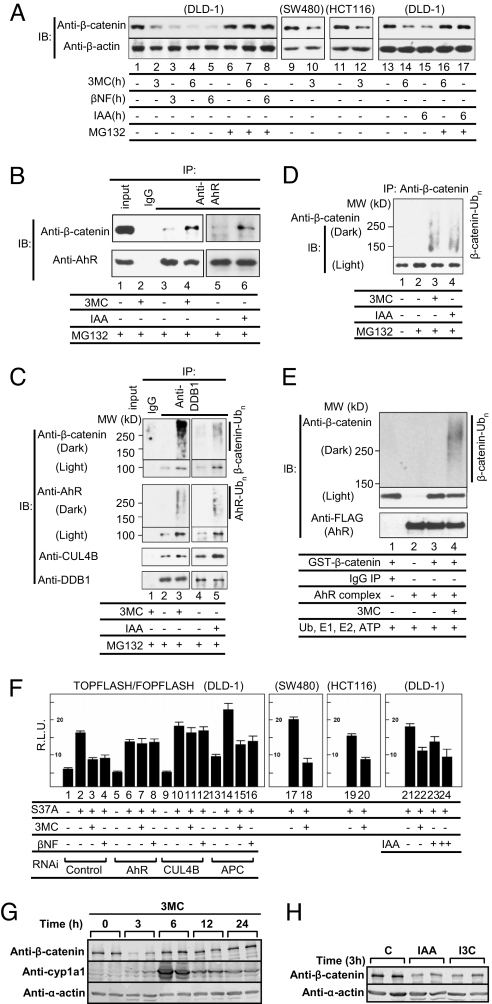
Next, we examined whether the AhR E3 ubiquitin ligase participates in the degradation of β-catenin (Fig. 3) as reported (6) for the degradation of ER and AR. On activation of AhR by exogenous ligands, 3MC or β-naphthoflavone (βNF), endogenous β-catenin protein levels markedly decreased in DLD-1 cells derived from a colon cancer and in other colon cancer-derived cells, SW480 and HCT116 (Fig. 3A). These results clearly show that β-catenin is degraded in an AhR ligand-dependent manner even in colon cancer-derived cells harboring mutations (24) in APC or β-catenin that stabilize β-catenin protein against APC-dependent degradation. These findings suggest that AhR participates in a previously undescribed mechanism of β-catenin degradation that is independent of the APC pathway. Also, after the addition of IAA, which is produced in the intestine from Trp by intestinal microbes (15), and was detected in the cecal contents by HPLC (Fig. S6H), AhR-dependent degradation of β-catenin was also observed (Fig. 3A; Fig. S6A). Degradation of β-catenin induced by xenobiotics or natural AhR ligands was abrogated in the presence of either the proteasome inhibitor MG132 (Fig. 3A) or AhR siRNA (Fig. S6A). We observed that the AhR ligands promoted selective degradation of β-catenin in the soluble fractions, but not in the membrane fraction of cells (Fig. S6B), suggesting that β-catenin involved in the Wnt signaling pathway is selectively degraded. Recognition of endogenous β-catenin by AhR was clearly ligand-dependent, as shown by coimmunoprecipitation assays (Fig. 3B). Also, AhR ligand-dependent assembly of the Cullin (CUL)4BAhR E3 ligase complex with β-catenin (Fig. 3C) was detected by immunoprecipitation assays using an antibody to DDB1 (6), a component of the E3 ubiquitin ligase complex of AhR, together with ligand-induced polyubiquitylation of β-catenin (Fig. 3 C and D) and self-ubiquitylation of AhR (Fig. 3C). AhR-mediated degradation of β-catenin was reconstituted in an in vitro ubiquitylation assay. In this assay, immunopurified CUL4BAhR complexes showed, as expected, E3 ubiquitin ligase activity toward ER (Fig. S6C) and purified GST-β-catenin (Fig. 3E; Fig. S6D). In both these cases, the E3 ubiquitin ligase activity was increased by addition of the ligand, 3MC (Fig. 3E; Fig. S6C). These data strongly suggest that the ligand-dependent E3 ubiquitin ligase activity of AhR participates in β-catenin degradation, and is consistent with the repression of the transcriptional activity of endogenous β-catenin by 3MC (Fig. S6E).
Fig. 3.
Novel AhR ligand-dependent ubiquitylation and proteasomal degradation of β-catenin. (A) Activated AhR promotes proteasomal degradation of β-catenin. Cells were incubated as indicated with 3MC (1 μΜ), βNF (1 μΜ), or IAA (100 μM) in the presence or absence of the proteasome inhibitor MG132 (10 μΜ) for 3 or 6 h. Cell lysates were subjected to Western blotting with antibodies indicated. (B) Ligand-dependent recognition of β-catenin by AhR. DLD-1 cells were incubated with 3MC or IAA and MG132 for 2 h. Then, the extracts were prepared and immunoprecipitated. (C) Ligand-dependent complex assembly of CUL4BAhR E3 ligase with β-catenin. DLD-1 cells were incubated with 3MC or IAA and MG132 for 2 h, after which the cell extracts were prepared and immunoprecipitated with an anti-DDB1 antibody to detect CUL4BAhR complexes with β-catenin. Western blottings were subjected to a long exposure (Dark) to detect polyubiquitylated forms of the proteins. (D) AhR ligand-induced ubiquitylation of β-catenin. DLD-1 cells were incubated with the indicated ligands and MG-132 for 6 h. (E) The AhR complex directly ubiquitylates β-catenin in vitro. The FLAG-HA-AhR-associated immunocomplex in the presence of CUL4BAhR components was mixed with recombinant GST-β-catenin (Fig. S6D) and His-ubiquitin, and an in vitro ubiquitylation assay was performed. (F) CUL4BAhR components are essential for AhR ligand-dependent repression of hyperactive β-catenin (S37A) transactivation. Cells were incubated as indicated with 3MC (1 μΜ), βNF (1 μΜ), or IAA (+, 10 μM; ++, 100 μM). All values are means ± SD for at least 3 independent experiments. (G) AhR ligand-dependent β-catenin degradation in vivo. AhR+/+ mice received a single i.p. injection of 3MC (4 mg/kg). The levels of proteins in the cecal epithelium were determined. (H) AhR+/+ mice received a single i.p. injection of IAA or I3C (25 mg/kg).
To substantiate AhR-dependent degradation of β-catenin in terms of its transcriptional activity and its relationship with the canonical APC-dependent degradation system, we performed reporter assays with TOPFLASH/FOPFLASH mediated by a hyperactive β-catenin (S37A) mutant (Fig. 3F) (25). The reporter activity was enhanced by the addition of β-catenin, and the enhanced reporter expression was repressed by the AhR ligands, 3MC, βNF, and IAA (P < 0.05). Repression of the transcriptional activity of β-catenin by AhR ligands was reversed by AhR or CUL4B siRNA, but not by APC siRNA, confirming that AhR is involved in a previously undescribed ligand-dependent mechanism of proteasomal degradation of β-catenin that is distinct from the canonical APC-dependent pathway (Fig. 3F; Fig. S6F).
We were interested to investigate whether β-catenin protein is reduced in vivo in the intestines of mice after AhR ligand treatment. AhR ligand-dependent degradation of the β-catenin protein was clearly observed in vivo in the intestines of mice with a peak at 3 h after i.p. injection of 3MC, whereas cyp1a1 expression was markedly enhanced as expected (Fig. 3G). This transient degradation of β-catenin is likely due to the rapid down-regulation of AhR after ligand activation (6). Also, this in vivo degradation of β-catenin by 3MC was AhR-dependent, because accumulated β-catenin levels in the cecal epithelia of AhR−/− mice were not altered by 3MC treatment (Fig. S6G). Also, in vivo degradation of β-catenin was observed after i.p. injection of the natural AhR ligands, IAA and I3C (Fig. 3H). HPLC analysis of cecal materials demonstrated that the production of natural AhR ligands [IAA (≈1.2 μM), TA (tryptamin) (≈7.2 μM), and indole (≈43 μM)] depended on the presence of intestinal microbes (Fig. S6H), and the concentrations of these ligands were in a range that effectively activates AhR. During 3MC treatment, β-catenin mRNA levels remained unchanged with a slight, but reproducible decrease in c-myc mRNA expression, whereas cyp1a1 mRNA levels were markedly enhanced (Fig. S6I). These in vivo observations are highly consistent with the in vitro experiments, and provide a basis for possible chemoprevention against intestinal carcinogenesis by using natural AhR ligands.
Cooperative Function Between Apc and AhR Pathways.
The tumor suppressor APC gene was originally discovered as a gene responsible for a hereditary cancer syndrome termed familial adenomatous polyposis (FAP) (26, 27). APC mutations are also found in most sporadic colorectal cancers (28) with an abnormal accumulation of β-catenin. The murine model of FAP, ApcMin/+ (multiple intestinal neoplasia/+), carries an Apc mutation (29). However, in contrast to FAP patients who develop tumors in the colon (28), these mice develop numerous adenomatous polyps mostly in the small intestine, although the reasons for this difference remain unknown.
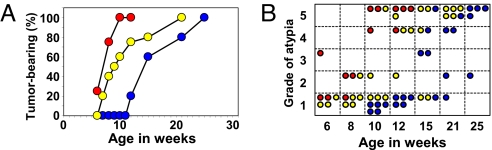
To investigate a functional association between the Apc- and AhR-mediated pathways of β-catenin degradation with regard to intestinal tumor development, we generated mice with compound mutations in both the Apc and AhR genes with the same genetic background. We observed no effect of AhR mutation on the expression of Apc, and vice versa (Fig. S7A). The tumor incidence in compound ApcMin/+·AhR-disrupted mutant mice was compared with that of single gene mutant ApcMin/+ mice. In the cecum (Fig. 4A), ApcMin/+ mice showed a tumor incidence of ≈50% of the total at 14 weeks of age that reached 100% at 25 weeks of age, whereas no tumors were found in AhR+/− mice (Fig. 1B). Remarkably, the compound ApcMin/+·AhR+/− mutant mice had a tumor incidence of 50% at 9–10 weeks of age, and were much more susceptible to cecal tumorigenesis than ApcMin/+ mice, which supports a cooperative tumor suppression function between the 2 genes. Compound ApcMin/+·AhR−/− mutant mice displayed this tendency more prominently, although in limited numbers because of difficulty in breeding. A similarly accelerated carcinogenesis in the small intestine at 7 and 8 weeks was observed in ApcMin/+·AhR+/− mice (Fig. 5D) compared with ApcMin/+ mice (Fig. 5B) (P < 0.001). In the compound mutant mice, the grade of atypia of cecal tumors progressed with age in a cooperative manner, reflecting a cooperative interaction between the AhR and Apc pathways (Fig. 4B).
Fig. 4.
Functional cooperation between Apc and AhR with regard to cecal tumor incidence. Macroscopic cecal tumor incidence by age in weeks (A) and summary of histological grades of atypia (B) that developed in ApcMin/+·AhR+/+ (blue circles), ApcMin/+·AhR+/− (yellow circles), and ApcMin/+·AhR−/− (red circles) mice. Four to 5 mice were used in each group.
Fig. 5.
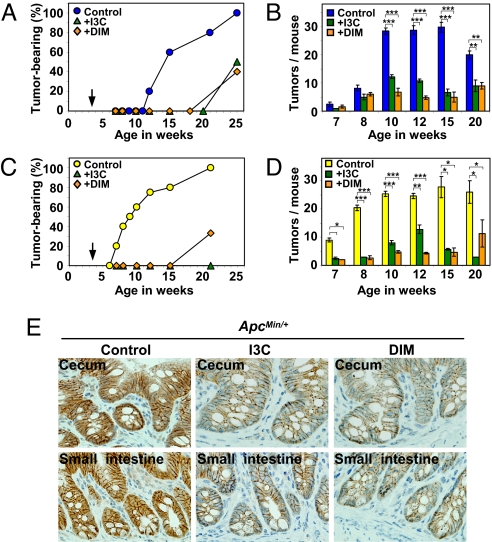
Natural AhR ligands suppress intestinal carcinogenesis. Four to 5 mice were used in each group. Cecal carcinogenesis in the ApcMin/+ (A) and ApcMin/+·AhR+/− (C) mice. Tumor development in mice fed a control diet (blue circles in A and yellow circles in C), 0.1% I3C-containing (green triangles) or 0.01% DIM-containing (beige diamondes) diet just after weaning of 3–4 weeks of age as noted by the arrows. Number of small intestinal polyps in ApcMin/+ (B, blue squares) or ApcMin/+·AhR+/− (D, yellow squares) mice fed a control diet. Number of polyps in the small intestines of mice fed an I3C-containing (green squares) or DIM-containing (beige squares) diet. Data are presented as means ± SD. *, P < 0.01; **, P < 0.001; ***, P < 0.0001. (E) Representative profile of immunohistochemical staining with an antibody against β-catenin in the intestines from 15-week-old ApcMin/+ mice fed a control or ligand-containing diet.
To determine how compound ApcMin/+·AhR-disrupted mutant mice were more susceptible to cecal tumorigenesis than ApcMin/+ mice, β-catenin levels were monitored in the cecum by Western blotting (Fig. S7B) and immunohistochemistry (Fig. S7C) at 6 to 8 weeks of age, when a morphologically normal epithelium was observed (Fig. 4B). And we found elevated levels of β-catenin in the cecum of both ApcMin/+·AhR−/− and ApcMin/+·AhR+/− mice compared with ApcMin/+·AhR+/+ mice, suggesting an association between the levels of β-catenin and tumor susceptibility. Expression levels of the β-catenin/TCF4 target genes, c-myc and cyclin D1, were concomitantly enhanced in ApcMin/+·AhR-disrupted mice, suggesting that AhR-mediated β-catenin degradation has a suppressive role in intestinal carcinogenesis in parallel to the Apc system.
Tumor Suppression by AhR Natural Ligands.
As described in Fig. 3, IAA and I3C accelerated β-catenin degradation in the intestine. We were interested to study whether natural AhR ligands actually suppress carcinogenesis in the cecum or small intestine in ApcMin/+ mice (Fig. 5). The chemoprevention (30) study was designed so that ApcMin/+ or ApcMin/+·AhR+/− mice were fed natural AhR ligand-containing diets, such as I3C (31) and DIM (32), immediately after weaning at 3–4 weeks of age. When fed the control diet, ApcMin/+ mice started to develop small intestinal polyps at 7 weeks of age with the number of tumors containing polyps plateauing (≈30 tumors per mouse) at ≈10 to 15 weeks (Fig. 5B), whereas the cecal tumor incidence was as described (Figs. 4A and 5A). However, when fed an I3C (0.1%)- or DIM (0.01%)-containing diet, ApcMin/+ mice showed a cecal tumor incidence of ≈50% of the total at 25 weeks of age (Fig. 5A) and a markedly reduced number of tumors in the small intestine (Fig. 5B). Similar chemopreventive effects were also clearly observed with the compound ApcMin/+·AhR+/− mutant mice (Fig. 5 C and D). However, no suppressive effect was observed in AhR−/− mice (Fig. S7D), suggesting that AhR ligand-dependent chemoprevention requires the presence of AhR.
Using immunohistochemical analysis, we showed a marked reduction of β-catenin except for the molecules associated with adherence junctions in the intestines of ApcMin/+ (Fig. 5E; Fig. S7F) and ApcMin/+·AhR+/− mice (Fig. S7 E and F) fed AhR ligand-containing diets compared with those fed a control diet. These results clearly demonstrate that chemoprevention of intestinal carcinogenesis by AhR ligands in ApcMin/+ and ApcMin/+·AhR+/− mice is due to β-catenin degradation mediated by the natural ligand-activated AhR E3 ubiquitin ligase.
Discussion
In this study, we provide both loss-of-function and gain-of-function data to show that the AhR mediates ligand-dependent degradation of β-catenin, leading to suppression of intestinal carcinogenesis. The AhR-mediated pathway of β-catenin degradation is independent of the canonical APC-mediated pathway, but functions cooperatively with it, because (i) AhR−/− mice develop colonic tumors mostly in the cecum, whereas numerous polyps develop mostly in the small intestine of ApcMin/+ mice; (ii) even in cells containing mutations in APC or β-catenin gene, β-catenin is clearly degraded in an AhR ligand-dependent manner; and (iii) experiments using siRNAs against AhR, its E3 ubiquitin ligase cofactor CUL4B, and APC clearly indicate the independency between the 2 pathways. The cooperative function is strongly confirmed by additional experiments, in which (i) accelerated carcinogenesis was observed in the compound ApcMin/+·AhR-disrupted mutant mice compared with ApcMin/+ mice, and (ii) AhR natural ligands suppress intestinal carcinogenesis in ApcMin/+ mice. These distinct roles are most likely because the AhR- and APC-dependent β-catenin degradation pathways are considered to be in different subcellular compartments (Fig. S8A); ligand-activated AhR translocates to the nucleus where it forms an ubiquitylation complex containing CUL4B (7) and the constitutively nuclear protein Arnt, whereas the APC-dependent pathway functions in the cytoplasm (33–35).
It is noteworthy that AhR−/− mice mainly develop tumors in the cecum, but not in the small intestine, whereas numerous polyps develop mostly in the small intestine of ApcMin/+ mutant mice (29). Our findings that AhR is abundantly expressed in Paneth cells of the small intestine, as well as the cecum near the ileocecal junction, and that abnormal β-catenin accumulation is observed in the intestines of AhR−/− mice, suggest that intestines of AhR−/− mice may be in a cancer-prone or precancerous state (23). Although it is still unknown why AhR−/− mice specifically develop cecal cancers, the host genetic predisposition to these cancers may be potentiated by stimuli from bacteria colonized in the cecum (36). Abnormal β-catenin accumulation, together with microbial interaction or subsequent inflammation, may promote cecal carcinogenesis in AhR−/− mice. In conjunction with the involvement of intestinal microbes, different structural and functional properties of intestinal epithelial cells (34) may also be associated with the specific development of cecal tumor in AhR−/− mice.
We show evidence that natural AhR ligands converted from dietary Trp and glucosinolates in the intestine are as efficient as exogenous AhR ligands in promoting degradation of endogenous β-catenin. These results provide a molecular basis for chemopreventive mechanisms against intestinal carcinogenesis that were observed in ApcMin/+ and ApcMin/+·AhR+/− mice fed diets containing the AhR ligands I3C and DIM. Also, our findings lend credence to previous reports on the chemopreventive effects on colorectal cancers in humans by cruciferous vegetables that contain a high content of glucosinolates (16–18), and suggest that AhR ligands define a potent strategy for dietary chemoprevention of intestinal cancer.
In conclusion, this study shows that AhR has a critical role in suppression of intestinal carcinogenesis by a previously undescribed ligand-dependent mechanism of proteasomal degradation of β-catenin, which functions independently of and cooperatively with the canonical APC-dependent pathway. AhR−/− mice provide a murine model for spontaneously developing tubular adenocarcinomas, which have the most common histologic characteristics of sporadic colorectal cancers in humans. Although the reasons remain to be established, reduced AhR expression was observed in 12 specimen of human cecal cancers and their surrounding tissues (Fig. S4). Together, we conclude that AhR−/− mice are a useful model to study human intestinal cancer, and will help us to investigate the molecular mechanisms of pathogenesis and chemoprevention of intestinal cancer.
Materials and Methods
Animal Experiments.
C57BL/6 wild-type and AhR-deficient (AhR−/−) (4) mice on the C57BL/6 background were obtained from CLEA Japan. ApcMin/+ mice (29) on a C57BL/6 genetic background were purchased from The Jackson Laboratory. Generation of germ-free (GF) mice or compound ApcMin/+·AhR-disrupted mutant mice, carcinogenesis, and chemoprevention studies were performed as described in the SI Materials and Methods. All animal experiments were approved by the Saitama Cancer Center Animal Care and Use Committee.
Biochemical Analyses.
Immunohistochemistry was performed on 4–5 μm sequential paraffin sections using the antibodies described. Total RNA was extracted from the intestines of AhR+/+ or AhR−/− mice using an Isogen kit (Nippon Gene), and RT-PCR was performed using TaKaRa RNA PCR kits (Takara Shuzo). Cell culture and transfection assays were performed using standard methods. Protein stability analysis and in vitro ubiquitylation assay were performed as previously reported (6). Sequences of the siRNAs used in this study and HPLC analysis are described in SI Materials and Methods.
Statistical Analyses.
Differences in survival in the mouse genotypes were analyzed using the Kaplan-Meyer method, and statistical analyses were performed with the log-rank test. We analyzed numeric data for statistical significance using the Student's t test. We considered P < 0.05 as significant.
Supplementary Material
Acknowledgments.
We thank Drs. T. Omura and M. Suganuma for valuable comments, and Ms. S. Nakabayashi for technical assistance. This work was supported in part by the Solution Oriented Research for Science and Technology Agency (K.K. and Y.F.-K), by grants-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (K.K), and by a grant for Scientific Research from the Ministry of Health, Labor, and Welfare of Japan (to Y.F.-K).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0902132106/DCSupplemental.
References
- 1.Gu YZ, Hogenesch J, Bradfield CA. The PAS superfamily: Sensors of environmental and developmental signals. Annu Rev Pharmacol. 2000;40:519–561. doi: 10.1146/annurev.pharmtox.40.1.519. [DOI] [PubMed] [Google Scholar]
- 2.Fujii-Kuriyama Y, Mimura J. Molecular mechanisms of AhR functions in the regulation of cytochrome P450 genes. Biochem Biophys Res Commun. 2005;338:311–317. doi: 10.1016/j.bbrc.2005.08.162. [DOI] [PubMed] [Google Scholar]
- 3.Nebert DW, Dalton TP. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat Rev Cancer. 2006;6:947–960. doi: 10.1038/nrc2015. [DOI] [PubMed] [Google Scholar]
- 4.Mimura J, et al. Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes Cells. 1997;2:645–654. doi: 10.1046/j.1365-2443.1997.1490345.x. [DOI] [PubMed] [Google Scholar]
- 5.Shimizu Y, et al. Benzopyrene carcinogenicity is lost in mice lacking the aryl hydrocarbon receptor. Proc Natl Acad Sci USA. 2000;97:779–782. doi: 10.1073/pnas.97.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohtake F, et al. Dioxin receptor is a ligand-dependent E3 ubiquitin ligase. Nature. 2007;446:562–566. doi: 10.1038/nature05683. [DOI] [PubMed] [Google Scholar]
- 7.Quintana FJ, et al. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 8.Veldhoen M, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 9.Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci USA. 2008;105:9721–9726. doi: 10.1073/pnas.0804231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikuta T, Kobayashi Y, Kawajiri K. Cell density regulates intracellular localization of aryl hydrocarbon receptor. J Biol Chem. 2004;279:19209–19216. doi: 10.1074/jbc.M310492200. [DOI] [PubMed] [Google Scholar]
- 11.Lee S, et al. Nuclear/cytoplasmic localization of the von Hippel-Lindau tumor suppressor gene product is determined by cell density. Proc Natl Acad Sci USA. 1996;93:1770–1775. doi: 10.1073/pnas.93.5.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang F, White RL, Neufeld KL. Cell density and phosphorylation control the subcellular localization of adenomatous polyposis coli protein. Mol Cell Biol. 2001;21:8143–8156. doi: 10.1128/MCB.21.23.8143-8156.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen LP, Bladfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol. 2008;21:102–116. doi: 10.1021/tx7001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heath-Pagliuso S, et al. Activation of the Ah receptor by tryptophan and tryptophan metabolites. Biochemistry. 1998;37:11508–11515. doi: 10.1021/bi980087p. [DOI] [PubMed] [Google Scholar]
- 15.Bjeldanes LF, et al. Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: Comparisons with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Proc Natl Acad Sci USA. 1991;88:9543–9547. doi: 10.1073/pnas.88.21.9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim YS, Milner JA. Targets for indole-3-carbinol in cancer prevention. J Nut Biochem. 2005;16:65–73. doi: 10.1016/j.jnutbio.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Bonnesen C, Eggleston IM, Hayes JD. Dietary indoles and isothiocyanates that are generated from cruciferous vegetables can both stimulate apoptosis and confer protection against DNA damage in human colon cell lines. Cancer Res. 2001;61:6120–6130. [PubMed] [Google Scholar]
- 18.Potter JD, Steinmetz K. Vegetables, fruit, and phytoestrogens as preventive agents. IARC Sci Publ. 1996;139:61–90. [PubMed] [Google Scholar]
- 19.Fernandez-Salguero PM, Ward JM, Sundberg JP, Gonzalez FJ. Lesions of aryl hydrocarbon receptor-deficient mice. Vet Pathol. 1997;34:605–614. doi: 10.1177/030098589703400609. [DOI] [PubMed] [Google Scholar]
- 20.McMillan BJ, Bradfield CA. The aryl hydrocarbon receptor sans xenobiotics: Endogenous function in genetic model system. Mol Pharmacol. 2007;72:487–498. doi: 10.1124/mol.107.037259. [DOI] [PubMed] [Google Scholar]
- 21.He TC, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 22.van Es JH, et al. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nat Cell Biol. 2005;7:381–386. doi: 10.1038/ncb1240. [DOI] [PubMed] [Google Scholar]
- 23.van de Wetering M, et al. The β-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241–250. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 24.Yang J, et al. Adenomatous polyposis coli (APC) differentially regulates β-catenin phosphorylation and ubiquitination in colon cancer cells. J Biol Chem. 1997;281:17751–17757. doi: 10.1074/jbc.M600831200. [DOI] [PubMed] [Google Scholar]
- 25.Liu C, et al. β-Trcp couples β-catenin phosphorylation-degradation and regulates Xenopus axis formation. Proc Natl Acad Sci USA. 1999;96:6273–6278. doi: 10.1073/pnas.96.11.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kinzler KW, et al. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 27.Nishisho I, et al. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991;253:665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- 28.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 29.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 30.Wattenberg LW. Chemoprevention of cancer. Cancer Res. 1985;45:1–8. [PubMed] [Google Scholar]
- 31.Xu M, et al. Protection by green tea, black tea, and indole-3-carbinol against 2-amino-3-methylimidazo[4,5-f]quinoline-induced DNA adducts and colonic aberrant crypts in the F344 rat. Carcinogenesis. 1996;17:1429–1434. doi: 10.1093/carcin/17.7.1429. [DOI] [PubMed] [Google Scholar]
- 32.Chen I, McDougal A, Wang F, Safe S. Aryl hydrocarbon receptor-mediated antiestrogenic and antitumorigenic activity of diindolylmethane. Carcinogenesis. 1998;19:1631–1639. doi: 10.1093/carcin/19.9.1631. [DOI] [PubMed] [Google Scholar]
- 33.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 34.Reya T, Clevers H. Wnt signaling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 35.Kitagawa M, et al. An F-box protein, FWD1, mediates ubiquitin-dependent proteolysis of β-catenin. EMBO J. 1999;18:2401–2410. doi: 10.1093/emboj/18.9.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maggio-Price L, et al. Helicobacter infection is required for inflammation and colon cancer in Smad3-deficient mice. Cancer Res. 2006;66:828–838. doi: 10.1158/0008-5472.CAN-05-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.