A new paradigm in the way we envision tissue change during carcinogenesis has evolved in recent years. From a clinical standpoint, this paradigm has altered how we view “at-risk” tissue. Rather than focusing on clinical lesions, we often discuss “field” changes involving the expansion of genetically and epigenetically altered cells within a tissue, and not necessarily centered on a clinically identifiable lesion. This change reflects the recognition that genetically altered fields of cells are not always clinically or histologically apparent, yet even when occult, can constitute a significant risk. This shift in perspective has caused a management conundrum. We can use molecular techniques to characterize field changes in an extremely detailed fashion; however, such evaluation depends on identifying areas for its use.
In this issue of the journal, Roblyer et al. (1) describe work with autofluorescence imaging, a field-assessment approach that may be an alternative and potential complement to lesion-focused assessments and may improve our ability to clinically distinguish normal from premalignant and malignant oral tissue in a real-time fashion. Generally, autofluorescence imaging uses higher-energy light to excite specific compounds in tissue (fluorophores) so that they re-emit lower-energy light that makes up the autofluorescence image of the tissue. The excitation light is produced by a filtered arch lamp, an array of light-emitting diodes, or a laser. Effective detection of the autofluorescence image requires blocking the excitation light from reaching the imaging sensor (camera or eye).
Autofluorescence imaging is already accepted by some health authorities as a standard of care for early lung cancer detection, and research devices are nearing clinical utility in many other organ sites, including the cervix, skin, esophagus, bladder, and colon (2–8). Several years ago, a form of qualitative autofluorescence imaging called direct fluorescence visualization (FV) became available for widespread use in the oral cavity with the commercial release of the VELscope® (LEDDental, Inc., White Rock, British Columbia, Canada), which is approved by the U.S. Food and Drug Administration and Health Canada. This simple handheld device uses a blue/violet light (400–460 nm) to illuminate oral tissue. A selective filter in the eyepiece allows the viewer to directly visualize the pale green autofluorescence that is given off by normal tissue (9). Abnormal or suspicious tissue shows decreased levels of normal autofluorescence and appears as a dark brown to black region in comparison with the brighter, green surrounding healthy tissue.
Although early results with autofluorescence in the oral cavity have been promising, published data to date have come mainly from oral specialists working in referral clinics. Roblyer and coworkers describe a potentially important step in the evolution of this approach toward facilitating its transfer to less-experienced clinicians, including those in community settings. Departing from the direct visual assessment of autofluorescence such as with a VELscope®, they coupled autofluorescence with digital imaging and digital processing of the images so as to move from a qualitative to an objective quantitative assessment. Although results are still preliminary, the data are interesting and suggest one way in which this approach could be extended to other clinical niches.
Changes in fluorescence reflect a complex interplay of alterations to fluorophores in tissue and structural changes in tissue morphology. The endogenous fluorophores most relevant to optical screening and diagnosis of precancer and cancer are those that excite in the spectrum from visible violet/blue (400–450 nm) to UV-A (315–400 nm) and have properties that have been spectroscopically correlated with disease progression (10–13). When tissue is illuminated in such a fashion, most of the fluorescence originates from collagen, its cross-links and elastin, which are located in the stroma and basement membrane, and a small fraction originates from the reduced form of NADH and the oxidized form of flavin adenine dinucleotide in epithelial cells. The interaction between the light source and tissue is also affected by characteristic changes of cancer development, such as alterations to epithelial thickness, nuclear morphology (dysplastic nuclei), and vascularization, that affect absorption and scattering of light.
What has autofluorescence told us to date about at-risk oral fields? Our group has been using direct FV to follow patients enrolled in the Oral Cancer Prediction Longitudinal Study in Vancouver, British Columbia. An intriguing early discovery was that this technology identified lesions not apparent under white-light examination yet containing dysplasia and/or cancer when biopsied (14). Equally remarkable, even lesions apparent under conventional white light frequently involved autofluorescence loss extending into the surrounding tissue with no apparent clinical change. In 2006, we showed for the first time that direct FV could be used in the operating room to identify subclinical high-risk fields (15). This study examined 20 consecutive patients undergoing surgical excision, documenting histologic and molecular changes within areas showing loss of autofluorescence in clinically normal tumor margins. All tumors showed autofluorescence loss, which extended beyond the clinically visible tumor boundary in 19 cases. Of note, autofluorescence loss was not uniformly distributed around the clinically apparent lesion, varying in extent and evident from 4 to 25 mm beyond the clinical parameter. It is striking that autofluorescence loss in these clinically occult margins was associated with both histologic change and the presence of high-risk molecular clones even in the majority of margins with low-grade or no dysplasia.
Will the use of FV to guide surgery reduce tumor recurrence? The literature documents a high frequency of recurrence at the primary site (10–30% of cases) of oral cancer (16–18). We have begun a longitudinal study to explore the effect of FV in defining the surgical margin on outcome of oral cancer surgery. Between 2004 and 2008, 60 patients with a ≤4 cm oral cancer entered the study. Each case was treated with surgical excision alone and was followed for at least 12 months. Thirty-eight patients had FV-guided surgery, with the surgical margin placed at 10 mm beyond the perimeter of autofluorescence loss. The remaining patients (control group) had the surgical margin placed at 10 mm beyond the tumor edge defined by standard white-light examination. Use of the FV-guided approach depended on the availability of the pathologist to attend the surgical procedure.
The FV-guided and control groups had similar distributions with respect to gender, smoking habits, anatomic lesion site, and follow-up time. To date, 7 of the 60 cases (12%) have developed a recurrence of severe dysplasia or worse neoplasia at the treated site, all in the control group (25% versus 0%, P = 0.002). Analyses of histologic and molecular alterations in tumor margins are ongoing (Fig. 1). These data suggest the utility of autofluorescence changes within this clinical setting and provide pilot support for planning a larger clinical trial aimed at establishing whether FV-guided surgery has value.
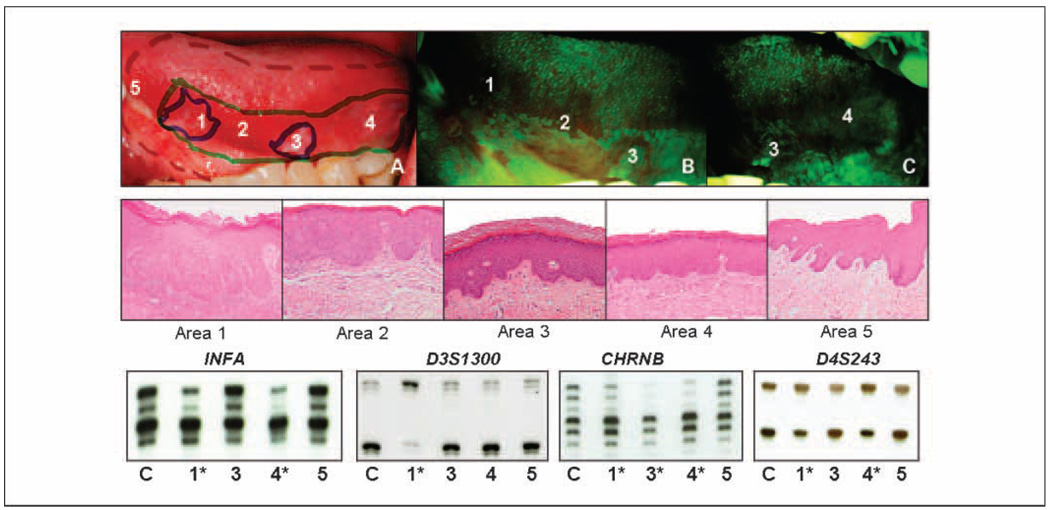
Fig. 1.
Mapping histologic and molecular change within an autofluorescence-defined, at-risk field. This figure illustrates the real-time mapping of a surgical field around a carcinoma in situ with subsequent integration of histologic and molecular information. High-grade histology and abnormal molecular clones are apparent in regions of autofluorescence loss lying outside of the clinically apparent tumor. A, delineation of three boundaries in the operating room (top): clinically apparent tumor (solid blue marker), autofluorescence loss (solid green marker), and surgical boundary (dotted red marker); the field in A is shown under white light, and in B and C under autofluorescence imaging. Areas 1 and 3 are clinically apparent and show autofluorescence loss, areas 2 and 4 are not clinically apparent but have autofluorescence loss, area 5 represents the anterior surgical margin, which is beyond the clinical tumor and boundaries of autofluorescence loss. Photomicrographs of areas indicated in A (middle, H&E staining with original magnification, ×100): area 1, carcinoma in situ; area 2, severe dysplasia; area 3, moderate epithelial dysplasia; area 4, mild-to-moderate epithelial dysplasia; area 5, no dysplasia. Loss of heterozygosity analysis at IFNA, D3S1300, CHRNB, and D4S243 (bottom). Numbers under each set of alleles correspond to areas in A (top; samples from area 2 were not available for analysis). *, presence of loss of heterozygosity in a sample.
We are also using FV to monitor the potential re-emergence of regions of autofluorescence loss at treated sites in the cases accrued to the longitudinal study and are currently completing an interim assessment of these monitoring results. Autofluorescence loss persists in some cases, increasing in size and intensity over time and giving rise to a clinical lesion containing dysplasia or cancer (examples in Fig. 2 and ref. 19). New lesions showing autofluorescence loss at some distance from the treated site have also been observed. Molecular analysis is being used to determine whether this is recurrent disease or a second primary (18, 20–22).
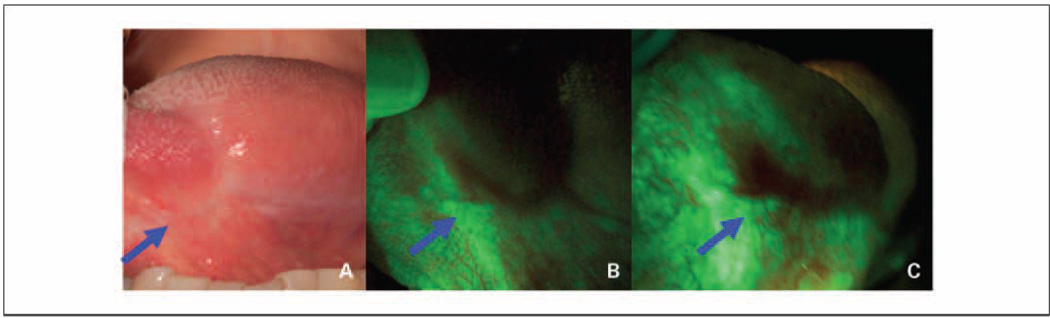
Fig. 2.
A 63-year-old female former smoker was examined for 6 mo (A and B) and 12 mo (C) after a surgical excision of severe epithelial dysplasia on the left lateral tongue. A, white-light image of a well-healed scar on the left lateral tongue (arrow); B, the anterior aspect of this scar (arrow) under fluorescent visualization showing a dark brown region of autofluorescence loss. C, at 12 mo, the same area (arrow) showed a persistent autofluorescence loss of increased size; at 20 mo after initial treatment (data not shown), a biopsy from the region of loss (arrow) showed carcinoma in situ.
What do the autofluorescence modifications of Roblyer et al. contribute to this field? Their study represents the first use of multiple fluorescence excitation and reflectance imaging to detect and delineate oral cancer and premalignant lesions. It reflects a growing trend toward multimodal and quantitative imaging for early cancer detection using color ratio imaging (6, 23, 24). The authors used this approach to select an optimum wavelength that may more precisely differentiate between oral cancers or premalignant lesions and confounders. The ability to exclude confounders in an objective fashion would be an important step towards making autofluorescence available to inexperienced users in community settings, in which potential confounders are more frequent than are oral malignancy and premalignancy.
Roblyer et al. used a wide-field multispectral digital microscope, which essentially is a modified dissecting microscope, to illuminate tissue with 365, 380, 405, and 450 nm excitation. The resultant autofluorescence images were recorded with a high-resolution charge-coupled device color camera (red, green, and blue channels). Sixty-seven subjects (56 patients and 11 normal volunteers) were evaluated with 276 measurements from 159 regions of interest. This data set was divided into a training and a test set; the training set was used to select the best illumination wavelength and color combination for discriminating normal from abnormal areas. A red channel-to-green channel ratio for 405 nm excitation provided the best discrimination between neoplastic (including dysplasia, carcinoma in situ, and cancer) and nonneoplastic areas (including inflammation and hyperplasia) in the training set, and its performance was validated in the test set. The classification algorithm developed in this way had 96% sensitivity and 96% specificity in the training set and 100% sensitivity and 91% specificity in the test set.
In a further effort to make the technology more accessible to primary care providers, the authors have produced a probability map that overlaps and highlights abnormal areas within a white-light image of the oral cavity. This probability map is based on the red-to-green ratio in the classifier and is used to indicate areas of high-likelihood of abnormality versus low-likelihood of abnormality. Disease-probability maps were compared with histologic diagnosis of tissue resected from the field of view. The authors noted that there was a qualitative agreement between the presence of dysplasia and cancer as indicated by the map and the corresponding histology.
Will the modifications proposed by Roblyer and coworkers facilitate the uptake, further evaluation, and quality of data obtained with autofluorescence in the community setting, as suggested by the authors? It is too soon to judge. However, these are important first steps in the evolution of this approach toward an improved ability to address clinical needs.
As mentioned by Roblyer et al., their present data represent a strong proof-of-principle for the approach. However, the range of lesions being evaluated in any clinical setting is notoriously heterogeneous, and only a fraction of these abnormalities were available to the researchers in the tertiary care setting. One of the most critical future steps will be the expansion of this analysis to a larger number of cases, carefully choosing a spectrum of lesions that reflects the wide range of clinical abnormalities encountered for each clinical use that is being targeted, for example, in screening versus diagnostic settings.
For screening, this spectrum should include changes that are common to the general population and that are known to confound autofluorescence assessment, such as inflammation (e.g., lichen planus), infection, and chronic trauma. The current assessment was limited to only a few such examples.
For diagnostic settings, the further characterization of dysplastic lesions and hyperplastic lesions detected with FV loss will be equally crucial. Our experience within the ongoing longitudinal study has been that nearly all high-grade dysplasias (severe dysplasia/carcinoma in situ) show FV loss. This finding needs to be confirmed more broadly in a multicenter study. In contrast, only a portion of low-grade (mild or moderate) dysplasia shows FV loss. Autofluorescence determinations need to be combined with molecular analyses to determine whether high-risk molecular clones are more prevalent in low-grade lesions showing autofluorescence loss (versus not; Fig. 1). These low-grade lesions also need to be followed over time to determine the likelihood of outcome for different combinations of molecular and autofluorescence changes. Last, the key to our understanding of autofluorescence-detected fields is the collection of concise and detailed descriptions of both lesions (including pictures) and test subjects (demographics, risk factors, and medical history). These data will allow for a later pooling of results within a meta-analysis framework. Critical information for premalignant disease includes the degree of dysplasia and whether the dysplasia occurs in patients with former oral cancers, as part of a cancer, or as a primary dysplasia (i.e., no history of oral cancer or dysplasia). Risk of progression is known to change with each of these aspects.
The Roblyer et al. development of probability maps is also intriguing. Further advances in this process will most likely involve a combination of technology change and the continuing integration of the data described in the previous paragraph. For example, although the autofluorescence images in the article are promising (1), they will need to become more robust to the effects of noise (random fluctuations of the data in the image), particularly in the darker areas, where small changes in the denominator (green channel) could result in large fluctuations of the ratio used to calculate the disease-probability values. To go to real-time probability mapping, software improvements will be needed for determining the red-to-green ratios and for displaying the color code (the range of map colors indicating low-to-high probability of neoplasia) as the images are being acquired. Nevertheless, this approach has promise for the quantitative establishment of the presence and boundaries of a lesion.
In summary, autofluorescence and other such imaging processes have the potential to make a significant impact on standards of care, influencing not only surgical margin assessment but also the evaluation of tissue alterations during chemoprevention, which relies on establishing lesion boundaries. Autofluorescence imaging has already begun to shed new light on tissue changes during cancer development (25). Other visualization technologies (e.g., in vivo confocal microscopy, molecular targeted optical contrast agents alone and in combination) are poised to enter this field and will be integrated in the future with molecular findings to further advance our understanding of disease processes. How this is to be done will be complex; for example, the field defined by existing optical agents such as toluidine blue (26) does not overlap completely with that defined by direct FV, and neither completely corresponds with a molecularly altered field. Only long-term follow-up of patients can unravel the clinical significance of these different views of the underlying neoplastic process.
A major challenge for any new technology, including that presented by Roblyer et al., is to develop a mechanism by which we can begin to collect these very diverse sets of information into a framework that will facilitate its use to address important clinical questions.
Acknowledgments
Grant support: National Institute of Dental and Craniofacial Research (R01 DE13124 and R01 DE17013) and the Canadian Institutes of Health Research (MOP-77663). Clinician Scientist Award from the Canadian Institutes of Health Research and a Scholar Award from the Michael Smith Foundation for Health Research (C.F. Poh).
Footnotes
Disclosure of Potential Conflicts of Interest
Dr. Calum MacAulay serves on the scientific advisory board of Remicalm LLC.
References
- 1.Roblyer D, Kurachi C, Stepanek V, et al. Objective detection and delineation of oral neoplasia using autofluorescence imaging. Cancer Prev Res (Phila Pa) 2009;2:423–431. doi: 10.1158/1940-6207.CAPR-08-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D'Hallewin MA, Bezdetnaya L, Guillemin F. Fluorescence detection of bladder cancer: a review. Eur Urol. 2002;42:417–425. doi: 10.1016/s0302-2838(02)00402-5. [DOI] [PubMed] [Google Scholar]
- 3.von Holstein CS, Nilsson AM, Andersson-Engels S, Willen R, Walther B, Svanberg K. Detection of adenocarcinoma in Barrett's oesophagus by means of laser induced fluorescence. Gut. 1996;39:711–716. doi: 10.1136/gut.39.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeng H, MacAulay C, Lui H, McLean DI. Fluorescence spectroscopy and imaging for skin cancer detection and evaluation. OSA Trends in Optics and Photonomics (Optical Society of America) 2000;38 SubB4. [Google Scholar]
- 5.de Leeuw J, van der Beek N, Neugebauer WD, Bjerring P, Neumann HA. Fluorescence detection and diagnosis of non-melanoma skin cancer at an early stage. Lasers Surg Med. 2009;41:96–103. doi: 10.1002/lsm.20739. [DOI] [PubMed] [Google Scholar]
- 6.Benavides JM, Chang S, Park SY, et al. Multispectral digital colposcopy for in vivo detection of cervical cancer. Opt Express. 2003;11:1223–1236. doi: 10.1364/oe.11.001223. [DOI] [PubMed] [Google Scholar]
- 7.Lam S, Kennedy T, Unger M, et al. Localization of bronchial intraepithelial neoplastic lesions by fluorescence bronchoscopy. Chest. 1998;113:696–702. doi: 10.1378/chest.113.3.696. [DOI] [PubMed] [Google Scholar]
- 8.Ramanujam N, Mitchell MF, Mahadevan A, et al. In vivo diagnosis of cervical intraepithelial neoplasia using 337-nm-excited laser-induced fluorescence. Proc Natl Acad Sci U S A. 1994;91:10193–10197. doi: 10.1073/pnas.91.21.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lane PM, Gilhuly T, Whitehead P, et al. Simple device for the direct visualization of oral-cavity tissue fluorescence. J Biomed Opt. 2006;11:24006. doi: 10.1117/1.2193157. [DOI] [PubMed] [Google Scholar]
- 10.Richards-Kortum R, Sevick-Muraca E. Quantitative optical spectroscopy for tissue diagnosis. Annu Rev Phys Chem. 1996;47:555–606. doi: 10.1146/annurev.physchem.47.1.555. [DOI] [PubMed] [Google Scholar]
- 11.Pavlova I, Sokolov K, Drezek R, Malpica A, Follen M, Richards-Kortum R. Microanatomical and biochemical origins of normal and precancerous cervical autofluorescence using laser-scanning fluorescence confocal microscopy. Photochem Photobiol. 2003;77:550–555. doi: 10.1562/0031-8655(2003)077<0550:maboon>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Drezek R, Brookner C, Pavlova I, et al. Autofluorescence microscopy of fresh cervical-tissue sections reveals alterations in tissue biochemistry with dysplasia. Photochem Photobiol. 2001;73:636–641. doi: 10.1562/0031-8655(2001)073<0636:AMOFCT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 13.Drezek R, Sokolov K, Utzinger U, et al. Understanding the contributions of NADH and collagen to cervical tissue fluorescence spectra: modeling, measurements, and implications. J Biomed Opt. 2001;6:385–396. doi: 10.1117/1.1413209. [DOI] [PubMed] [Google Scholar]
- 14.Poh CF, Ng SP, Williams PM, et al. Direct fluorescence visualization of clinically occult high-risk oral premalignant disease using a simple hand-held device. Head Neck. 2007;29:71–76. doi: 10.1002/hed.20468. [DOI] [PubMed] [Google Scholar]
- 15.Poh CF, Zhang L, Anderson DW, et al. Fluorescence visualization detection of field alterations in tumor margins of oral cancer patients. Clin Cancer Res. 2006;12:6716–6722. doi: 10.1158/1078-0432.CCR-06-1317. [DOI] [PubMed] [Google Scholar]
- 16.Leemans CR, Tiwari R, Nauta JJ, van der Waal I, Snow GB. Recurrence at the primary site in head and neck cancer and the significance of neck lymph node metastases as a prognostic factor. Cancer. 1994;73:187–190. doi: 10.1002/1097-0142(19940101)73:1<187::aid-cncr2820730132>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 17.Brennan JA, Mao L, Hruban RH, et al. Molecular assessment of histopathological staging in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332:429–435. doi: 10.1056/NEJM199502163320704. [DOI] [PubMed] [Google Scholar]
- 18.Tabor MP, Brakenhoff RH, Ruijter-Schippers HJ, Kummer JA, Leemans CR, Braakhuis BJ. Genetically altered fields as origin of locally recurrent head and neck cancer: a retrospective study. Clin Cancer Res. 2004;10:3607–3613. doi: 10.1158/1078-0432.CCR-03-0632. [DOI] [PubMed] [Google Scholar]
- 19.Poh CF, MacAulay CE, Lorande DM, Williams PM, Zhang L, Rosin MP. Squamous cell carcinoma and precursor lesions: diagnosis and screening in a technical era. Periodontology. 2009 doi: 10.1111/j.1600-0757.2011.00386.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bedi GC, Westra WH, Gabrielson E, Koch W, Sidransky D. Multiple head and neck tumors: evidence for a common clonal origin. Cancer Res. 1996;56:2484–2487. [PubMed] [Google Scholar]
- 21.Tabor MP, Brakenhoff RH, Ruijter-Schippers HJ, et al. Multiple head and neck tumors frequently originate from a single preneoplastic lesion. Am J Pathol. 2002;161:1051–1060. doi: 10.1016/S0002-9440(10)64266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jang SJ, Chiba I, Hirai A, Hong WK, Mao L. Multiple oral squamous epithelial lesions: are they genetically related? Oncogene. 2001;20:2235–2242. doi: 10.1038/sj.onc.1204311. [DOI] [PubMed] [Google Scholar]
- 23.Park SY, Follen M, Milbourne A, et al. Automated image analysis of digital colposcopy for the detection of cervical neoplasia. J Biomed Opt. 2008;13 doi: 10.1117/1.2830654. 014029. [DOI] [PubMed] [Google Scholar]
- 24.Zeng H, McWilliams A, Lam S. Opticl spectroscopy and imaging for early lung cancer detection: a review. Photodiagnosis Photodyn Ther. 2004;1:111–122. doi: 10.1016/S1572-1000(04)00042-0. [DOI] [PubMed] [Google Scholar]
- 25.Westra WH, Sidransky D. Fluorescence visualization in oral neoplasia: shedding light on an old problem. Clin Cancer Res. 2006;12:6594–6597. doi: 10.1158/1078-0432.CCR-06-2253. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, Williams M, Poh CF, et al. Toluidine blue staining identifies high-risk primary oral premalignant lesions with poor outcome. Cancer Res. 2005;65:8017–8021. doi: 10.1158/0008-5472.CAN-04-3153. [DOI] [PubMed] [Google Scholar]