Abstract
N-acetyl 5-aminosalicylic acid (5-AcASA) that was intracellularly formed from 5-aminosalicylic acid (5-ASA) at 200 μM was discharged 5.3, 7.1, and 8.1-fold higher into the apical site than into the basolateral site during 1, 2, and 4-hour incubations, respectively, in Caco-2 cells grown in Transwells. The addition of flavonols (100 μM) such as fisetin and quercetin with 5-ASA remarkably decreased the apically directed efflux of 5-AcASA. When 5-ASA (200 μM) was added to Caco-2 cells grown in tissue culture dishes, the formation of 5-AcASA decreased, and, in addition, the formed 5-AcASA was found to be accumulated within the cells in the presence of such flavonols. Thus, the decrease in 5-AcASA efflux by such flavonols was attributed not only to the inhibition of N-acetyl-conjugation of 5-ASA but to the predominant cellular accumulation of 5-AcASA. Various flavonoids also had both of the effects with potencies that depend on their specific structures. The essential structure of flavonoids was an absence of a hydroxyl substitution at the C5 position on the A-ring of flavone structure for the inhibitory effect on the N-acetyl-conjugation of 5-ASA, and a presence of hydroxyl substitutions at the C3′ or C4′ position on the B-ring of flavone structure for the promoting effect on the cellular accumulation of 5-AcASA. Both the decrease in 5-AcASA apical efflux and the increase in 5-AcASA cellular accumulation were also caused by MK571 and indomethacin, inhibitors of MRPs, but not by quinidine, cyclosporin A, P-glycoprotein inhibitors, and mitoxantrone, a BCRP substrate. These results suggest that certain flavonoids suppress the apical efflux of 5-AcASA possibly by inhibiting MRPs pumps located on apical membranes in Caco-2 cells.
1. Introduction
Sulfasalazine used in the therapy of inflammatory bowel diseases, such as ulcerative colitis and Crohn's disease [1, 2]. Ingested sulfasalazine passes to the colon without being absorbed in intestine and is split into 5-aminosalicylic acid (5-ASA) and sulfapyridine by colonic bacteria [1, 2]. Most of 5-ASA is metabolized by N-acetyl-conjugation in the form of N-acetyl 5-aminosalicylic acid (5-AcASA) in the colonic epithelia, while sulfapyridine is quickly absorbed from the colon and metabolized in the liver [3–5]. It has been proposed that 5-ASA, the active moiety of sulfasalazine, exerts an antiinflammatory activity by inhibiting prostaglandin synthesis in colonic mucosa [6, 7]. Some reports have shown that 5-AcASA has a potency as an inhibitor of prostaglandin synthesis comparable to that of 5-ASA [7], and therapeutically active when administered by enema to patients with ulcerative colitis [8]. However, 5-AcASA formed in colonic epithelia is immediately secreted into mucosal lumen and excreted in feces [9–11]. Thus, 5-AcASA is considered the portion that has already exerted therapeutical action within the bowel tissue [1–3, 9–11]. Zhou et al. reported that 5-AcASA was exclusively transported from the basolateral to the apical direction using human colon-derived Caco-2 cells [11]. However, the mechanism underlying the cellular transport of 5-AcASA has not extensively elucidated. It is well known that flavonoids (Figure 1), plant-derived compounds, alter the function of efflux transporters such as P-glycoprotein, that is, present in epithelium cells [12–14]. Recently, several researchers reported the inhibitory interaction of flavonoids with multidrug resistance-associated proteins (MRPs) that are responsible for active secretion of pharmacologically relevant drugs [15–20]. In this study, the effect of flavonoids and transporter inhibitors on the cellular efflux of 5-AcASA that was intracellularly formed from 5-ASA was examined in Caco-2 cells. Certain flavonoids and MRPs inhibitors displayed strong potency in decreasing the preferential apical efflux of 5-AcASA and in increasing the cellular accumulation of 5-AcASA in Caco-2 cells.
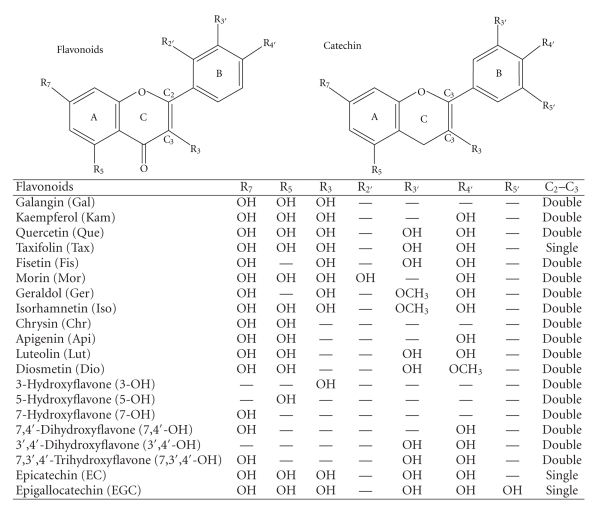
Figure 1.
Structure of flavonoids.
2. Materials and Methods
2.1. Materials
Materials and chemical reagents were purchased from the following companies: Transwells from Corning Costar (Cambridge, MA, USA); tissue culture dishes from Becton Dickinson Com. (Falcon; USA); flavonoids from Funakoshi Co. (Tokyo, Japan); 5-ASA and quinidine from Sigma-Aldrich Com. (Japan); MK571 from Alexis Biochemicals (Lausen, Switzerland); mitoxantrone from LKT Laboratories (MA, USA); indomethacin and other chemicals used from Wako Pure Chemical Co. (Osaka, Japan); and the Develosil RPAQUEOUS C-30-UG-3 column (4.6 I.D. × 150 mm) from Nomura Chemical Co. (Aichi, Japan). Cyclosporin A was purchased from Sigma-Aldrich Com. and Wako Pure Chemical Co. 5-AcASA was synthesized by the reaction of 5-ASA with acetic anhydride, as described by other researchers [21].
2.2. Efflux of 5-AcASA from Caco-2 Cells
Caco-2 cells were purchased from the Riken (no. RCB0988) and used as previously described [22]. The cell line was cultured in Dulbecco's modified Eagle's medium containing 12% fetal calf serum and penicillin-streptomycin-amphotericin B. The suspended cells were seeded on 6-well- polycarbonate Transwell inserts (0.4 μm mean pore size, 4.7 cm2 growth area) at a density of 5 × 104 cells/dish, and then placed in an incubator in an atmosphere of 5% CO2–95% air at 37°C. The Caco-2 cells in the Transwell were grown for 3 weeks in Dulbecco's modified Eagle's medium containing fetal calf serum. The monolayers with transepithelial electric resistance of more than 250 Ω cm2 were used for transport studies. 5-ASA in a stock solution at 50 mM was added to the apical chamber at a final concentration of 200 μM after 10 minutes of the addition of flavonols. After incubation for 2 and 4 hours at 37°C, 50 μL of the medium from both of the chambers was mixed with 50 μL of 0.5 M perchloric acid.
2.3. Cellular Accumulation of 5-AcASA
Caco-2 cell line at passage of 40 was used for the experiments. The suspended cells in Dulbecco's modified Eagle's medium containing 12% fetal calf serum and penicillin-streptomycin-amphotericin B were seeded on 35 mm plastic culture dishes at a density of 5 × 104 cells/dish. After seeding, the cells were cultured in a 37°C incubator under 5% CO2–95% air at 37°C for two weeks until the cells were fully differentiated into confluent enterocyte-like monolayers. Flavonoids, 5-ASA and other chemicals were dissolved in dimethyl sulfoxide and added to the medium at definite concentrations, with the final concentration of dimethyl sulfoxide about 1%. After incubation for 2 hours, the cell monolayers were washed twice with Hanks balanced solution and harvested. The adequate volume of the medium and cell suspensions was treated with the same volume of 0.5 M perchloric acid.
2.4. HPLC Analysis
Chromatographic separation and quantitative determination were carried out according to the HPLC analytical methods described previously [23]. A 0.1 mL aliquot of perchloric acid-treated sample was neutralized with 25 μL of 1 M NaOH solution and 25 μL of 0.5 M Tris-HCl buffer (pH 7.4), and the total volume was adjusted to 0.5 mL with HPLC elution solvent. A 50 μL aliquot of sample was injected onto a Develosil C-30-UG-3 (4.6 I.D. × 150 mm) column adjusted to 40°C, and 5-AcASA was separated by solution with a mixture of acetonitrile (4%) and 20 mM phosphate buffer (pH 5.0 solution) using a CCPD HPLC system equipped with an FS-8020 fluorescence detector (Tosoh Co., Japan). The flow rate of the mobile phase was 1.0 mL/min, and elution of 5-ASA and 5-AcASA was monitored at a fluorescence excitation wavelength of 310 nm and an emission wavelength of 480 nm. 5-ASA and 5-AcASA were eluted at 2.7 and 11.5 minutes, respectively. The quantitative determination of 5-AcASA was based upon the integration of fluorescence peak areas.
2.5. Statistical Analysis
The data in figures are given as the mean ± S.D. of four to five experiments. Differences among the mean values were assessed by Dunnett's test using Stat-100 (BIOSOFT, UK) or Student's t-test. A P value of < 0.05 was considered significant.
3. Results
The incubation of Caco-2 cells with 5-ASA formed only one peak of 5-ASA metabolite, which was identified as 5-AcASA by the same retention time as the synthesized standard in HPLC. The N-acetyl-conjugative reaction of 5-ASA in Caco-2 cells was saturated above 1 mM of 5-ASA. The effect of flavonols and inhibitors of transporters on 5-AcASA efflux was examined using Caco-2 cell monolayers grown in Transwells which contained 1.5 and 2.6 mL Dulbecco's modified Eagle's medium in the apical and basolateral chambers, respectively. 5-ASA was loaded at 200 μM in the apical chamber and 5-AcASA discharged from both of the apical and basolateral sites was measured. After 1, 2, and 4-hour incubation, amounts of 5-AcASA were 1.01, 2.05, and 5.04 nmoles in the apical chamber and 0.19, 0.29, and 0.62 nmoles in the basolateral chamber, respectively (Table 1). The apical efflux of 5-AcASA was 5.32, 7.07, and 8.13-fold higher than the basolateral efflux at 1, 2, and 4-hour incubation, respectively. When fisetin and quercetin (100 μM) were added with 5-ASA to Caco-2 cells, the apical efflux of 5-AcASA decreased remarkably (Table 1). The basolateral efflux of 5-AcASA rather increased in the presence of such flavonols. The ratios for the apical to the basolateral efflux of 5-AcASA actually decreased to 0.52 and 0.68 at 1 hour, 0.77 and 0.85 at 2 hours, and 0.92 and 1.05 at 4-hour incubation, in the presence of fisetin and quercetin, respectively. Morin had a weaker effect than fisetin and quercetin. MK571, a MRPs inhibitor, showed a similar effect to quercetin; however, quinidine, a P-glycoprotein inhibitor, had no effects.
Table 1.
The effect of flavonols and transporter inhibitors on the apical and basolateral efflux of N-acetyl 5-aminosalicylic acid in Caco-2 cells. Caco-2 cells grown in Transwells were incubated with 200 μM 5-ASA for 1, 2, and 4 hours in the presence of flavonols and transporter inhibitors at the concentration of 100 μM. Api: apical efflux of 5-AcASA, Baso: basolateral efflux of 5-AcASA. Each value represents the mean ± SD of four to five experiments.
| Control | Fisetin | Quercetin | Morin | MK571 | Quinidine | ||
|---|---|---|---|---|---|---|---|
| 1hr | Api (nmol) | 1.01 ± 0.14 | 0.16 ± 0.06** | 0.32 ± 0.07** | 0.87 ± 0.03 | 0.47 ± 0.13** | 0.91 ± 0.16 |
| Baso (nmol) | 0.19 ± 0.02 | 0.31 ± 0.09** | 0.47 ± 0.08** | 0.43 ± 0.01** | 0.45 ± 0.04** | 0.18 ± 0.03 | |
| Api/Baso | 5.32 ± 0.38 | 0.52 ± 0.11** | 0.68 ± 0.06** | 2.02 ± 0.04** | 1.04 ± 0.19** | 5.06 ± 0.21 | |
| 2hr | Api (nmol) | 2.05 ± 0.28 | 0.36 ± 0.13** | 0.80 ± 0.21** | 1.64 ± 0.15 | 1.05 ± 0.05** | 1.96 ± 0.59 |
| Baso (nmol) | 0.29 ± 0.04 | 0.47 ± 0.11** | 0.94 ± 0.07** | 0.66 ± 0.06** | 0.70 ± 0.03** | 0.28 ± 0.07 | |
| Api/Baso | 7.07 ± 0.19 | 0.77 ± 0.16** | 0.85 ± 0.16** | 2.48 ± 0.04** | 1.50 ± 0.11** | 7.00 ± 0.18 | |
| 4hr | Api (nmol) | 5.04 ± 0.61 | 0.89 ± 0.25** | 2.16 ± 0.35** | 3.62 ± 0.94 | 2.30 ± 0.22** | 4.61 ± 0.72 |
| Baso (nmol) | 0.62 ± 0.08 | 0.97 ± 0.21** | 2.05 ± 0.17** | 1.15 ± 0.20* | 1.14 ± 0.09** | 0.59 ± 0.10 | |
| Api/Baso | 8.13 ± 0.27 | 0.92 ± 0.16** | 1.05 ± 0.23** | 3.15 ± 0.10** | 2.02 ± 0.11** | 7.81 ± 0.06 | |
Significant difference from control *P < .05, **P < .01.
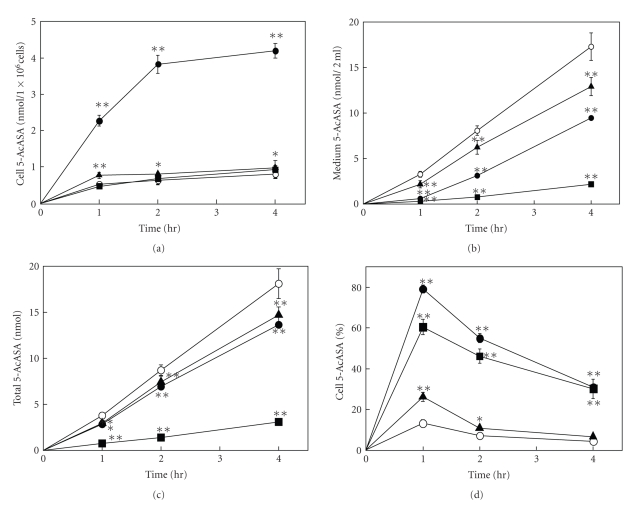
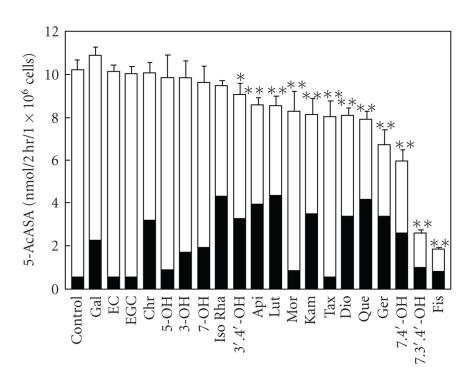
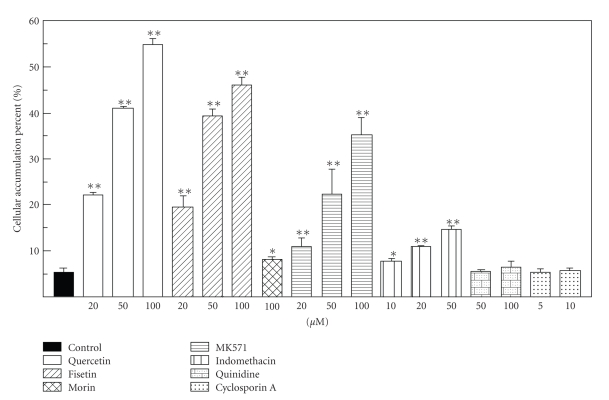
Figure 2 shows the time course of the amount of 5-AcASA in the cells, medium, and their total (cells plus medium), and the percentage of cellular accumulation of 5-AcASA at 1, 2, and 4-hour incubation in the presence of flavonols (100 μM) with 5-ASA (200 μM) in Caco-2 cells grown in tissue culture dishes. 5-AcASA was formed at the rate of 4 nmol/h/1 × 106 cells during a 4 h-incubation period in the control cells. Flavonoids are potent inhibitors of N-acetyltransferase [23]. Fisetin remarkably decreased the formation of 5-AcASA from 5-ASA in Caco-2 cells. Furthermore, a large amount of 5-AcASA was found in the cells treated by quercetin. The amount of 5-AcASA inside the control cells was 12 percent of the total 5-AcASA at a 1 h-incubation and decreased to 6.3 and 3.2 percents at 2 and 4-hour incubation, respectively. The cellular accumulation rate increased by several-fold than that of the control cells by quercetin and fisetin, and increased slightly by morin during a 4 h-incubation period. Figure 3 shows the amount of 5-AcASA in the cells and medium in the presence of various flavonoids at a 2 h-incubation. Flavonoids that lack a hydroxyl substitution at the C5 position on the A-ring had a strong inhibitory effect on the N-acetyl-conjugation of 5-ASA. The total 5-AcASA formed in the presence of fisetin, 7,3′,4′-OH flavone, 7,4′-OH flavone and geraldol decreased to 16.3, 23.3, 54.3, and 68.3 percents of that of the control cells, respectively. Furthermore, most of flavonols and flavones caused an abundant cellular accumulation of 5-AcASA inside the cells. The cellular 5-AcASA accumulation was 52.7 percent of the total formed in the presence of quercetin, the most effective one among flavonoids tested (Table 2). Flavonoids that lack a C2-3 double bond or a carboxyl group at the C4 position on the C-ring, such as catechins and taxifolin, had no effects. The structural feature required for the potent effect on the cellular 5-AcASA accumulation was a presence of hydroxyl group on the B-ring of flavone structure. The effect of inhibitors or substrate of transporters on the cellular 5-AcASA accumulation was compared with flavonols at a 2 h-incubation with 200 μM of 5-ASA in Caco-2 cells (Figure 4). MK-571 and indomethacin, MRPs inhibitors [24–26], increased in concentration-dependent manner the cellular 5-AcASA accumulation, while they did not affect the formation of 5-AcASA. MK-571 was more effective than indomethacin and showed equivalent efficacy to quercetin and fisetin. On the other hand, qunidine, a P-glycoprotein inhibitor, and cyclosporine A, an inhibitor of both P-glycoprotein and MRPs [27, 28], did not affect the cellular 5-AcASA accumulation. Mitoxantrone, a breast cancer resistance protein (BCRP) substrate [29], had no effects either at the concentration of 20 μM (data not shown).
Figure 2.
(a) The time course curve of N-acetyl 5-aminosalicylic acid in Caco-2 cells, (b) the medium and (c) the total, and (d) the cellular accumulation percent. Caco-2 cells grown in tissue culture dishes were incubated with 200 μM 5-ASA for 1, 2, and 4 hours in the absence ( ) and the presence of quercetin (
) and the presence of quercetin ( ), fisetin (
), fisetin ( ), and morin (
), and morin ( ) at the concentration of 100 μM. Cell 5-AcASA (%): (cells/cells plus medium) × 100. Each point represents the mean ± SD of four to five experiments. Significant difference from control *P < .05, **P < .01.
) at the concentration of 100 μM. Cell 5-AcASA (%): (cells/cells plus medium) × 100. Each point represents the mean ± SD of four to five experiments. Significant difference from control *P < .05, **P < .01.
Figure 3.
The amount of N-acetyl 5-aminosalicylic acid in Caco-2 cells and the medium. Caco-2 cells grown in tissue culture dishes were incubated with 200 μM 5-ASA for 2 hours in the presence of flavonoids at the concentration of 100 μM. 5-ASA in cells (Closed column), 5-ASA in medium (Open column). Each bar represents the mean ± SD of four to five experiments. Significant difference from control *P < .05, **P < .01.
Table 2.
The cellular accumulation percent of in N-acetyl 5-aminosalicylic acid Caco-2 cells. Caco-2 cells grown in tissue culture dishes were incubated with 200 μM 5-ASA for 2 hours in the presence of flavonoids at the concentration of 100 μM. Cellular accumulation percent: (cells/cells plus medium) × 100. Each value represents the mean ± SD of four to five experiments.
| Flavonoids | Cellular accumulation (%) | Flavonoids | Cellular accumulation (%) |
|---|---|---|---|
| Control | 5.5 ± 0.8 | 7,3′,4′- OH flavone | 38.7 ± 4.2** |
| Epicatechin | 5.7 ± 0.3 | Diosmetin | 41.8 ± 2.4** |
| Epigallocatechin | 5.8 ± 0.3 | Fisetin | 42.7 ± 1.2** |
| Taxifolin | 6.9 ± 0.6 | 7,4′-OH flavone | 42.8 ± 4.5** |
| 5-OH flavone | 9.2 ± 0.2** | Kaempferol | 43.1 ± 0.7** |
| Morin | 9.9 ± 0.3** | Isorhamnetin | 45.4 ± 4.1** |
| 3-OH flavone | 17.4 ± 0.9** | Apigenin | 45.7 ± 2.3** |
| 7-OH flavone | 20.1 ± 3.8** | Geraldol | 50.2 ± 1.1** |
| Galangin | 21.3 ± 3.1** | Luteolin | 50.7 ± 3.2** |
| Chrysin | 31.7 ± 3.5** | Quercetin | 52.7 ± 2.5** |
| 3′,4′-OH flavones | 36.7 ± 3.6** |
Significant differences from control *P < .05, **P < .01.
Figure 4.
The effect of flavonols and transpoter inhibitors on the cellular accumulation of N-acetyl 5-aminosalicylic acid in Caco-2 cells. Caco-2 cells grown in tissue culture dishes were incubated with 200 μM 5-ASA for 2 hours in the presence of flavonols and transporter inhibitors at the concentration of 100 μM. Cellular accumulation percent : (cells/cells plus medium) × 100. Each bar represents the mean ± SD of four to five experiments. Significant difference from control *P < .05, **P < .01.
4. Discussion
5-AcASA that was formed from 5-ASA in the interior of cells was discharged preferentially to the apical direction compared to the basolateral direction in Caco-2 cells grown in Transwells. Quercetin and fisetin remarkably decreased the apical efflux of 5-AcASA, while morin did with a less potency. The amount of 5-AcASA in Caco-2 cells and the medium was measured during a 4 h-incubation with 5-ASA in the presence of such flavonols. Flavonoids are effective inhibitors of N-acetyl-conjugation of 5-ASA in rat liver cytosol preparation [23]. Fisetin, in particular, exhibited strong inhibitory activity on 5-AcASA formation in Caco-2 cells. Thus, the inhibition of 5-AcASA formation is likely to contribute largely to the decrease in the 5-AcASA efflux in the case of fisetin. However, quercetin showed a much weaker inhibitory effect on the 5-AcASA formation than fisetin. Surprisingly, the formed 5-AcASA was found to be accumulated inside the cells treated by flavonols. For quercetin, the cellular accumulation of 5-AcASA coincides with the decrease in 5-AcASA apical efflux. An increase in the basolateral efflux of 5-AcASA during an incubation of Transwells is probably due to the extensive cellular accumulation of 5-AcASA particularly in quercetin-treated cells.
A large group of flavonoids were examined for their inhibitory effects on the 5-AcASA formation as well as their promoting effects on the cellular 5-AcASA accumulation. A key chemical determinant necessary for exerting the strong inhibitory effect on the N-acetyl-conjugation of 5-ASA was a lack of hydroxyl substitution at the C5 position on the A-ring of flavone structure such as fisetin and 7,3′,4′-OH favone. On the other hand, the structural requirement for the promoting effect on cellular 5-AcASA accumulation was a presence of hydroxyl substitution at the C3′ or C4′ position on the B-ring of flavone structure. Therefore, the inhibition of 5-AcASA formation and the promotion of cellular 5-AcASA accumulation by flavonoids seem to be caused by different mechanisms.
The results mentioned above suggest that 5-AcASA is pumped out by an active efflux transporter located on the apical membrane and certain flavonoids appear to play an important replacing role in the apical-directed transport of 5-AcASA in Caco-2 cells. Flavonoids are well-known modulators of the cellular transport of various substances mediated by P-glycoprotein which is localized on apical membranes in polarized cells [12–14]. Recently, several researchers reported the interaction of flavonoids with MRPs transporters. Walgren et al. reported that the efflux of quercetin 4′-beta-glucoside across Caco-2 cell monolayers was mediated by MRP2 [24]. Van Zanden et al. studied on the inhibitory effect of quercetin on MRPs pump-mediated efflux of calcein and vincristine, well-known MRPs substrates, in the MRP1 and MRP2 transfected MDCK cells [18–20]. They mentioned that MRP2 displayed higher selectivity for flavonoid-type inhibition than MRP1. Phase II metabolites of various drugs conjugated to glutathione, glucuronate, or sulfate are generally considered to be transported by MRPs-like transporters [30–32]. MRPs were characterized as the canalicular multispecific organic anion transporters that function in terminal secretion into bile canaliculus of endo- and xenobiotics such as acetaminophen metabolites, bilirubin glucuronides, 2,4-dinitrophoenyl-S-glutathione, 17β-glucuronosyl estradiol, and 4-methylumbelliferyl glucuronide that are conjugated in hepatocytes [33–35]. The transcellular transport of acetyl-conjugated 5-ASA from the basolateral site to the apical site in Caco-2 cell was first reported by Zhou et al. [11]. However, the transporter-mediated efflux of 5-AcASA has not been investigated thoroughly. To address the interest in involvement of transporters that are responsible for the 5-AcASA apical efflux in Caco-2 cells, several inhibitors of transporters were examined for their suppressing effect on the 5-AcASA apical efflux and promoting effect on the cellular 5-AcASA accumulation. MK571 and indomethacin, inhibitors of MRPs had similar effects to flavonoids. Quinidine, a P-glycoprotein inhibitor, and Cyclosporine A, an inhibitor of P-glycoprotein and MRPs [27, 28], showed no effects. Absence of inhibitory activity of Cyclosporine A may be explained by substrate specificity of 5-AcASA for MRPs. Mitoxantrone, a substrate of BCRP [29], had no effects either. These results suggest that 5-AcASA is possibly pumped out by an MRPs-like transporter and certain flavonoids inhibit their efflux-pump activity in Caco-2 cells.
Flavonoids are part of the human diet and possess many health benefits with low toxicity [36, 37]. However, flavonoids are poorly absorbable compounds from the digestive tract in vertebrates [38, 39]. When quercetin was given p.o. to the rats (630 mg/kg), approximately 20% of the total dose was absorbed from the digestive tract, more than 30% was decomposed in the intestinal microflora, and approximately 30% was excreted unchanged in the feces during 72 hours [38]. After a single oral dose of quercetin in humans (4 g), approximately 53% of the dose was recovered unchanged in the feces. Thus it was concluded that 1% of the original 4 g dose of quercetin was absorbed [39]. In this study, flavonoids were added at the concentration range from 20 to 100 μM only into the apical compartment of Caco-2 cells in Transwells that faces to intestinal lumen in vivo. A high luminal level around 100 μM of flavonoids is expected to be achieved with a single oral administration of a few hundred mg of flavonoids in humans.
5-ASA, an active moiety of sulfasalazine, is immediately secreted into the luminal side from intestinal epithelia following extensive N-acetyl-conjugation, and is finally excreted into feces [3–5]. Zhou et al. [11] reported that at luminal levels below 200 μg/mL (concentrations that are typically achieved by controlled release dosage forms), intestinal secretion of 5-AcASA accounts for more than 50% of the total 5-ASA elimination. Thus, 5-AcASA has been considered to be therapeutically nonactive portion [1–3, 9–11]. However, 5-AcASA has still antiinflammatory potential if the drug retains within the intestinal tissues [8]. The efficacy of 5-ASA therapy correlates with tissue delivery of 5-ASA, that is, determined by N-acetylation and cellular discharge. The present study showed that certain flavonoids have the inhibitory effect on N-acetyl-conjugation of 5-ASA and the suppressive effect on the 5-AcASA apical efflux in Caco-2 cells. Viewed in this light, both of these effects of flavonoids seem to be desirable in the treatment of inflammatory bowel diseases, since coadministration of flavonoids with 5-ASA is expected to increase the tissue levels of 5-ASA and 5-AcASA in intestine.
References
- 1.Das KM, Eastwood MA, McManus JP, Sircus W. Adverse reactions during salicylazosulfapyridine therapy and the relation with drug metabolism and acetylator phenotype. The New England Journal of Medicine. 1973;289(10):491–495. doi: 10.1056/NEJM197309062891001. [DOI] [PubMed] [Google Scholar]
- 2.Ardizzone S, Porro GB. Comparative tolerability of therapies for ulcerative colitis. Drug Safety. 2002;25(8):561–582. doi: 10.2165/00002018-200225080-00003. [DOI] [PubMed] [Google Scholar]
- 3.Allgayer H, Ahnfelt NO, Kruis W, et al. Colonic N-acetylation of 5-aminosalicylic acid in inflammatory bowel disease. Gastroenterology. 1989;97(1):38–41. doi: 10.1016/0016-5085(89)91412-1. [DOI] [PubMed] [Google Scholar]
- 4.Ricart E, Taylor WR, Loftus EV, et al. N-acetyltransferase 1 and 2 genotypes do not predict response or toxicity to treatment with mesalamine and sulfasalazine in patients with ulcerative colitis. American Journal of Gastroenterology. 2002;97(7):1763–1768. doi: 10.1111/j.1572-0241.2002.05838.x. [DOI] [PubMed] [Google Scholar]
- 5.Bat B, Lodowska J, Orchel A, et al. Evaluation of biotransformation of sulphasalazine in the colon epithelial Caco-2 cells. Acta Poloniae Pharmaceutica. 2004;61:8–10. [PubMed] [Google Scholar]
- 6.Sharon P, Ligumsky M, Rachmilewitz D, Zor U. Role of prostaglandins in ulcerative colitis. Enhanced production during active disease and inhibition by sulfasalazine. Gastroenterology. 1978;75(4):638–640. [PubMed] [Google Scholar]
- 7.Hawkey CJ, Truelove SC. Inhibition of prostaglandin synthetase in human rectal mucosa. Gut. 1983;24(3):213–217. doi: 10.1136/gut.24.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willoughby CP, Piris J, Truelove SC. The effect of topical N-acetyl-5-aminosalicylic acid in ulcerative colitis. Scandinavian Journal of Gastroenterology. 1980;15(6):715–719. doi: 10.3109/00365528009181520. [DOI] [PubMed] [Google Scholar]
- 9.Layer PH, Goebell H, Keller J, Dignass A, Klotz U. Delivery and fate of oral mesalamine microgranules within the human small intestine. Gastroenterology. 1995;108(5):1427–1433. doi: 10.1016/0016-5085(95)90691-6. [DOI] [PubMed] [Google Scholar]
- 10.Bondesen S. Intestinal fate of 5-aminosalicylic acid: regional and systemic kinetic studies in relation to inflammatory bowel disease. Pharmacology and Toxicology. 1997;81(supplement 2):1–28. doi: 10.1111/j.1600-0773.1997.tb01944.x. [DOI] [PubMed] [Google Scholar]
- 11.Zhou SY, Fleisher D, Pao LH, Li C, Winward B, Zimmermann EM. Intestinal metabolism and transport of 5-aminosalicylate. Drug Metabolism and Disposition. 1999;27(4):479–485. [PubMed] [Google Scholar]
- 12.Di Pietro A, Conseil G, Pérez-Victoria JM, et al. Modulation by flavonoids of cell multidrug resistance mediated by P-glycoprotein and related ABC transporters. Cellular and Molecular Life Sciences. 2002;59(2):307–322. doi: 10.1007/s00018-002-8424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang S, Morris ME. Effects of the flavonoids biochanin A, morin, phloretin, and silymarin on P-glycoprotein-mediated transport. Journal of Pharmacology and Experimental Therapeutics. 2003;304(3):1258–1267. doi: 10.1124/jpet.102.044412. [DOI] [PubMed] [Google Scholar]
- 14.Takano M, Yumoto R, Murakami T. Expression and function of efflux drug transporters in the intestine. Pharmacology and Therapeutics. 2006;109(1-2):137–161. doi: 10.1016/j.pharmthera.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Leslie EM, Mao Q, Oleschuk CJ, Deeley RG, Cole SPC. Modulation of multidrug resistance protein 1 (MRP1/ABCC1) transport and ATpase activities by interaction with dietary flavonoids. Molecular Pharmacology. 2001;59(5):1171–1180. doi: 10.1124/mol.59.5.1171. [DOI] [PubMed] [Google Scholar]
- 16.Bobrowska-Hägerstrand M, Wróbel A, Mrówczyńska L, et al. Flavonoids as inhibitors of MRP1-like efflux activity in human erythrocytes. A structure-activity relationship study. Oncology Research. 2003;13(11):463–469. doi: 10.3727/000000003108747983. [DOI] [PubMed] [Google Scholar]
- 17.Łania-Pietrzak B, Hendrich AB, Zugaj J, Michalak K. Metabolic O-demethylation does not alter the influence of isoflavones on the biophysical properties of membranes and MRP1-like protein transport activity. Archives of Biochemistry and Biophysics. 2005;433(2):428–434. doi: 10.1016/j.abb.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Van Zanden JJ, Wortelboer HM, Bijlsma S, et al. Quantitative structure activity relationship studies on the flavonoid mediated inhibition of multidrug resistance proteins 1 and 2. Biochemical Pharmacology. 2005;69(4):699–708. doi: 10.1016/j.bcp.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Van Zanden JJ, De Mul A, Wortelboer HM, et al. Reversal of in vitro cellular MRP1 and MRP2 mediated vincristine resistance by the flavonoid myricetin. Biochemical Pharmacology. 2005;69(11):1657–1665. doi: 10.1016/j.bcp.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Van Zanden JJ, van der Woude H, Vaessen J, et al. The effect of quercetin phase II metabolism on its MRP1 and MRP2 inhibiting potential. Biochemical Pharmacology. 2007;74(2):345–351. doi: 10.1016/j.bcp.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Brendel E, Meineke I, Witsch D, Zschunke M. Simultaneous determination of 5-aminosalicylic acid and 5-acetylaminosalicylic acid by high-performance liquid chromatography. Journal of Chromatography. 1987;385:299–304. doi: 10.1016/s0021-9673(01)94644-8. [DOI] [PubMed] [Google Scholar]
- 22.Sugihara N, Toyama K, Michihara A, Akasaki K, Tsuji H, Furuno K. Effect of benzo[a]pyrene on P-glycoprotein-mediated transport in Caco-2 cell monolayer. Toxicology. 2006;223(1-2):156–165. doi: 10.1016/j.tox.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Mizoyama Y, Takaki H, Sugihara N, Furuno K. Inhibitory effect of flavonoids on N-acetylation of 5-aminosalicylic acid in cultured rat hepatocytes. Biological and Pharmaceutical Bulletin. 2004;27(9):1455–1458. doi: 10.1248/bpb.27.1455. [DOI] [PubMed] [Google Scholar]
- 24.Walgren RA, Karnaky KJ, Jr., Lindenmayer GE, Walle T. Efflux of dietary flavonoid quercetin 4′-β-glucoside across human intestinal Caco-2 cell monolayers by apical multidrug resistance-associated protein-21. Journal of Pharmacology and Experimental Therapeutics. 2000;294(3):830–836. [PubMed] [Google Scholar]
- 25.Hong J, Lambert JD, Lee S-H, Sinko PJ, Yang CS. Involvement of multidrug resistance-associated proteins in regulating cellular levels of (-)-epigallocatechin-3-gallate and its methyl metabolites. Biochemical and Biophysical Research Communications. 2003;310(1):222–227. doi: 10.1016/j.bbrc.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Schrickx J, Lektarau Y, Fink-Gremmels J. Ochratoxin A secretion by ATP-dependent membrane transporters in Caco-2 cells. Archives of Toxicology. 2006;80(5):243–249. doi: 10.1007/s00204-005-0041-5. [DOI] [PubMed] [Google Scholar]
- 27.Honda Y, Ushigome F, Koyabu N, et al. Effects of grapefruit juice and orange juice components on P-glycoprotein- and MRP2-mediated drug efflux. British Journal of Pharmacology. 2004;143(7):856–864. doi: 10.1038/sj.bjp.0706008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takasuna K, Hagiwara T, Watanabe K, et al. Optimal antidiarrhea treatment for antitumor agent irinotecan hydrochloride (CPT-11)-induced delayed diarrhea. Cancer Chemotherapy and Pharmacology. 2006;58(4):494–503. doi: 10.1007/s00280-006-0187-8. [DOI] [PubMed] [Google Scholar]
- 29.Yanase K, Tsukahara S, Mitsuhashi J, Sugimoto Y. Functional SNPs of the breast cancer resistance protein-therapeutic effects and inhibitor development. Cancer Letters. 2006;234(1):73–80. doi: 10.1016/j.canlet.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 30.Jedlitschky G, Leier I, Buchholz U, Barnouin K, Kurz G, Keppler D. Transport of glutathione, glucuronate, and sulfate conjugates by the MRP gene-encoded conjugate export pump. Cancer Research. 1996;56(5):988–994. [PubMed] [Google Scholar]
- 31.Konig J, Nies AT, Cui Y, Leier I, Keppler D. Conjugate export pumps of the multidrug resistance protein (MRP) family: localization, substrate specificity, and MRP2-mediated drug resistance. Biochimica et Biophysica Acta. 1999;1461(2):377–394. doi: 10.1016/s0005-2736(99)00169-8. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki H, Sugiyama Y. Single nucleotide polymorphisms in multidrug resistance associated protein 2 (MRP2/ABCC2): its impact on drug disposition. Advanced Drug Delivery Reviews. 2002;54:1319–1331. doi: 10.1016/s0169-409x(02)00075-3. [DOI] [PubMed] [Google Scholar]
- 33.Gotoh Y, Suzuki H, Kinoshita S, Hirohashi T, Kato Y, Sugiyama Y. Involvement of an organic anion transporter (canalicular multispecific organic anion transporter/multidrug resistance-associated protein 2) in gastrointestinal secretion of glutathione conjugates in rats. Journal of Pharmacology and Experimental Therapeutics. 2000;292(1):433–439. [PubMed] [Google Scholar]
- 34.Slitt AL, Cherrington NJ, Maher JM, Klaassen CD. Induction of multidrug resistance protein 3 in rat liver is associated with altered vectorial excretion of acetaminophen metabolites. Drug Metabolism and Disposition. 2003;31(9):1176–1186. doi: 10.1124/dmd.31.9.1176. [DOI] [PubMed] [Google Scholar]
- 35.Zamek-Gliszczynski MJ, Hoffmaster KA, Humphreys JE, Tian X, Nezasa K-I, Brouwer KLR. Differential involvement of Mrp2 (Abcc2) and Bcrp (Abcg2) in biliary excretion of 4-methylumbelliferyl glucuronide and sulfate in the rat. Journal of Pharmacology and Experimental Therapeutics. 2006;319(1):459–467. doi: 10.1124/jpet.106.101840. [DOI] [PubMed] [Google Scholar]
- 36.Havsteen B. Flavonoids, a class of natural products of high pharmacological potency. Biochemical Pharmacology. 1983;32(7):1141–1148. doi: 10.1016/0006-2952(83)90262-9. [DOI] [PubMed] [Google Scholar]
- 37.Cook NC, Samman S. Flavonoids—chemistry, metabolism, cardioprotective effects, and dietary sources. Journal of Nutritional Biochemistry. 1996;7(2):66–76. [Google Scholar]
- 38.Ueno I, Nakano N, Hirono I. Metabolic fate of [14C] quercetin in the ACl rat. Japanese Journal of Experimental Medicine. 1983;53(1):41–50. [PubMed] [Google Scholar]
- 39.Gugler R, Leschik M, Dengler HJ. Disposition of quercetin in man after single oral and intraveneous doses. European Journal of Clinical Pharmacology. 1975;9:229–234. doi: 10.1007/BF00614022. [DOI] [PubMed] [Google Scholar]