Summary
Sleep Disordered Breathing (SDB) is highly prevalent in elderly populations and is thought to reflect, at least in part, age-dependence. Several studies suggest that SDB in elderly populations may hold different functional outcomes relative to SDB in middle-aged populations. Risk factors for SDB specific for the elderly remain uncertain. In this report, we examined changes in SDB, body weight and pulmonary function in 103 individuals over an average interval of 7 years to determine whether changes in these measures covaried. In-lab polysomnography was performed on members of an elderly cohort (Bay Area Sleep Cohort) on two separate occasions (Time 1, Time 2) with multiple nights of measurement typically made on each occasion. Results indicated that: a) SDB progressed over time in both men and women; b) changes in body weight were unrelated to the progression in SDB; c) relative declines in lung volumes (Forced Vital Capacity, Forced Expiratory Volume in 1.0 second) were associated with relative increases in SDB, with the effects slightly stronger in men. These data suggest that age-dependence in one commonly ascribed aging biomarker (lung function) were coupled to increments in SDB. Maintenance of healthy lung function into old age may confer some protective benefits in the development of age-dependent SDB.
Background: Age Dependence
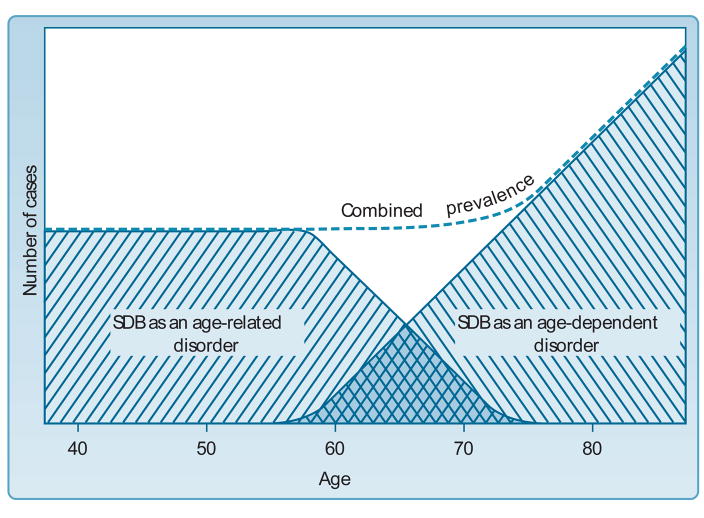
Age dependence refers to a physiologic vulnerability whose likelihood increases with chronological age (1). Sleep Disordered Breathing (SDB) has been proposed as a condition whose prevalence at least partially reflects such age-dependence (2–4). During middle-age, SDB is thought to represent an age-related phenomenon conferring a distinct window of vulnerability (2) (see Figure 1, ref 5) in association with various morbidities. Indeed, a large number of analyses from the Sleep Heart Health Study (SHHS) have suggested that cardiovascular outcomes of SDB, such as hypertension, stroke, endothelial dysfunction and myocardial infarct, may be more likely to occur in middle-aged, rather than older populations (6–10), although data from other elderly cohorts (11,12) who may be less subject to survivor bias by virtue of enrolling subjects either at a younger age or by not excluding elderly individuals who may be unusually healthy, often do not concur.
Figure 1.
Heuristic Model Suggesting SDB as an age-dependent condition in elderly persons, relative to SDB as an age-related condition, primarily affecting middle-aged adults (from ref 5). Note that such a model predicts incident cases of SDB in old age should increase with advancing years (see age results in Table 4)
A key feature of age-dependence is that effects of advancing age are assumed broadly to operate in concert with other physiologic systems undergoing aging (13, 14). Sehl and Yates (15) have articulated and refined this biomarker concept still further by examining rates of change (typically as yearly percentage change in function in the measurement of a particular marker) across a wide variety of organ systems, including measurements of endocrine, musculoskeletal, autonomic, and cardiovascular function, to name but a few. Because SDB is multi-determined, age-dependent changes in such systems could be important for understanding risk factors for the condition (Figure 2) (5). Of particular interest when discussing SDB and aging are age-dependent declines in pulmonary function, most typically indexed as changes in Forced Vital Capacity (FVC) and Forced Expiratory Volume in 1 second (FEV1). Age-dependent change in FVC and FEV1 have been well documented and consist of change of about 25–40 mL per year based on cross-sectional data (16–18). Whether such changes have any bearing upon SDB in old age remain unexplored.
Figure 2.
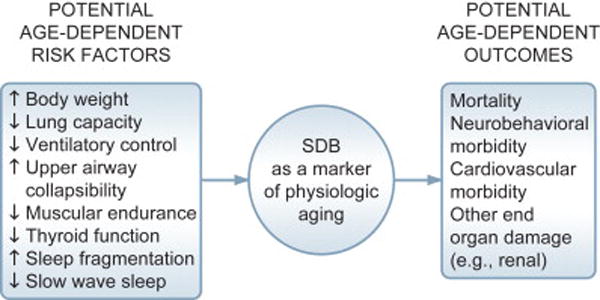
Potential age-dependent risk factors and outcomes of SDB in old age (from ref 5)
Introduction to the Bay Area Sleep Cohort (BASC)
The existence of a previously established, prospectively studied cohort (Bay Area Sleep Cohort, BASC), which has been described in greater detail previously (11, 19–21), has allowed us to gain some understanding of age dependence in SDB using longitudinal data. The vast majority of our knowledge about SDB in adulthood and old age derives from cross-sectional studies. Our goals in the current analyses of BASC were: a) to document whether SDB increases over time within individuals and determine the rate of those changes; and b) to examine relationships between two candidate biomarkers of age dependence, SDB and lung volumes, when both are studied over time. We hypothesized that steeper rates of decline in FVC and FEV1 would be associated with steeper rates of increases in SDB.
BASC originated as a convenience sample of independently living middle aged and elderly subjects (primarily Caucasian) residing in the mid-Peninsula region south of San Francisco recruited between the years 1974 and 1985. All participants gave informed consent and the study was approved by the Institutional Review Board. Individuals with serious health problems (history of cancer, myocardial infarct, stroke, cardiac surgery, neurodegenerative diseases) were excluded from the cohort at entry. Individuals with controlled medical conditions (e.g., hypertension, arthritis) were allowed. All subjects in this report remained healthy throughout the observation period and were free from neurodegenerative diseases (e.g., dementia) or congestive heart failure at time of follow-up. BASC represented a population somewhat overselected for insomnia at entry, as many subjects were subsequently enrolled in pharmacologic trials. More completion descriptions of the original 256 members of the cohort are provided in previous publications (11, 21). None of these individuals underwent treatment of SDB during the window of observation presented here.
The subjects presented below are 103 individuals whose sleep was studied polysomnographically on 2 separate occasions (hereafter labeled Time 1 and Time 2) a minimum of 60 months apart (mean = 82.2 months, SD = 12.5, range 60–121). Table 1 compares Baseline characteristics at time of entry for the 103 participants in this follow-up from the 153 individuals not participating in this follow-up and indicates that individuals reported on here were less likely to have self-reported poor sleep but were otherwise comparable in terms of demographics and comorbid cardiovascular and psychiatric comorbidity, as indexed by medication usage. Mean age of the 103 participants at Time 1 was 66.2 (8.2) and consisted of 34 men and 69 women; men were significantly older than women (68.9 [8.3] versus 64.9 [8.0], t = 2.38, p = .019).
Table 1.
Comparison of Baseline (Entry) Data from Subjects Included and Subjects Not Included in Current Analyses
| Included (n = 103) | Not Included (n = 153) | Comparison | |
|---|---|---|---|
| Variable | |||
| X (SD) Age (at entry) (yrs) | 64.7 (8.7) | 65.5 (9.6) | t = .69, p = .49 |
| % Male | 33.0 | 32.0 | χ2 = .03, p = .87 |
| % with Insomnia | 44.7 | 66.7 | χ2 = 12.22, p = .0005 |
| % receiving CV Rx | 16.5 | 24.8 | χ2 = 2.53, p = .11 |
| % receiving Psych Rx | 14.6 | 19.0 | χ2 = .83, p = .36 |
| X (SD) BMI (kg/m2) | 24.2 (3.6) | 25.0 (4.0) | t = 1.62, p = .11 |
| X (SD) TST (PSG) (mins) | 362.2 (84.9) | 358.0 (93.2) | t = .36, p = .72 |
| X (SD) SE (PSG) (%) | 74.5 (14.8) | 73.2 (15.8) | t = .70, p = .48 |
| X (SD) PLMSI (events/hour) | 14.2 (23.8) | 19.7 (28.5) | t = 1.52, p = .13 |
| X (SD) typical sleep duration (mins) (self-report) | 407.8 (74.2) | 373.8 (86.7) | t = 3.10, p = .002 |
NOTES: CV Rx = Cardiovascular medication, includes hydrochlorothiazide, beta blockers, alpha blockers, calcium-channel blockers, angiotensin-converting enzyme inhibitors, lipid-lowering agents, nitrates, diuretics, and cardiac arrhythmia medications including those containing digitalis; Psych Rx = Psychoactive medication, includes anxiolytics, sedative/hypnotics, anti-depressants, neuroleptics; BMI = Body Mass Index; TST = Total Sleep Time; SE = Sleep Efficiency; SL = Sleep Latency to first epoch of stage 2; PLMSI = Periodic Leg Movements in Sleep per sleep hour.
All 103 individuals underwent in-lab polysomnography (PSG), height and weight measurements, and pulmonary function tests (PFTs) at Time 1 and Time 2 evaluations. Most participants (58 at Time 1, 98 at Time 2) had 2 more nights of polysomnography, and in those cases, nights were averaged within subjects. For those subjects with only one night of recording (45 at Time 1, 5 at Time 2), data from that single night were used in all subsequent calculations.
Because concurrent use of spirometry was not always available at the time of Baseline into BASC, the Time 1 measurements here represent data during the 1974–1985 entry window for only a subset of the 103 participants (n = 62). For the remaining 41 cases, the Time 1 data presented here represent polysomnographic, spirometric and body weight data collected at a date subsequent to entry into BASC, but at least 60 months apart from the Time 2 measurement. All Time 2 data were derived from the 1987 to 1992 round of follow-up.
Polysomnography (PSG)
Polysomnography (PSG) was performed with Grass Model 78 polysomnographs (Grass Instruments, Quincy, MA) and scored on paper by trained technologists not familiar with the body weight or spirometry data of the participants. Details of the montage can be found elsewhere (11). Breathing disturbance in sleep was quantified as the apnea/hypopneas index (AHI), with apneas classified by a complete cessation of breathing and hypopneas classified by a reduction in airflow of at least 50% from the immediately preceding baseline. For the analyses described here, we did not rely on a minimal desaturation criterion or an arousal criterion to qualify hypopneas. Previous analyses from this cohort showed a similar pattern of results in association with outcomes regardless of hypopnea definition (11). AHI as a summed metric retains high inter-rater reliability within our laboratory recording and scoring procedures (22). The majority of events (74 %) were scored as obstructive/mixed rather than central. All scoring was performed blind to patients’ spirometry results.
Body Mass Index
Heights (m) and weights (kg) were derived from a standard clinic scale with participants full clothed but with shoes removed. Body Mass Index (BMI) was calculated as weight/height2.
Spirometry
Spirometry was performed at Time 1 and Time 2 for all BASC subjects included in the current report. Standard maneuvers followed American Thoracic Society Snowbird Workshop guidelines for measurements of Forced Vital Capacity (FVC) and Forced Expiratory Volume in 1 second (FEV1), employing the best of 3 trials (23). Studies were done in standing position using nose clips. Following the procedures of Knudson et al (24), we used each subject’s highest FVC and FEV1, even if they were derived from 2 different maneuvers (25).
At Time 1, spirometry was performed with a portable, bellows unit (Waters Spirometer, Model Pulmonarie 10, Jones, Oakbrook, IL), which was regularly checked with a 3 L calibration syringe. Time 2 testing was performed in the Stanford University Medical Center Pulmonary Function Laboratory using a water-based volume displacement spirometer (Collins DS system, Warren Collins, Braintree, MA). In order to adjust for possible effects of use of different equipment at Time 1 and Time 2, 65 of the 103 participants at Time 2 were re-tested with the portable spirometer the same day as the in-hospital measurements were made. Linear regression demonstrated an extremely high concordance between the portable and in-lab measurements (for FVC, r2= .928, F = 700.22, p < .0001, slope =1.10, intercept = .208; for FEV1, r2 = .933, F = 882.91, p < .0001, slope = 1.08, intercept = .110) and suggested that the portable unit slightly, but consistently, underestimated volumes. In order to correct for this small underestimation of FVC and FEV1 on Time 1 measurements, all Time 1 spirometric measurements were adjusted to correct for these differences (for FVC, portable FVC × 1.10 + .208; for FEV1, portable FEV1 × 1.08 + .111).
Findings from the current analyses of BASC
Table 2 shows Time 1 and Time 2 data on BASC subjects; simple t-tests suggested that AHI increased over time for both men and women. For all time dependent measures (i.e., AHI, BMI, FVC, and FEV1), we also calculated rates of change as the Time 2 value of each measurement minus the Time 1 value of each measurement divided by number of months of follow-up. These rates were then multiplied by 12 to derive a rate of change per year. AHI data are presented as both raw and log transformed (calculated as log[1 + AHI] to correct for skew) values (see Table 2), although only the latter were used for correlational analyses.
Table 2.
Change in Time-Dependent Variables
| Time 1 | Time 2 | Change | |
|---|---|---|---|
| Variable | |||
| AHI (events/hour) | |||
| Women | 1.95 (2.77) | 4.51 (6.67) | t = 3.86, p = .0003 |
| Men | 9.69 (11.30) | 16.11 (17.40) | t = 3.40, p = .0018 |
| Both | 4.51 (7.74) | 8.34 (12.56) | t = 4.91, p < .0001 |
| LogAHI | |||
| Women | .79 (.70) | 1.34 (.78) | t = 6.53, p < .0001 |
| Men | 1.88 (1.03) | 2.34 (1.04) | t = 4.09, p = .0003 |
| Both | 1.15 (.96) | 1.67 (.99) | t = 7.72, p < .0001 |
| Weight (kg) | |||
| Women | 64.24 (12.20) | 65.52 (14.13) | t = 1.62, p = .109 |
| Men | 75.15 (11.60) | 74.35 (12.87) | t = .79, p = .433 |
| Both | 67.84 (13.01) | 68.44 (14.28) | t = .94, p = .35 |
| BMI (kg/m2) | |||
| Women | 24.01 (4.04) | 24.51 (4.92) | t = 1.69, p = .096 |
| Men | 24.18 (2.51) | 23.90 (2.98) | t = .87, p = .39 |
| Both | 24.07 (3.59) | 24.31 (4.37) | t = 1.05, p = .30 |
| FVC (L) | |||
| Women | 2.79 (.66) | 2.50 (.65) | t = 6.33, p < .0001 |
| Men | 3.93 (.84) | 3.64 (.84) | t = 2.77, p = .009 |
| Both | 3.17 (.90) | 2.88 (.90) | t = 6.30, p < .0001 |
| FEV1 (L) | |||
| Women | 2.05 (.57) | 1.88 (.55) | t = 4.54, p < .0001 |
| Men | 2.77 (.62) | 2.57 (.68) | t = 1.76, p = .087 |
| Both | 2.29 (.67) | 2.11 (.68) | t = 4.04, p < .0001 |
NOTE: Data presented as X (SD)
Rates of change in AHI over time are shown in Table 3 for both men and women and suggest slightly higher rates of change for men. Overall the median rate of change in AHI was .188 events/hour/year (range: −2.50 to + 5.69) with 81 cases showing increases, 20 cases showing decreases and 2 without change. These data indicated that BASC members were far more likely to increase, rather than decrease, their AHI over time.
Table 3.
Gender Differences in Yearly Rate of Change
| Women (n = 69) | Men (n = 34) | Combined (n = 103) | Comparison | |
|---|---|---|---|---|
| Variable | ||||
| BMI (kg/m2/year) | .064 (.331) | −.046 (.332) | .027 (.333) | t = 1.58, p = .12 |
| FVC (L/year) | −.040 (.053) | −.047 (.103) | −.043 (.073) | t = .33, p = .74 |
| FEV1 (L/year) | −.024 (.044) | −.032 (.106) | −.027 (.070) | t = .40, p = .69 |
| AHI (events/hr/year) | .36 (.82) | 1.10 (.1.94) | .61 (1.33) | t = 2.12, p = .04 |
| LogAHI (events/hr/year) | .076 (.100) | .076 (.109) | .076 (.103) | t = 0.0, p = .99 |
NOTES: Values in first 3 columns represent X (SD) of change rate (in units shown); final column shows 2-group t-tests for gender comparisons
With regards to body weight, BASC women gained (within subjects analyses) on average 1.28 kg (SD = 6.53) where as men lost −.80 kg (SD 5.86) over the years of observation (mean group values shown on Table 2). These changes were not statistically significant. Rates of change in FVC and FEV1 averaged 47 and 32 mL/year for men, respectively, and 40 and 24 mL/year for women, respectively (see Table 3), approximating rates of change in these measures in previously published normative data (16–18).
Table 4 shows data relevant to age dependence in AHI. Over the 7 years of observation, steeper rates of decline in yearly rates for both FVC and FEV1 were related to increased rates in AHI, those relationships somewhat more pronounced for men than for women. These data suggest a critical association between these two biomarkers (SDB and lung volumes) within BASC. Yearly rates of change in AHI were marginally related to older age at entry in men but not in women.
Table 4.
Correlations between Yearly Log Transformed Rate of Change in AHI and Key Variables
| Women (n = 69) | Men (n = 34) | Combined (n = 103) | |
|---|---|---|---|
| Variable | |||
| Time 1 Age | .139 (.254) | .324 (.062) | .199 (.044) |
| BMI Yearly Change (kg/m2/year) | −.052 (.669) | −.206 (.242) | −.104 (.295) |
| FVC Yearly Change (L/year) | −.114 (.351) | −.414 (.015) | −.187 (.059) |
| FEV1 Yearly Change (L/year) | −.214 (.078) | −.208 (.238) | −.183 (.064) |
NOTES: Pearson correlations shown; p-values in parentheses
Implications of the Current Findings: Spirometry and Age Dependence in SDB
Numerous cross-sectional studies have generally suggested little utility in traditional spirometric measures in predicting individuals with SDB. Although initial enthusiasm for the determination of sleep-related upper airway obstruction from measures such as flow volume loops was high (e.g., 26), most measures derived from routine PFTs were considered to hold little prognostic value in sleep clinic patients (27). Studies examining the pathophysiology of SDB in old age have instead focused on age-dependent changes upper airway structure or function (28–31). Placed in this context, the current results suggesting that declining FVC and FEV1 may be associated with incident SDB over time may appear surprising.
More careful consideration of the complex interactive effects between upper and lower airway function in SDB, however, yields a potential basis for such an effect. Cross-sectional area in the upper airway has been long known to be dependent upon lung volumes (32–36), and experimental evidence has shown that increased functional residual capacity (by experimentally applied negative extrathoracic pressures) reduces SDB (33). Recent mechanistic studies examining muscle activity and upper airway collapsibility in normal subjects subjected to manipulations in extrathoracic pressure have confirmed that as lung volumes increased, collapsibility decreased, though the role of altered genioglossus activity appears unlikely to be the basis of the effect (37). A more likely explanation involves the mechanism sometimes referred to as the “tracheal tug,” which has been modeled in dogs (38) and is thought to represent the caudal displacement of the trachea, which results in stiffening of the upper airway musculature from mechanical forces. These findings suggest that the development of SDB in older adults might at least partially derive from such mechanical factors (e.g., declines in chest wall compliance and loss of elastic recoil [39]) that could manifest in steeper rates of decline in FVC and FEV1. In this regard, it may be informative to re-examine the largely negative findings of relationships between routine pulmonary function measures and SDB. For example, one large case series of PSG and PFT data in sleep apnea indicated that FVC and FEV1 decreased with increased severity of SDB (40). However, those effects disappeared when age was controlled, and the effects interpreted largely as indicating that lung volumes were of minimal significance in the prediction of SDB. Data such as these are compatible with the longitudinal data presented here, in which age was a time-dependent covariate (by definition) and the passage of time (aging) was associated with lung-volume dependent increases in SDB. Our data are also consistent with reports from a small cohort of healthy older individuals, for whom elevated Baseline AHI measurements (AHI > 5) showed persistent relationship to reduced pulmonary function (41, 42).
We did not note effects of body weight changes in relation to incident SDB in BASC. In cross-sectional analyses from the Sleep Heart Health Study (43), elevated BMI was noted to confer a decreased magnitude of risk for SDB in older age groups, and a similarly reduced role of changes in BMI for incident SDB was noted in SHHS longitudinal data over a 5-year interval (44). In longitudinal population-based data encompassing younger subjects, both the Wisconsin Sleep Cohort Study (WSCS) (45) and the Cleveland Family Study (CFS) (46, 47) noted that increases and decreases in body weight over time were associated with increases and decreases in AHI, respectively, though in CFS, the magnitude of body weight effects were tempered among older cohort members (46). Regardless of differential strength of associations with body weight changes, all of these studies uniformly reported increases in AHI over time, which is consistent with what we have noted in BASC. Some previous longitudinal studies of sleep in older adults did not find increased SDB over time (48, 49), but did not rely on laboratory-based polysomnography and used smaller samples than we are reporting here. In contrast to all of these previous studies, BASC affords a somewhat unique perspective on the SDB of old age by virtue of simultaneous PFT measurements and repeated nights of PSG.
What are the implications of a possible impact of lung volumes in the prediction of long-term development of SDB and/or even its prevention? Maintenance of healthy pulmonary function throughout the human life span has long been recognized to be related to avoidance of aversive environmental exposures, avoidance of smoking and appropriate nutrition in the presence of ideal body weight. Salutary influences on lung function, such as exercise, may have beneficial effects on sleep disordered breathing that are not dependent upon body weight (50–52). To the extent that lung volumes predict development of SDB over time, maintenance of healthy aerobic capacity might be protective to some extent for development of SDB in old age.
Acknowledgments
Support:
AG-020269; AG-06066; AG-02504
I gratefully acknowledge the following individuals for their invaluable assistance in conducting and analyzing data on BASC: Farzaneh Pour Ansari, Sophia A. Greer, Ann Pursley Kollrack, Julia Zarcone Pattmore, Laura-Beth Straight, as well as the Pulmonary Function Laboratory at Stanford University Medical School,
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brody JA, Schneider EL. Diseases and disorders of aging: an hypothesis. J Chron Dis. 1986;39:871–76. doi: 10.1016/0021-9681(86)90035-4. [DOI] [PubMed] [Google Scholar]
- 2.Young T. Age dependence of sleep disordered breathing. In: Kuna ST, Suratt PM, Remmers JE, editors. Sleep and Respiration in Aging Adults. Elsevier; New York: 1991. pp. 161–70. [Google Scholar]
- 3.Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16:40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- 4.Bliwise DL. Chronological age, physiological age, and mortality in sleep apnea (editorial) Sleep. 1996;19:275–276. doi: 10.1093/sleep/19.4.275. [DOI] [PubMed] [Google Scholar]
- 5.Bliwise DL. Normal aging. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4. Philadelphia: Saunders/Elsevier; 2005. pp. 24–38. [Google Scholar]
- 6.Haas DC, Foster GL, Nieto FJ, et al. Age-dependent associations between sleep-disordered breathing and hypertension: importance of discriminating between systolic/diastolic hypertension and isolated systolic hypertension in the Sleep Heart Health Study. Circulation. 2005;111:614–25. doi: 10.1161/01.CIR.0000154540.62381.CF. [DOI] [PubMed] [Google Scholar]
- 7.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea and hypertension in a large community-based study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 8.Nieto FJ, Herrington DM, Redline S, et al. Sleep apnea and markers of vascular endothelial function in a large community sample of older adults. Am J Respir Crit Care Med. 2004;169:354–60. doi: 10.1164/rccm.200306-756OC. [DOI] [PubMed] [Google Scholar]
- 9.Newman AB, Nieto FJ, Guidry U, et al. Relation of sleep-disordered breathing to cardiovascular disease risk factors: the Sleep Heart Health Study. Am J Epidemiol. 2001;154:50–59. doi: 10.1093/aje/154.1.50. [DOI] [PubMed] [Google Scholar]
- 10.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 11.Endeshaw YW, Bloom HL, Bliwise DL. Sleep-disordered breathing and cardiovascular disease in the Bay Area Sleep Cohort. Sleep. 2008;31:563–8. doi: 10.1093/sleep/31.4.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehra R, Stone KL, Blackwell T, et al. Prevalence and correlates of sleep-disordered breathing in older men: osteoporotic fractures in men sleep study. J Am Geriatr Soc. 2007;55:1356–64. doi: 10.1111/j.1532-5415.2007.01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dement WC, Miles LE, Bliwise DL. Physiological markers of aging: human sleep pattern changes. In: Reff ME, Schneider EL, editors. Biological Markers of Aging. Washington, DC: U.S. Department of Health and Human Services; Apr, 1982. pp. 177–87. (NIH Publication 82-2221) [Google Scholar]
- 14.Kannel WB, Hubert H. Vital capacity as a biomarker of aging. In: Reff ME, Schneider EL, editors. Biological Markers of Aging. Washington, DC: U.S. Department of Health and Human Services; Apr, 1982. pp. 145–160. (NIH Publication 82-2221) [Google Scholar]
- 15.Sehl ME, Yates FE. Kinetics of human aging: I. rates of senescence between ages 30 and 70 years in healthy people. J Gerontol: Biol Sci. 2001;56A:B198–B208. doi: 10.1093/gerona/56.5.b198. [DOI] [PubMed] [Google Scholar]
- 16.Morris JF, Koski A, Johnson LC. Spirometric standards for healthy nonsmoking adults. Am Rev Respir Dis. 1971;103:57–67. doi: 10.1164/arrd.1971.103.1.57. [DOI] [PubMed] [Google Scholar]
- 17.Dhar S, Shastri SR, Lenora RAK. Aging and the respiratory system. Med Clin North Am. 1976;60:1121–139. doi: 10.1016/s0025-7125(16)31871-5. [DOI] [PubMed] [Google Scholar]
- 18.Smith WDF, Cunningham DA, Patterson, et al. Forced expiratory volume, height and demispan in Canadian men and women aged 55–86. J Gerontol: Med Sci. 1992;47:M40–44. doi: 10.1093/geronj/47.2.m40. [DOI] [PubMed] [Google Scholar]
- 19.Bliwise DL, Bliwise NG, Partinen M, et al. Sleep apnea and mortality in an aged cohort. Am J Public Health. 1988;78:544–7. doi: 10.2105/ajph.78.5.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bliwise DL, Feldman DE, Bliwise NG, et al. Risk factors for sleep disordered breathing in heterogeneous geriatric populations. J Amer Geriatr Soc. 1987;35:132–41. doi: 10.1111/j.1532-5415.1987.tb01342.x. [DOI] [PubMed] [Google Scholar]
- 21.Bliwise DL. Sleep and aging. In: Pressman MR, Orr WC, editors. Understanding Sleep: The Evaluation and Treatment of Sleep Disorders. Washington D.C: American Psychological Association; 1997. pp. 441–464. [Google Scholar]
- 22.Bliwise D, Bliwise NG, Kraemer HC, et al. Measurement error in visually scored electrophysiological data: respiration during sleep. J Neurosci Method. 1984;12:49–56. doi: 10.1016/0165-0270(84)90047-5. [DOI] [PubMed] [Google Scholar]
- 23.American Thoracic Society ATS. Statement—Snowbird Workshop on Standardization of Spirometry. Am Rev Respir Dis. 1979;119:831–38. doi: 10.1164/arrd.1979.119.5.831. [DOI] [PubMed] [Google Scholar]
- 24.Knudson RJ, Lebowitz MD, Holberg CJ, et al. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127:725–34. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- 25.American Thoracic Society. Standardization of spirometry—1987 update. Am Rev Respir Dis. 1987;136:1285–98. doi: 10.1164/ajrccm/136.5.1285. [DOI] [PubMed] [Google Scholar]
- 26.Sanders MH, Martin RJ, Pennock BE, et al. The detection of sleep apnea in the awake patient: the “sawtooth” sign. JAMA. 1981;243:2414–8. [PubMed] [Google Scholar]
- 27.Katz I, Zamel N, Slutsky AS, et al. An evaluation of flow-volume curves as a screening test for obstructive sleep apnea. Chest. 1990;98:337–40. doi: 10.1378/chest.98.2.337. [DOI] [PubMed] [Google Scholar]
- 28.Brown IG, Zamel N, Hoffstein V. Pharyngeal cross-sectional area in normal men and women. J Appl Physiol. 1986;61:890–95. doi: 10.1152/jappl.1986.61.3.890. [DOI] [PubMed] [Google Scholar]
- 29.Martin SE, Marthur R, Marshall I, et al. The effects of age, sex, obesity and posture on upper airway size. Eur Respir J. 1997;10:2087–90. doi: 10.1183/09031936.97.10092087. [DOI] [PubMed] [Google Scholar]
- 30.McGinty D, Littner M, Beahm E, et al. Sleep related breathing disorders in older men: a search for underlying mechanisms. Neurobiol Aging. 1982;3:337–50. doi: 10.1016/0197-4580(82)90022-7. [DOI] [PubMed] [Google Scholar]
- 31.White DF, Lombard RM, Cadieux RJ, et al. Pharyngeal resistance in normal humans: influence of gender, age and obesity. J Appl Physiol. 1985;58:365–71. doi: 10.1152/jappl.1985.58.2.365. [DOI] [PubMed] [Google Scholar]
- 32.Brooks LJ, Byard PJ, Helms RC, et al. Relationship between lung volume and tracheal area as assessed by acoustic reflection. J Appl Physiol. 1988;64:1050–4. doi: 10.1152/jappl.1988.64.3.1050. [DOI] [PubMed] [Google Scholar]
- 33.Series F, Cormier Y, Lampron N, et al. Influence of lung volume in sleep apnoea. Thorax. 1989;44:52–7. doi: 10.1136/thx.44.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Series F, Cormier Y, Desmeules M. Influence of passive changes of lung volume on upper airways. J Appl Physiol. 1990;68:2159–64. doi: 10.1152/jappl.1990.68.5.2159. [DOI] [PubMed] [Google Scholar]
- 35.Series F, Marc I. Influence of lung volume dependence of upper airway resistance during continuous negative airway pressure. J Appl Physiol. 1994;77:840–44. doi: 10.1152/jappl.1994.77.2.840. [DOI] [PubMed] [Google Scholar]
- 36.Stauffer JL, White DP, Zwillich CW. Pulmonary function in obstructive sleep apnea: relationships to pharyngeal resistance and cross-sectional area. Chest. 1990;97:302–07. doi: 10.1378/chest.97.2.302. [DOI] [PubMed] [Google Scholar]
- 37.Stanchina ML, Malhotra A, Fogel RB, et al. The influence of lung volume on pharyngeal mechanics, collapsibility, and genioglossus muscle activation during sleep. Sleep. 2003;26:851–6. doi: 10.1093/sleep/26.7.851. [DOI] [PubMed] [Google Scholar]
- 38.Van de Graaff WB. Thoracic influence on upper airway patency. J Appl Physiol. 1988;65:2124–31. doi: 10.1152/jappl.1988.65.5.2124. [DOI] [PubMed] [Google Scholar]
- 39.Pack AI, Millman RP. Changes in control of ventilation, awake and asleep, in the elderly. J Am Geriatr Soc. 1986;34:533–44. doi: 10.1111/j.1532-5415.1986.tb04247.x. [DOI] [PubMed] [Google Scholar]
- 40.Hoffstein V, Oliver Z. Pulmonary function and sleep apnea. Sleep Breath. 2003;7:159–165. doi: 10.1007/s11325-003-0159-8. [DOI] [PubMed] [Google Scholar]
- 41.Phillips BA, Berry DT, Lipke-Molby TC. Sleep-disordered breathing in healthy, aged persons. Fifth and final year follow-up. Chest. 1996;110:654–8. doi: 10.1378/chest.110.3.654. [DOI] [PubMed] [Google Scholar]
- 42.Phillips BA, Berry DTR, Schmitt FA, et al. Sleep-disordered breathing in healthy aged persons: two- and three-year follow-up. Sleep. 1994;17:411–15. doi: 10.1093/sleep/17.5.411. [DOI] [PubMed] [Google Scholar]
- 43.Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 44.Newman AB, Foster G, Givelber R, et al. Progression and regression of sleep-disordered breathing with changes in weight. Arch Intern Med. 2005;165:2408–13. doi: 10.1001/archinte.165.20.2408. [DOI] [PubMed] [Google Scholar]
- 45.Peppard PE, Young T, Palta M, et al. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015–21. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 46.Redline S, Schluchter MD, Larkin EK, et al. Predictors of longitudinal change in sleep-disordered breathing in a nonclinic population. Sleep. 2003;26:703–9. doi: 10.1093/sleep/26.6.703. [DOI] [PubMed] [Google Scholar]
- 47.Tishler PV, Larkin EK, Schluchter MD, et al. Incidence of sleep-disordered breathing in an urban adult population; the relative importance of risk factors in the development of sleep-disordered breathing. JAMA. 2003;289:2230–37. doi: 10.1001/jama.289.17.2230. [DOI] [PubMed] [Google Scholar]
- 48.Ancoli-Israel S, Kripke DF, Klauber MR, et al. Natural history of sleep disordered breathing in community dwelling elderly. Sleep. 1993;16:S25–29. doi: 10.1093/sleep/16.suppl_8.s25. [DOI] [PubMed] [Google Scholar]
- 49.Ancoli-Israel S, Gehrman P, Kripke DF, et al. Long-term follow-up of sleep disordered breathing in older adults. Sleep Med. 2001;2:511–16. doi: 10.1016/s1389-9457(00)00096-4. [DOI] [PubMed] [Google Scholar]
- 50.Giebelhaus V, Strohl KP, Lormes W, et al. Physical exercise as an adjunct therapy in sleep apnea—an open trial. Sleep Breath. 2000;4:173–6. doi: 10.1007/s11325-000-0173-z. [DOI] [PubMed] [Google Scholar]
- 51.Norman JF, Von Essen SG, Fuchs RH, et al. Exercise training effect on obstructive sleep apnea syndrome. Sleep Res Online. 2000;3:121–9. [PubMed] [Google Scholar]
- 52.Peppard PE, Young T. Exercise and sleep disordered breathing: an association independent of body habitus. Sleep. 2004;27:480–4. doi: 10.1093/sleep/27.3.480. [DOI] [PubMed] [Google Scholar]