Abstract
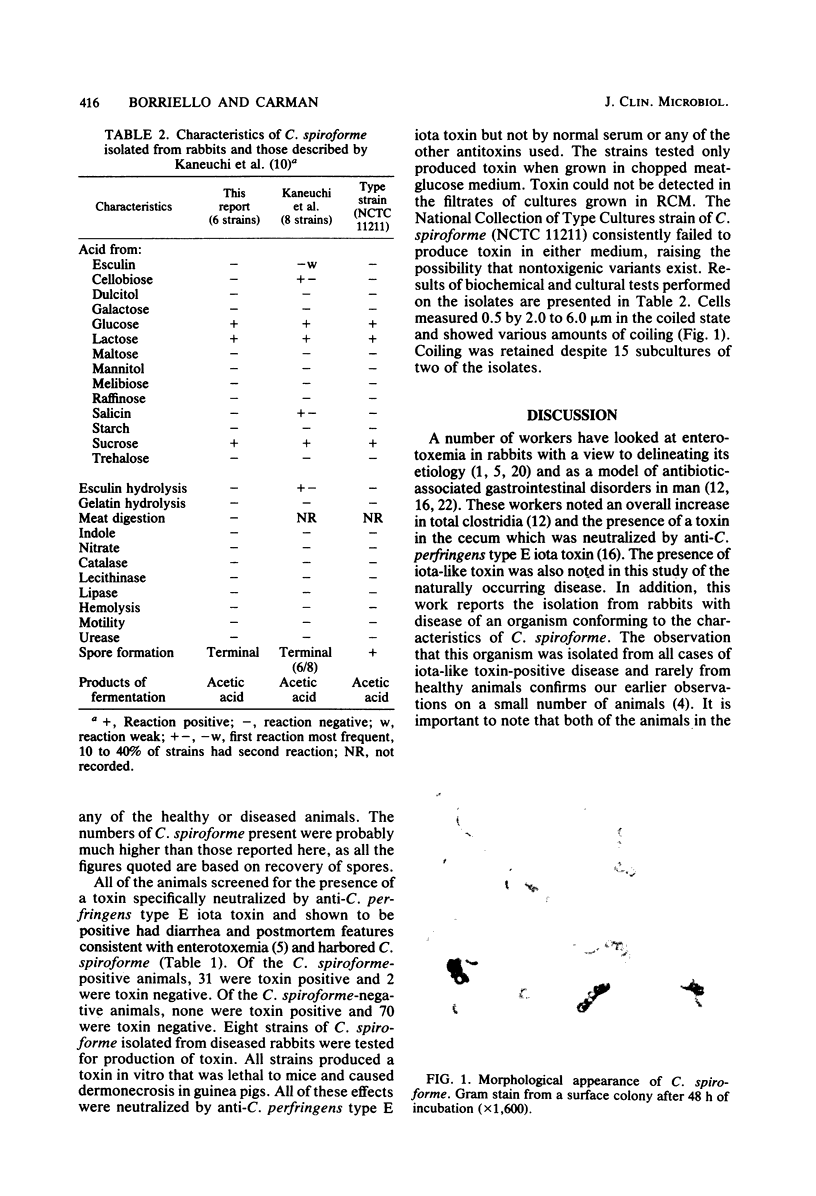
A helically coiled, anaerobic, gram-positive sporeforming bacillus, identified as Clostridium spiroforme, was isolated from the cecal contents of all of 27 rabbits with spontaneous diarrhea, at a mean concentration of 10(6.0) spores per g of material. All of these rabbits also had a toxin present in their cecal contents that was neutralized by anti-Clostridium perfringens type E iota toxin, but not by other clostridial antitoxins. In addition, four rabbits with clindamycin-associated colitis were positive for C. spiroforme at a mean concentration of 10(4.5). All of these animals also had iota-like toxin present. Iota-like toxin was not detected in the cecal contents of 72 healthy animals, although C. spiroforme was found in two of these animals at a mean concentration of 10(6.0). C. spiroforme was shown to produce a toxin in vitro that was lethal to mice and caused dermonecrosis in guinea pigs. In all cases, this toxin was neutralized by anti-C. perfringens type E iota toxin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLADEN H. A., NYLEN M. U., FITZGERALD R. J. INTERNAL STRUCTURES OF A EUBACTERIUM SP. DEMONSTRATED BY THE NEGATIVE STAINING TECHNIQUE. J Bacteriol. 1964 Sep;88:763–770. doi: 10.1128/jb.88.3.763-770.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskerville M., Wood M., Seamer J. H. Clostridium perfringens type E enterotoxaemia in rabbits. Vet Rec. 1980 Jul 5;107(1):18–19. doi: 10.1136/vr.107.1.18. [DOI] [PubMed] [Google Scholar]
- Borriello S. P., Honour P. Simplified procedure for the routine isolation of Clostridium difficile from faeces. J Clin Pathol. 1981 Oct;34(10):1124–1127. doi: 10.1136/jcp.34.10.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton P., Fernie D. S. Enterotoxaemia involving Clostridium perfringens iota toxin in a hysterectomy-derived rabbit colony. Lab Anim. 1980 Oct;14(4):347–351. doi: 10.1258/002367780781071049. [DOI] [PubMed] [Google Scholar]
- Fitzgerald R. J., McBride J. A., Jordon H. V. Helically coiled micro-organism from caecum contents of the rat. Nature. 1965 Mar 13;205(976):1133–1134. doi: 10.1038/2051133a0. [DOI] [PubMed] [Google Scholar]
- Gordon J. H., Dubos R. The anaerobic bacterial flora of the mouse cecum. J Exp Med. 1970 Aug 1;132(2):251–260. doi: 10.1084/jem.132.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazenberg M. P., Custers-van Lieshout L. M., Engels W., Kock-van Dalen A. C. The clostridial flora of conventional mice. Z Versuchstierkd. 1977;19(3):167–174. [PubMed] [Google Scholar]
- Katz L., LaMont J. T., Trier J. S., Sonnenblick E. B., Rothman S. W., Broitman S. A., Rieth S. Experimental clindamycin-associated colitis in rabbits. Evidence of toxin-mediated mucosal damage. Gastroenterology. 1978 Feb;74(2 Pt 1):246–252. [PubMed] [Google Scholar]
- Koopman J. P., Kennis H. M. Differentiation of bacteria isolated from mouse ceca. Z Versuchstierkd. 1977;19(3):174–181. [PubMed] [Google Scholar]
- Koopman J. P., van Oeveren J. P., Janssen F. G. Use of combusted natural gas to cultivate the anaerobic bacterial flora from the cecum contents of mice. Appl Microbiol. 1973 Oct;26(4):584–588. doi: 10.1128/am.26.4.584-588.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koransky J. R., Allen S. D., Dowell V. R., Jr Use of ethanol for selective isolation of sporeforming microorganisms. Appl Environ Microbiol. 1978 Apr;35(4):762–765. doi: 10.1128/aem.35.4.762-765.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMont J. T., Sonnenblick E. B., Rothman S. Role of clostridial toxin in the pathogenesis of clindamycin colitis in rabbits. Gastroenterology. 1979 Feb;76(2):356–361. [PubMed] [Google Scholar]
- Larson H. E., Price A. B., Borriello S. P. Epidemiology of experimental enterocecitis due to Clostridium difficile. J Infect Dis. 1980 Sep;142(3):408–413. doi: 10.1093/infdis/142.3.408. [DOI] [PubMed] [Google Scholar]
- Lawrence G., Shann F., Freestone D. S., Walker P. D. Prevention of necrotising enteritis in Papua New Guinea by active immunisation. Lancet. 1979 Feb 3;1(8110):227–230. doi: 10.1016/s0140-6736(79)90764-5. [DOI] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl Microbiol. 1974 May;27(5):961–979. doi: 10.1128/am.27.5.961-979.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton N. M., Holmes H. T., Riggs R. J., Cheeke P. R. Enterotoxemia in rabbits. Lab Anim Sci. 1978 Oct;28(5):536–540. [PubMed] [Google Scholar]
- Pollock H. M., Rintala L. Unusual coiled gram-positive anaerobe isolated from a gutter wall abscess. J Clin Microbiol. 1977 Dec;6(6):642–644. doi: 10.1128/jcm.6.6.642-644.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehg J. E., Pakes S. P. Implication of Clostridium difficile and Clostridium perfringens iota toxins in experimental lincomycin-associated colitis of rabbits. Lab Anim Sci. 1982 Jun;32(3):253–257. [PubMed] [Google Scholar]
- Wensinck F., Ruseler-van Embden J. G. The intestinal flora of colonization-resistant mice. J Hyg (Lond) 1971 Sep;69(3):413–421. doi: 10.1017/s0022172400021665. [DOI] [PMC free article] [PubMed] [Google Scholar]