Abstract
Wolbachia bacteria in mosquitoes induce cytoplasmic incompatibility (CI), where sperm from Wolbachia-infected males can produce inviable progeny. The wPip strain in the Culex pipiens group of mosquitoes produces a complexity of CI crossing types. Several factors are thought to be capable of influencing the expression of CI including Wolbachia strain type and host genotype. In this study, the unidirectional CI that occurs between 2 C. pipiens complex laboratory strains, Col and Mol, was further investigated by nuclear genotype introgression. The unidirectional CI between Col and Mol was not found to be influenced by host genetic background, in contrast to a previous introgression study carried out using bidirectionally incompatible C. pipiens group strains. A line containing both wPip strain variants superinfection was also generated by embryonic cytoplasmic transfer. The same crossing type as the parental Col strain was observed in the superinfected line. Quantitative polymerase chain reaction demonstrated a low density of the injected wPipMol variant in the superinfected line after 18 generations, which was considered likely to be responsible for the crossing patterns observed. The Wolbachia density was also shown to be lower in the parental Mol strain males compared with Col strain males, and no inverse relationship between WO phage and Wolbachia density could be detected.
Keywords: cytoplasmic incompatibility, Wolbachia
Wolbachia are gram-negative endosymbiotic bacteria that infect numerous invertebrates including insects and nematodes. In their insect hosts, Wolbachia are maternally inherited and behave as reproductive parasites by manipulating host reproduction (Werren 1997). Reproductive alterations include parthenogenesis (Stouthamer et al. 1999), feminization (Rousset et al. 1992), and male killing (Hurst et al. 2000). In mosquitoes and many other insects, Wolbachia cause cytoplasmic incompatibility (CI) that results in the generation of inviable progeny when sperm from a Wolbachia-infected male fertilizes an uninfected egg. CI imparts a reproductive advantage to infected females allowing Wolbachia to rapidly invade host populations (Turelli and Hoffmann 1991) to reach very high population frequencies, and Wolbachia therefore has potential for use in pest population replacement strategies (Sinkins and O'Neill 2000).
Wolbachia were first reported in the reproductive tissues of Culex (Cx) pipiens mosquitoes (Hertig and Wolbach 1924), and the association between incompatibility and maternal cytoplasmic inheritance was confirmed using crossing experiments with strains cured of Wolbachia using tetracycline treatment (Yen 1975). The C. pipiens complex of sibling species includes the formally recognized species C. pipiens and Culex quinquefasciatus, the former more temperate in distribution and primarily bird feeding and the latter the main urban vector of human Bancroftian lymphatic filariasis across the tropics, plus an underground autogenous form for which we will use the full species designation Culex molestus following the population genetic study of Fonseca et al. (2004). Almost all C. pipiens complex populations assayed are infected at close to 100% with the wPip strain of Wolbachia (Cornel et al. 2003; Rasgon and Scott 2004). The wPip strain induces unusually complex crossing types in C. pipiens including partial or complete CI that can be unidirectional or bidirectional (e.g., Magnin et al. 1987; O'Neill and Paterson 1992; Guillemaud et al. 1997; Sinkins et al. 2005; Walker et al. 2007), but there is very low genetic variability in Wolbachia between populations (Guillemaud et al. 1997; Sinkins et al. 2005), making this a good system for attempting to elucidate the molecular basis of CI. A successful small field trial or local population eradication by release of incompatible males was conducted in Burma (Laven 1967), although there are questions concerning the sustainability of this control approach. Genetic replacement strategies targeting vector competence could also depend on CI and achieving a better understanding of how complex patterns of incompatibility between infected populations are generated would be important in this respect.
The mechanisms of Wolbachia-induced CI are not well characterized. CI is now generally accepted to involve sperm modification that interferes with the process of karyogamy and a rescue component provided by the Wolbachia present in the egg, which restores normal male and female pronuclear karyogamy in compatible crosses (Werren 1997). There are several factors that are thought to influence the expression of CI in insects including Wolbachia density (Bordenstein et al. 2006), Wolbachia strain type (Sasaki and Ishikawa 2000; Sakamoto et al. 2005), and host genotype (McGraw et al. 2001; Sasaki et al. 2005). The bidirectional CI between the Pel and Bei strains of C. quinquefasciatus was previously shown to be influenced by host modifying effects by introgressing the Pel nuclear genome into a Bei cytoplasmic background (Sinkins et al. 2005). In this comparative study, the unidirectional CI that occurs between 2 wPip-infected C. pipiens complex laboratory strains, Mol (C. molestus from China) and Col (C. quinquefasciatus from Colombia), was investigated using a similar process of nuclear genotype introgression. In addition, a wPip strain variant superinfection was generated for the first time using these 2 mosquito strains as donor and recipient, to investigate the effects on CI/crossing type.
Materials and Methods
Mosquito Colonies and Crossing Experiments
Culex pipiens complex laboratory strains Mol (C. molestus, China) and Col (C. quinquefasciatus, Colombia) were reared using standard mosquito rearing procedures at low larval densities in insectary conditions (26 °C, 70% relative humidity) with a 12:12 h light:dark circadian cycle. Mass crossing experiments were carried out using 50 virgin individuals of each sex. Virgin male and female mosquitoes were obtained through isolation and sexing of pupae. The F1 generation progeny from crosses were analyzed by calculating the percentage of hatched embryos from a minimum of 8 egg rafts, each containing between 50 and 110 eggs per raft, as a measure of the CI phenotype. Female spermathecae were examined for the presence of sperm if the hatch rate was zero to confirm insemination. Unidirectional incompatibility between Mol and Col strains allowed nuclear replacement by crossing Col males to females containing the Mol cytoplasmic background. In the backcrossing experiment, F1 females from the cross between Mol females and Col males were backcrossed with Col males, and offspring females backcrossed with Col males for a further 4 generations. From the third backcross generation, males were crossed with Col females to examine whether crossing type was maintained.
Discrimination of wPip Strain Variants
The Mol and Col strains are infected with the wPip strain of Wolbachia. No sequence polymorphism has been found for ftsZ (Guillemaud et al. 1997) or the highly variable Wolbachia surface protein (wsp) genes for the Wolbachia present in the Mol and Col strains (Sinkins et al. 2005). Further sequence analysis of wPip ankyrin repeat domain (ANK) genes revealed variation in both nucleotide sequence and predicted amino acid sequence for only 2 prophage-associated ANK genes. One of these genes, pk1, shows nucleotide sequence variability between the Col and Mol colonies, and discrimination of the wPipMol variant in the Mol colony from the wPipCol variant in the Col colony was carried out using pk1 primers previously described (Sinkins et al. 2005).
Microinjection of C. pipiens Embryos
Microinjection of Culex embryos was carried out using a method developed for injection of Anopheles embryos (Bossin and Benedict 2005). Donor Mol and recipient Col preblastoderm embryos were aligned against a thin hydrophilic blotting membrane in contact with moist filter paper. Microinjection was carried out under ×100 magnification using a FemtoJet microinjector system (eppendorf) with type II femtotip microinjection needles (eppendorf). A Narishige micromanipulator attached to a Nikon compound microscope was used to manipulate the microinjection needles. After breakage of the needle tip against the membrane, cytoplasm was withdrawn from the donor wPipMol-infected embryos and subsequently injected into the posterior poles of the recipient wPipCol-infected embryos. G0 females were mated to colony Col males and blood fed to establish isofemale lines.
Quantitative Polymerase Chain Reaction
Quantitative polymerase chain reaction (qPCR) analysis was carried out on 5 individual DNA extracts of C. pipiens colony adult mosquitoes. DNA was extracted using a modified version of the Livak buffer method with ethanol precipitation (Collins et al. 1987). Estimation of Wolbachia density was undertaken by comparing in vivo gene copy numbers: ftsZ gene copy counts were used to estimate the total Wolbachia abundance in the DNA extracts. The single copy C. pipiens gene was used to normalize the data, controlling for variation in the amount of DNA extracted or mosquito size. In order to estimate the relative density of the wPipMol variant in the superinfected line compared with the parental Mol strain, relative copy numbers of the wPipMol pk1 gene variant were measured. The pk1 gene is present in 3 of the integrated prophage copies in the wPip (Pel strain) genome sequence (Walker et al. 2007), and copy number was corrected for in the analysis. Bacteriophage WO density was estimated by comparing in vivo copy numbers of the phage capsid orf7 gene (Masui et al. 2000) to ftsZ copy numbers.
Long oligonucleotide standards were designed along with corresponding primer sets, and standards were diluted from 106 copies to 101 copies for each gene for generation of standard curves. Primer sequences were as follows: qS7F CGCAGCTGATCATCCAT, qS7R ATCGCTCCGAATGTAGAGATGCTA (standard = 120 bp); qftsZF TGG TTGGTAAGGTGCAGCAG, qftsZR CCAGTACCACCACCCATACC (ftsZ standard = 102 bp); qpk1MolF ATTTGGCAGCTGGTATTGGA, qpk1MolR CATCGGTGTC TGATTTCGTG (standard = 101bp); qorf7F AAGTAGACCGACTATATGAAATGTTT, qorf7R GCCAAAATATAGACCTGCTTCTG (standard = 98 bp).
The relative quantity of template DNA was estimated using an Opticon 2 Continuous Fluorescence Detection System (GRI) together with QuantiTect SYBR Green PCR Kit (Qiagen, Valencia, CA). Template DNA was PCR amplified using primers at 10 μM, and 1.0 μl of DNA was added in a total volume of 20 μl per reaction. qPCR cycling conditions consisted of an initial denaturation step of 95 °C for 15 min followed by 40 cycles of denaturating at 95 °C (15 s) and primer annealing at 50 °C (30 s). A melting curve was analyzed to check for any nonspecific amplification or primer dimers. Included in the assays were DNA extracts from the Wolbachia-uninfected Pel U colony and sterilized water controls to confirm the absence of contaminating DNA.
Results and Discussion
Crossing and Introgression
Crossing experiments between wPip-infected C. pipiens complex laboratory strains Mol and Col previously revealed unidirectional incompatibility (Walker et al. 2007). Col females mated to Mol males resulted in 0.44 ± 0.19% embryo hatch in contrast to 93.49 ± 1.17% hatch when Mol females mated with Col males. To investigate whether host modifying effects could influence this crossing pattern, as was previously observed in a cross between 2 bidirectionally incompatible Culex strains (Sinkins et al. 2005), 5 generations of backcrossing, introgressing the Col nuclear genotype into a wPipMol-infected cytoplasm expected to result in around 97% Col genotype, was undertaken as shown in Table 1. The resulting line was fully anautogenous (requiring a blood meal for egg laying, unlike the autogenous C. molestus).
Table 1.
The effect of introgression of host genome between Mol (Culex molestus, Beijing, China) and Col (Culex quinquefasciatus, Colombia) laboratory strains on crossing type
| Cross/backcross, female × male |
Progeny name |
Test cross, female × male |
% hatch (no. rafts/embryos) |
|---|---|---|---|
| Mol × Col | F1 | — | — |
| F1 × Col | BCI | — | — |
| BCI × Col | BCII | — | — |
| BCII × Col | BCIII | Col × BCIII | 0 (48/6427) |
| CIII × Col | BCIV | Col × BCIV | 0 (42/5743) |
| BCIV × Col | Col × BCV | 0.65 (22/2780) |
F1 females from the cross between Mol females and Col males were back-crossed with Col males, and offspring females backcrossed with Col males for a further 4 generations. From the third backcross generation (BCIII), males were crossed with Col females and embryo hatch rates counted.
As shown in Table 1, close to complete incompatibility was observed when males from the introgressed line were crossed with Col females, showing no evidence for a host modifying effect in this particular example of CI. Host modifying effects on CI are thus not universal in this complex but dependent on the particular host strain variant combination being introgressed, and this pattern may contribute to the complexity of crossing type variation in the complex.
Introgression and replacement of the Col nuclear genotype into wPipMol-infected cytoplasm also confirm that the interstrain incompatibilities between Mol and Col are not controlled by host nuclear divergence but by Wolbachia. This introgression experiment has in effect created a novel crossing type in C. quinquefasciatus because the C. molestus nuclear genome has been largely replaced. The wPipMol variant would be expected to be able to spread through populations of C. quinquefasciatus of the Col crossing type.
wPip Strain Variant Superinfection
In order to create a line containing both the wPipMol and wPipCol variants, Col embryos were injected with wPipMol-infected preblastoderm embryo cytoplasm; of 350 injected embryos, there was a 15.1% hatch rate and 8.9% survival of injected embryos to eclosion (31/350). To detect the presence of the wPipMol variant in the Col G0 females and resulting isofemale lines, PCR analysis was carried out using primers that were designed to discriminate between variants of the WO prophage-associated pk1 gene present in the 2 wPip strains (Sinkins et al. 2005). An isofemale line, termed wPipCol(wPipMol), was successfully established and maintained.
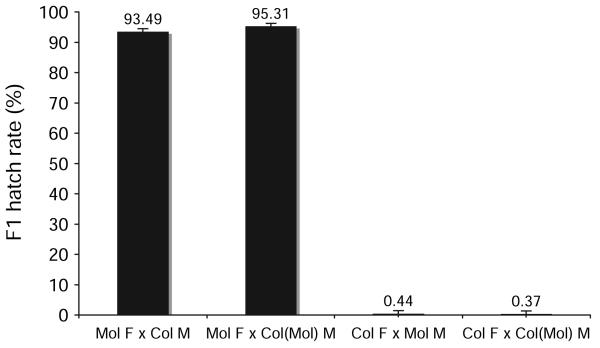
Crossing experiments were undertaken with generation 11 (G11) of the superinfected wPipCol(wPipMol) line to analyze the effect on CI crossing type. PCR analysis for the pk1Mol variant confirmed the presence of the wPipMol variant in all 5 G11 males assayed, suggesting no heterogeneity in the line. As shown in Figure 1, the very low embryo hatch resulting from crossing wPipCol(wPipMol) G11 females to wPipMol males was similar to the low hatch when wPipCol females were crossed to wPipMol males. The presence of the wPipMol variant in wPipCol(wPipMol) females would be expected to result in compatibility with Mol males, based on the model that the females contain the correct “rescue” factor for both singly and superinfected males (Sinkins et al. 1995). However, crossing analysis revealed that the wPipMol variant in females of the superinfected line was unable to rescue the modification factor in sperm produced by wPipMol males. Likewise, crossing the wPipCol(wPipMol)-infected G11 males with wPipCol-infected females produced a high hatch rate, very similar to the hatch seen when wPipMol females were crossed to wPipCol males. Thus, the presence of the wPipMol variant did not produce CI in this cross as had been expected.
Figure 1.
Crossing analysis to determine the effect of the wPipMol variant in the superinfected wPipCol(wPipMol) line. Crossing experiments were performed with 50 virgin males (M) and 50 virgin females (F), and the hatch rate of the F1 progeny was calculated from a minimum of 10 egg rafts, each containing 50–120 eggs per raft, as a measure of CI.
Density of wPip Strain Variants
A possible explanation for the crossing data described is that the wPipMol variant did not reach a sufficiently high density in the superinfected line to be able to induce CI when superinfected males were crossed to Col females or to rescue CI when superinfected females were crossed to Mol males. qPCR density assays were carried out on the G18 generation to allow a stable equilibrium density to be reached. Although all individual G18 males assayed were positive for the wPipMol based on PCR followed by gel electrophoresis, a very low comparative density of the wPipMol pk1 variant was detected by qPCR as shown by the pk1Mol:ftsZ gene ratios, equivalent to 1:250 (Table 2). Although naturally occurring Wolbachia strain superinfections such as that seen in Aedes albopictus can be stable despite significant density differences between strains (Dutton and Sinkins 2004), the density differences between them were lower than those seen here.
Table 2.
qPCR analysis to estimate the wPipMol density in the superinfected wPipCol(wPipMol) line
| Strain/line | Overall wPip density (ftsZ: S7) |
wPipMol relative density (pk1Mol:ftsZ) |
|---|---|---|
| Mol | 0.12 ± 0.04 | 1.278 ± 0.189 |
| Col | 0.39 ± 0.11 | 0 ± 0 |
| wPipCol(wPipMol) G18 | 0.42 ± 0.12 | 0.004 ± 0.001 |
qPCR was carried out on 4 individual DNA extracts of adult Col and Mol male mosquitoes and on 8 adult male mosquitoes of the G18 generation of the wPipCol(wPipMol) line. The overall Wolbachia density was estimated from the ftsZ:S7 gene copy ratio, and the relative density of the wPipMol variant was estimated using the gene copy ratio of pk1Mol:ftsZ.
The quantitative PCR assay is expected to amplify both integrated WO prophage copies of the pk1 genes in the Wolbachia genome and any lytic phage particles containing phage DNA that may be produced from those copies containing pk1. Thus, an alternative explanation is that the WO phage copy or copies containing the pk1 gene in the wPipMol variant may have infected a small subset of the wPipCol variant Wolbachia, rather than the transfer and transmission of whole wPipMol bacteria. There are no other markers outside of the phage regions available that can discriminate between these strains, if indeed any such markers exist. However, we believe this alternative hypothesis to be unlikely, based on a set of inter-Culex microinjection experiments using extracts filtered to exclude whole Wolbachia but not any phage particles, which did not result in any detectable conversions of pk1 type (data not shown). In any case, the same hypothesis would apply whether whole bacteria or just phage particles have in fact been transferred—that the titre reached was probably not high enough for crossing type conversion to occur—and either way the resulting line can be regarded as superinfected.
Wolbachia and Bacteriophage WO Density in Parental Col and Mol Strains
As shown in Table 2, the Wolbachia density in Mol males was significantly lower than for Col males (P = 0.03, Student's t-test). The wPipMol variant may have a slower growth rate compared with wPipCol, and this could be responsible for it being unable to establish itself to a sufficiently high density when indirect intrahost competition with wPipCol. Mol males carrying the lower density wPipMol variant are incompatible with females carrying wPipCol (Walker et al. 2007). This pattern is the opposite of what would be expected if density differences were causally responsible for the different patterns of CI observed between these strains. The density of Wolbachia in C. pipiens testes was also found to be strain dependent and did not appear to influence CI in other C. pipiens strains (Duron et al. 2007).
A threshold level of Wolbachia density (and/or even distribution within the testes) may be required for induction of complete CI. However, if Wolbachia density differences influence the penetrance of CI only when the density falls below a threshold level, the varying wPip densities between the Mol and Col strains may all be above this threshold and therefore irrelevant with respect to CI induction/rescue. However, when the Wolbachia variants are superinfecting the same host, then density of the wPipMol variant may have fallen below this necessary threshold due to effects such as direct competition between strains.
The overall density of bacteriophage WO was also assessed in Col and Mol, measured as a ratio of the phage capsid orf7 gene copy number to Wolbachia ftsZ copy number (because not all prophage copies contain pk1), and was not significantly different between Mol males (8.04 ± 2.42) and Col males (7.07 ± 2.18) (P = 0.43, Student's t-test). The densities of bacteriophage WO in males were not inversely correlated with Wolbachia density (Pearson r = −0.35) with no evidence for increased bacteriophage WO lytic activity in Mol males. These data suggest that the inverse relationship between phage density and Wolbachia density, and tripartite association as an explanation for unidirectional CI presented for Nasonia by Bordenstein et al. (2006), do not apply in this case. The observed phage copy numbers per Wolbachia are broadly consistent with the 5 prophage regions containing the orf7 gene identified in the wPip (Pel strain) genome, implying that bacteriophage WO is primarily temperate rather than lytic in wPip.
The development of intraspecific transfer of wPip strain variants by microinjection, coupled with the development of specific PCR assays to separate these variants and estimate their density (Sinkins et al. 2005; Walker et al. 2007) provide useful tools to investigate the control of CI in the C. pipiens complex. Future experiments can now be contemplated within the Culex genus involving other wPip variants with more similar growth rates/relative densities; the effects of introducing novel Wolbachia strains from other species can also be investigated. Meanwhile, the replacement of host genotype by introgression has provided a novel crossing type in C. quinquefasciatus, furthering understanding of CI in the complex and potentially providing a platform for CI-based mosquito control strategies.
Acknowledgments
Funding
Wellcome Trust (GR079059MA); Royal Society/KC Wong Educational Foundation China Postdoctoral Fellowship to S.S.
References
- Bordenstein SR, Marshall ML, Fry AJ, Kim U, Wernegreen JJ. The tripartite associations between bacteriophage, Wolbachia, and arthropods. PLoS Pathog. 2006;2:e43. doi: 10.1371/journal.ppat.0020043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossin H, Benedict M. MR4 vector component technical manual. Malaria Research and Reference Reagent Resource Center; Manassas (VA): 2005. Microinjection method for anopheles embryos, larval and pupal techniques V2 (2005) [Google Scholar]
- Collins FH, Mendez MA, Rasmussen MO, Mehaffey PC, Besansky NJ, Finnerty V. A ribosomal RNA gene probe differentiates member species of the Anopheles gambiae complex. Am J Trop Med Hyg. 1987;37:37–41. doi: 10.4269/ajtmh.1987.37.37. [DOI] [PubMed] [Google Scholar]
- Cornel AJ, McAbee RD, Rasgon J, Stanich MA, Scott TW, Coetzee M. Differences in extent of genetic introgression between sympatric Culex pipiens and Culex quinquefasciatus (Diptera: Culicidae) in California and South Africa. J Med Entomol. 2003;40:36–51. doi: 10.1603/0022-2585-40.1.36. [DOI] [PubMed] [Google Scholar]
- Duron O, Fort P, Weill M. Influence of aging on cytoplasmic incompatibility, sperm modification and Wolbachia density in Culex pipiens mosquitoes. Heredity. 2007;98:368–374. doi: 10.1038/sj.hdy.6800948. [DOI] [PubMed] [Google Scholar]
- Dutton TJ, Sinkins SP. Strain-specific quantification of Wolbachia density in Aedes albopictus and effects of larval rearing conditions. Insect Mol Biol. 2004;13:317–322. doi: 10.1111/j.0962-1075.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- Fonseca DM, Keyghobadi N, Malcolm CA, Mehmet C, Schaffner F, Mogi M, Fleischer RC, Wilkerson RC. Emerging vectors in the Culex pipiens complex. Science. 2004;303:1535–1538. doi: 10.1126/science.1094247. [DOI] [PubMed] [Google Scholar]
- Guillemaud T, Pasteur N, Rousset F. Contrasting levels of variability between cytoplasmic genomes and incompatibility types in the mosquito Culex pipiens. Proc Biol Sci. 1997;264:245–251. doi: 10.1098/rspb.1997.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertig M, Wolbach S. Studies on rickettsia-like microorganisms in insects. J Med Res. 1924;44:329–374. [PMC free article] [PubMed] [Google Scholar]
- Hurst GD, Johnson AP, Schulenburg JH, Fuyama Y. Male-killing Wolbachia in Drosophila: a temperature-sensitive trait with a threshold bacterial density. Genetics. 2000;156:699–709. doi: 10.1093/genetics/156.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laven H. Eradication of Culex pipiens fatigans through cytoplasmic incompatibility. Nature. 1967;216:383–384. doi: 10.1038/216383a0. [DOI] [PubMed] [Google Scholar]
- Magnin M, Pasteur N, Raymond M. Multiple incompatibilities within populations of Culex pipiens L. in southern France. Genetica. 1987;74:125–130. doi: 10.1007/BF00055223. [DOI] [PubMed] [Google Scholar]
- Masui S, Kamoda S, Sasaki T, Ishikawa H. Distribution and evolution of bacteriophage WO in Wolbachia, the endosymbiont causing sexual alterations in arthropods. J Mol Evol. 2000;51:491–497. doi: 10.1007/s002390010112. [DOI] [PubMed] [Google Scholar]
- McGraw EA, Merritt DJ, Droller JN, O'Neill SL. Wolbachia-mediated sperm modification is dependent on the host genotype in Drosophila. Proc Biol Sci. 2001;268:2565–2570. doi: 10.1098/rspb.2001.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill SL, Paterson HE. Crossing type variability associated with cytoplasmic incompatibility in Australian populations of the mosquito Culex quinquefasciatus Say. Med Vet Entomol. 1992;6:209–216. doi: 10.1111/j.1365-2915.1992.tb00608.x. [DOI] [PubMed] [Google Scholar]
- Rasgon JL, Scott TW. An initial survey for Wolbachia (Rickettsiales: Rickettsiaceae) infections in selected California mosquitoes (Diptera: Culicidae) J Med Entomol. 2004;41:255–257. doi: 10.1603/0022-2585-41.2.255. [DOI] [PubMed] [Google Scholar]
- Rousset F, Bouchon D, Pintureau B, Juchault P, Solignac M. Wolbachia endosymbionts responsible for various alterations of sexuality in arthropods. Proc Biol Sci. 1992;250:91–98. doi: 10.1098/rspb.1992.0135. [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Ishikawa Y, Sasaki T, Kikuyama S, Tatsuki S, Hoshizaki S. Transinfection reveals the crucial importance of Wolbachia genotypes in determining the type of reproductive alteration in the host. Genet Res. 2005;85:205–210. doi: 10.1017/S0016672305007573. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Ishikawa H. Transinfection of Wolbachia in the Mediterranean flour moth, Ephestia kuehniella, by embryonic microinjection. Heredity. 2000;85(Pt 2):130–135. doi: 10.1046/j.1365-2540.2000.00734.x. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Massaki N, Kubo T. Wolbachia variant that induces two distinct reproductive phenotypes in different hosts. Heredity. 2005;95:389–393. doi: 10.1038/sj.hdy.6800737. [DOI] [PubMed] [Google Scholar]
- Sinkins SP, Braig HR, O'Neill SL. Wolbachia superinfections and the expression of cytoplasmic incompatibility. Proc Biol Sci. 1995;261:325–330. doi: 10.1098/rspb.1995.0154. [DOI] [PubMed] [Google Scholar]
- Sinkins SP, O'Neill SL. Wolbachia as a vehicle to modify insect populations. In: Handler AM, James AA, editors. Insect transgenesis: methods and applications. CRC Press; Boca Raton (FL): 2000. pp. 271–288. [Google Scholar]
- Sinkins SP, Walker T, Lynd AR, Steven AR, Makepeace BL, Godfray HC, Parkhill J. Wolbachia variability and host effects on crossing type in Culex mosquitoes. Nature. 2005;436:257–260. doi: 10.1038/nature03629. [DOI] [PubMed] [Google Scholar]
- Stouthamer R, Breeuwer JA, Hurst GD. Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu Rev Microbiol. 1999;53:71–102. doi: 10.1146/annurev.micro.53.1.71. [DOI] [PubMed] [Google Scholar]
- Turelli M, Hoffmann AA. Rapid spread of an inherited incompatibility factor in California Drosophila. Nature. 1991;353:440–442. doi: 10.1038/353440a0. [DOI] [PubMed] [Google Scholar]
- Walker T, Klasson L, Sebaihia M, Sanders MJ, Thomson NR, Parkhill J, Sinkins SP. Ankyrin repeat domain-encoding genes in the wPip strain of Wolbachia from the Culex pipiens group. BMC Biol. 2007;5:39. doi: 10.1186/1741-7007-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren JH. Biology of Wolbachia. Annu Rev Entomol. 1997;42:587–609. doi: 10.1146/annurev.ento.42.1.587. [DOI] [PubMed] [Google Scholar]
- Yen JH. Transovarial transmission of Rickettsia-like microorganisms in mosquitoes. Ann N Y Acad Sci. 1975;266:152–161. doi: 10.1111/j.1749-6632.1975.tb35096.x. [DOI] [PubMed] [Google Scholar]