Abstract
Purpose: Phlebitis caused by intravenous infusion of antineoplastic agents is one of the critical problems when anticancer therapy is prolonged. We have already reported that both rapid infusion and dilution of the injection solution were effective methods for reducing phlebitis caused by vinorelbine (VNR) in rabbits. The aim of this study was to explore other practical methods for preventing phlebitis caused by VNR and doxorubicin (DXR) in a rabbit model. VNR is often used with cisplatin, and dexamethasone (DEX) has been co-administered for prevention of cisplatin-induced nausea. DXR is used with prednisolone (PSL) in the CHOP regimen for the treatment of non-Hodgkin's lymphoma. Therefore, the present study investigated the prevention of phlebitis due to VNR with DEX and that due to DXR with PSL.
Methods: VNR and DXR were diluted with normal saline to prepare test solutions at concentrations of 0.6 mg/mL and 1.4 mg/mL, respectively. Each test solution was infused into the auricular veins of rabbits. Two days after VNR infusion and three days after DXR infusion, the veins were evaluated histopathologically. The effect of DEX on VNR-induced phlebitis was evaluated by infusion of DEX before or after VNR. The effect of PSL on DXR-induced phlebitis was similarly evaluated by co-infusion of PSL.
Results: The histopathological features of phlebitis caused by the antineoplastic agents differed between VNR and DXR: VNR did not cause the loss of venous endothelial cells, but caused inflammatory cell infiltration, edema, and epidermal degeneration. In contrast, DXR caused the loss of venous endothelial cells and chrondrocyte necrosis. Pre-treatment and post-treatment with DEX significantly decreased VNR-induced phlebitis compared with the control group and pre-treatment was particularly effective. Co-infusion of PSL also significantly decreased phlebitis caused by DXR, but its effect was less marked.
Conclusion: The present findings suggested that pre-treatment with DEX may be a useful method for preventing phlebitis due to VNR, and that co-infusion of PSL has the potential to prevent phlebitis caused by DXR.
Keywords: antineoplastic agents, phlebitis, vinca alkaloids, anthracyclines, corticosteroid, rabbit ear vein, vinorelbine, doxorubicin
Introduction
Chemotherapy, including novel antineoplastic agents, is becoming increasingly effective for cancer and is performed widely, but adverse drug reactions are still a critical problem. The completion of chemotherapy regimens is an important factor that determines the prognosis of patients, but it is often the case that treatment is discontinued due to adverse drug reactions. Among such reactions, phlebitis induced by intravenous infusion of antineoplastic agents reduces the completion of chemotherapy. The causative factors of phlebitis include the pH and osmotic pressure of the solution, the size of the vein used, the size and material of the catheter, and the infusion periods 1. A number of methods for avoiding phlebitis have been reported 2, 3, but none are completely effective. Thus, there is an urgent need to develop new methods to prevent and alleviate phlebitis. We already showed that rapid infusion and dilution of VNR are effective for reducing phlebitis 4. In this study, we investigated other practical methods for preventing phlebitis.
VNR is a semi-synthetic vinca alkaloid derived from vinblastine that is used to treat non-small cell lung cancer and breast cancer, and a high incidence of phlebitis (16-33%) after a 6-min infusion of VNR has been reported 5-7. VNR is often used together with cisplatin and DEX, which is given to prevent nausea and vomiting caused by cisplatin. DXR is an anthracycline antineoplastic agent that is widely used to treat non-Hodgkin's lymphoma as one component of the CHOP regimen, along with PSL. It is also known to cause phlebitis after intravenous infusion 3.
Corticosteroids have been suggested to be effective for prevention of phlebitis, due to their anti-inflammatory action 8-11. Tononi et al. reported that post-treatment with DEX reduced phlebitis caused by VNR 12, although they did not show the actual data. Thus, to demonstrate that corticosteroids can prevent the development of phlebitis after anti-cancer chemotherapy, we investigated the effects of DEX and PSL on VNR- and DXR-induced phlebitis, respectively, in a rabbit model.
Materials and Methods
Animals
Male Japanese white rabbits (Std; JW, Japan SLC, Inc., Shizuoka, Japan) weighing from 2.3 to 3.5 kg were housed in individual cages in an animal room maintained at 23 ± 3 °C and 55 ± 10 % relative humidity with ventilation 13-16 times/hr and a 12-hr light-dark cycle. The rabbits were allowed free access to diet and water, except during infusion with the test solutions. This study was approved by the animal experiments committee of Mukogawa Women's University.
Drugs
Navelbine® Injection (VNR) and Adriacin® Injection (DXR) were kindly provided by Kyowa Hakko Kirin Co. (Tokyo, Japan). A 10 mg/mL vial of VNR was diluted with normal saline (Otsuka Normal Saline, Otsuka Pharmaceutical Factory, Inc., Tokushima, Japan) to provide a 0.6 mg/mL solution, while a 10 mg vial of DXR was dissolved and diluted with normal saline to provide a 1.4 mg/mL solution. DEX (Decadron®, 8 mg Banyu Pharmaceutical Co., Tokyo, Japan) and PSL (Predonine®, 20mg Shionogi & Co., Osaka, Japan) were used to prevent VNR- and DXR-induced phlebitis, respectively, after being prepared at a concentration of 0.8 mg/mL and 1 mg/mL in normal saline.
The dose of VNR and DXR were determined at 3 and 4 times the clinical dose from the preliminary experiments as the highest dose that caused phlebitis in all animals without producing other adverse events. As a result, DEX and PSL were administered at 3 and 4 times the clinical dose, respectively.
Experimental procedure
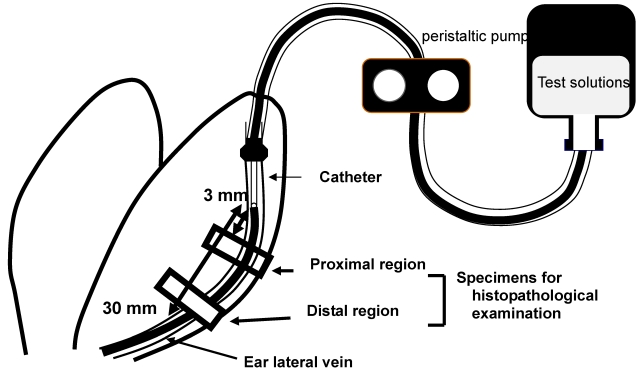
The effects of rapid infusion and dilution of the injection solution on phlebitis caused by VNR was evaluated in a rabbit model, as reported elsewhere 4. Briefly, test solutions of VNR or DXR were infused into both ears of a rabbit to compare different infusion conditions. The rabbits were euthanized with sodium pentobarbital (Nembutal®, Dainippon Sumitomo Pharmaceutical Co., Osaka, Japan) at 2 days after VNR infusion or 3 days after DXR infusion. Two samples of the ear vein were obtained, including the region at 3-10 mm (proximal) and that at 20-30 mm (distal) from the catheter tip, and these were fixed in phosphate-buffered 10% formalin (Figure 1). Cross-sections of the ear vein were cut and stained with hematoxylin and eosin. Histopathological evaluation was performed by a single observer who was blinded to the treatment of the specimen, and the findings were graded with respect to loss of venous endothelial cells, inflammatory cell infiltration, edema, and thrombus, while adding epidermal degeneration 13 that is not included in the criteria of Kuwahara 4.
Figure 1.
Sites of histopathological examination. Two regions of the ear vein, one located at 3-10 mm from the catheter tip (proximal region) and the other located at 20-30 mm from it (distal region), were sampled at 2 or 3 days after VNR or DXR infusion, respectively.
Effect of DEX on VNR-induced phlebitis
A 0.6-mg/mL solution of VNR was infused over 30 min at 5 mL/kg/hr into the ear vein. To clarify the effect of DEX on phlebitis caused by VNR infusion, a 0.8-mg/mL solution of DEX was infused for 15 min at 5 mL/kg/hr just before (pre-treatment) or just after (post-treatment) the infusion of VNR. In the control group, normal saline was infused before and after VNR. Tononi et al. reported that post-treatment with DEX reduced phlebitis caused by VNR, so we tried to clarify the difference in the effect of DEX administration before VNR or after VNR.
Effect of PSL on DXR-induced phlebitis
A 1.4 mg/mL solution of DXR was infused for 120 min at 2 mL/kg/hr with 1-mg/mL of PSL or normal saline (the control group) at 2 mL/kg/hr into the ear vein. In our preliminary experiments, co-administration of PSL exhibited a superior effect compared with pretreatment, so we investigated the efficacy of PSL co-administration in this study.
Statistical analysis
The grade of each histopathological finding was analyzed by the Wilcoxon rank sum test in each experiment, and p<0.05 was considered to indicate a significant difference.
Results
Effect of DEX of VNR-induced phlebitis
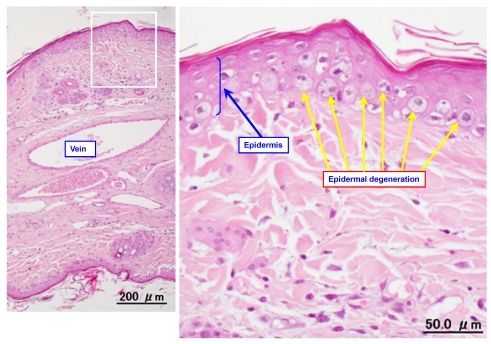
Figure 2 shows a representative photomicrograph of an ear vein after VNR infusion. Table 1 summarizes the histopathological findings after infusion of VNR with or without pre-treatment or post-treatment with DEX. Infusion of a 0.6-mg/mL solution of VNR for 30 min at 5 mL/kg/hr (the control group) caused slight loss of venous endothelial cells (Grade 1) in the proximal part of the vein in 2 out of 8 animals. In addition, there was inflammatory cell infiltration (Grades 1-3) in the proximal part of the vein in all 8 animals and in the distal part of the vein in 7 of the 8 animals. Edema (Grades 1-3) was found in the proximal part of the vein in 6 of the 8 animals and in the distal part of the vein in 7 of the 8 animals. Epidermal degeneration (Grades 1-3) was found in both the proximal and distal parts of the vein in all 8 animals. When infusion of DEX was done before VNR (pre-treatment with DEX), there was slight loss of venous endothelial cells (Grade 1) at the distal region of the vein in 2 of the 8 animals, inflammatory cell infiltration (Grades 1-2) at the proximal region in 2 animals and at the distal region in 1 animal, slight edema (Grade 1) at the proximal region in 1 animal, and epidermal degeneration (Grades 1-2) at both the proximal and distal regions in all 8 animals. When DEX was infused after VNR (post-treatment with DEX), there was inflammatory cell infiltration (Grades 1-3) at the proximal region of the vein in 4 animas and at the distal region in 3 of the 8 animals, edema (Grade 3) at the proximal region in 1 animal and edema (Grade 2) at the proximal region in 2 animals, and epidermal degeneration (Grades 1-3) at the proximal in all 8 animals and at the distal regions in 6 animals. With regard to the loss of venous endothelial cells, neither pre-treatment nor post-treatment with DEX led to a significant difference. With regard to inflammatory cell infiltration, edema, and epidermal degeneration, pre-treatment with DEX reduced these changes significantly at both the proximal and distal regions of the vein. However, post-treatment with DEX had a weaker inhibitory effect on VNR-induced phlebitis than the pre-treatment.
Figure 2.
Typical photomicrographs of an ear vein after VNR infusion. A 0.6-mg/mL solution of VNR was infused into the ear vein for 30 min at 5 mL/kg/hr, and the vein was subjected to pathological examination after 2 days.
Table 1.
Effect of pre-treatment and post-treatment with DEX on the histopathological grade in 8 rabbits at 2 days after VNR infusion.
| Region | Loss of venous endothelial cells | p value | Inflammatory cell infiltration | p value | Edema | p value | Thrombus | p value | Epidermal degeneration | p value | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | ||||||
| Proximal | Control | 6 | 2 | 0 | 0 | 0 | 1 | 3 | 4 | 2 | 3 | 2 | 1 | 8 | 0 | 0 | 0 | 0 | 2 | 4 | 2 | |||||
| Pre-treatment with DEX | 8 | 0 | 0 | 0 | N.S. | 6 | 1 | 1 | 0 | <0.01 | 7 | 1 | 0 | 0 | <0.05 | 8 | 0 | 0 | 0 | N.S. | 0 | 7 | 1 | 0 | <0.05 | |
| Post-treatment with DEX | 8 | 0 | 0 | 0 | N.S. | 4 | 3 | 0 | 1 | <0.01 | 7 | 0 | 0 | 1 | N.S. | 8 | 0 | 0 | 0 | N.S. | 0 | 5 | 1 | 2 | N.S. | |
| Distal | Control | 8 | 0 | 0 | 0 | 1 | 2 | 3 | 2 | 1 | 2 | 2 | 3 | 8 | 0 | 0 | 0 | 0 | 3 | 3 | 2 | |||||
| Pre-treatment with DEX | 6 | 2 | 0 | 0 | N.S. | 7 | 1 | 0 | 0 | <0.01 | 8 | 0 | 0 | 0 | <0.01 | 8 | 0 | 0 | 0 | N.S. | 0 | 8 | 0 | 0 | <0.05 | |
| Post-treatment with DEX | 8 | 0 | 0 | 0 | N.S. | 5 | 0 | 1 | 2 | N.S. | 6 | 0 | 2 | 0 | <0.05 | 8 | 0 | 0 | 0 | N.S. | 2 | 4 | 1 | 1 | N.S. | |
Numbers in the table represent the number of observations. P values show a significant difference from control. N.S.; not significant.
Effect of PSL on DXR-induced phlebitis
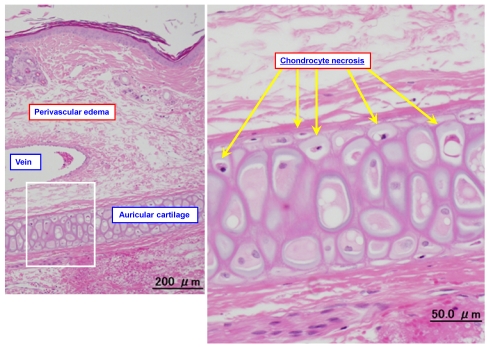
Figure 3 shows representative photomicrograph of an ear vein after DXR infusion. Table 2 summarizes the histopathological findings obtained after infusion of DXR with PSL or normal saline (the control group). Infusion of a 1.4-mg/mL solution of DXR for 120 min at 2 mL/kg/hr with normal saline (the control group) at 2 mL/kg/hr caused the loss of venous endothelial cells (Grades 1-2) at the proximal region of the vein in 3 animals and at the distal region in 5 out of 7 animals, inflammatory cell infiltration (Grades 1-2) at the proximal and distal regions in all 7 animals, and edema (Grades 1-3) at the proximal and distal regions in all 7 animals. No thrombus or epidermal degeneration was found in any of the animals. In addition, chrondrocyte necrosis was observed after treatment with DXR (Figure 3).
Figure 3.
Typical photomicrographs of an ear vein after DXR infusion. A 1.4-mg/mL solution of DXR was infused into the ear vein for 60 min at 2 mL/kg/hr, and the vein was subjected to pathological examination after 3 days.
Table 2.
Effect of co-infusion of PSL on the histopathological grade in 7 rabbits at 3 days after DXR infusion.
| Region | Loss of venous endothelial cells | p value | Inflammatory cell infiltration | p value | Edema | p value | Thrombus | p value | Epidermal degeneration | p value | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | ||||||
| Proximal | with normal saline | 4 | 3 | 0 | 0 | N.S. | 0 | 3 | 4 | 0 | N.S. | 0 | 1 | 3 | 3 | <0.05 | 7 | 0 | 0 | 0 | N.S. | 7 | 0 | 0 | 0 | N.S. |
| with PSL | 6 | 1 | 0 | 0 | 0 | 5 | 1 | 1 | 0 | 4 | 3 | 0 | 7 | 0 | 0 | 0 | 7 | 0 | 0 | 0 | ||||||
| Distal | with normal saline | 2 | 4 | 1 | 0 | N.S. | 0 | 3 | 4 | 0 | N.S. | 0 | 1 | 2 | 4 | N.S. | 7 | 0 | 0 | 0 | N.S. | 7 | 0 | 0 | 0 | N.S. |
| with PSL | 5 | 1 | 1 | 0 | 1 | 2 | 4 | 0 | 1 | 1 | 2 | 3 | 7 | 0 | 0 | 0 | 7 | 0 | 0 | 0 | ||||||
Numbers in the table represent the number of observations. P values indicate a significant difference between the normal saline and PSL groups. N.S.; not significant.
With regard to the loss of venous endothelial cells and inflammatory cell infiltration, co-infusion of PSL tended to reduce the effects of DXR, although the improvement was not statistically significant. A significant difference was only found for edema of the proximal part of the vein. It was suggested that co-infusion of PSL could be tried as a preventive method for DXR-induced phlebitis, but with limited efficacy.
Discussion
The histopathological features of phlebitis differed between VNR and DXR infusion in the present rabbit model. VNR did not cause the loss of venous endothelial cells, which is a common finding in phlebitis 14, but caused inflammatory cell infiltration, edema, and epidermal degeneration. DXR (an anthracycline anticancer agent) caused the loss of venous endothelial cells, and also produced chrondrocyte necrosis. Epirubicin was reported to have the same effects on chrondrocyte necrosis 15, suggesting that these findings may be characteristic of phlebitis caused by anthracyclines. The chrondrocyte necrosis is peculiar to animal experiments using rabbit auricular vein and therefore is not directly reflected in patients, but they suggested that this finding may be a useful and specific index to evaluate perivascular tissue damages caused by this kind of agents 15.
We have already shown that both rapid administration and dilution of the infusion solution is effective for preventing VNR-induced phlebitis 4. In addition, the present study suggested that pre-treatment and post-treatments with DEX were also effective methods. There have been a few reports that administration of steroids is useful for preventing irritation and phlebitis caused by intravenous infusion of hypertonic solutions in animals 16. As mentioned above, there has only been one report that DEX can reduce phlebitis after the infusion of VNR, but without any evidence to support this claim 12. Therefore, this is the first study to show that treatment with DEX is effective for preventing phlebitis caused by the infusion of VNR. In addition, our data suggested that the pre-treatment with DEX was more effective than post-treatment. Although the underlying mechanism by which DEX prevents phlebitis is not clear, it might be related to the well-known anti-inflammatory effects of steroids. However, further studies will be needed to determine this.
We also investigated the effect of PSL on DXR-induced phlebitis. Co-infusion of PSL caused a decrease in the grade of phlebitis due to DXR. Both DXR and PSL are used to treat non-Hodgkin's lymphoma in the CHOP regimen, so co-infusion of PSL could be a practical method for preventing DXR-induced phlebitis, albeit with minimal efficacy.
In conclusion, the histological features of phlebitis caused by antineoplastic agents differ between VNR and DXR. Our data suggested that pre-treatment with DEX was a useful preventive method for VNR-induced phlebitis, and that co-infusion of PSL could potentially prevent phlebitis caused by DXR.
References
- 1.Kuwahara T, Asanami S, Kubo S. Experimental infusion phlebitis: tolerance osmolality of peripheral venous endothelial cells. Nutrition. 1998;14:496–501. doi: 10.1016/s0899-9007(98)00037-9. [DOI] [PubMed] [Google Scholar]
- 2.Nakayama S, Matsubara N, Sakai T. et al. The incidence of phlebitis in patients administered vinorelbine by intravenous bolus injection--a retrospective study. Gan To Kagaku Ryoho. 2002;29:633–635. [PubMed] [Google Scholar]
- 3.Curran CF, Luce JK, Page JA. Doxorubicin-associated flare reactions. Oncol Nurs Forum. 1990;17:387–389. [PubMed] [Google Scholar]
- 4.Kohno E, Murase S, Nishikata M. et al. Methods of preventing vinorelbine-induced phlebitis: an experimental study in rabbits. Int J Med Sci. 2008;5:218–223. doi: 10.7150/ijms.5.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoh K, Niho S, Goto K. et al. Randomized trial of drip infusion versus bolus injection of vinorelbine for the control of local venous toxicity. Lung Cancer. 2007;55:337–341. doi: 10.1016/j.lungcan.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 6.de Lemos ML. Vinorelbine and venous irritation: optimal parenteral administration. J Oncol Pharm Pract. 2005;11:79–81. doi: 10.1191/1078155205jp146oa. [DOI] [PubMed] [Google Scholar]
- 7.Yoh K, Niho S, Goto K. et al. High body mass index correlates with increased risk of venous irritation by vinorelbine infusion. Jpn J Clin Oncol. 2004;34:206–209. doi: 10.1093/jjco/hyh029. [DOI] [PubMed] [Google Scholar]
- 8.Polak A. Hydrocortisone in the prevention of transfusion thrombophlebitis. Lancet. 1956;270:484–485. doi: 10.1016/s0140-6736(56)90538-4. [DOI] [PubMed] [Google Scholar]
- 9.Kohlhardt S. Heparin, hydrocortisone, and prevention of peripheral infusion thrombophlebitis. Nutrition. 1994;10:261–263. [PubMed] [Google Scholar]
- 10.De Cock C, Vermeij P, Stijnen T. On the efficacy of low dose prednisolone and heparin sodium in the prevention of infusion thrombophlebitis. A double-blind trial. Pharm Weekbl Sci. 1984;6:88–90. doi: 10.1007/BF01953960. [DOI] [PubMed] [Google Scholar]
- 11.Suga Y, Kumazaki M, Nishigami J. et al. Improvement of epirubicin-induced phlebitis to switch from liquid preparation to lyophilized formulation. Gan To Kagaku Ryoho. 2009;36:93–96. [PubMed] [Google Scholar]
- 12.Tononi A, Panzini I, Oliverio G. et al. Vinorelbine chemotherapy in non small cell lung cancer: experience in elderly patients. J Chemother. 1997;9:304–308. doi: 10.1179/joc.1997.9.4.304. [DOI] [PubMed] [Google Scholar]
- 13.Kuwahara T, Asanami S, Tamura T. et al. Effects of pH and osmolality on phlebitic potential of infusion solution for peripheral parenteral nutrition. J Toxicol Sci. 1998;23:77–85. doi: 10.2131/jts.23.77. [DOI] [PubMed] [Google Scholar]
- 14.Fonkalsrud EW, Murphy J, Smith FG Jr. Effect of pH in glucose infusions on development of thrombophlebitis. J Surg Res. 1968;8:539. doi: 10.1016/0022-4804(68)90005-x. [DOI] [PubMed] [Google Scholar]
- 15.Anami S, Nishikata M, Matsuyama K. et al. Rapid infusion or dilution is effective in reducing phlebitis caused by epirubicin injection: experimental study in rabbits. Asian J Pharm Sci. in press. [Google Scholar]
- 16.Dubick MA, Wade CE. Evaluation of the local irritation potential of hypertonic saline-dextran (HSD) in mice and rabbits. J Appl Toxicol. 2004;24:409–413. doi: 10.1002/jat.943. [DOI] [PubMed] [Google Scholar]