Abstract
CD22 is a member of the B cell receptor family and is implicated in B cell function and development. It is expressed on multiple forms of B cell lymphoma and is an attractive cancer therapeutic target. We report here the identification of two fully human anti-CD22 antibodies using phage display methodology. Both antibodies exhibit specific binding to cell surface-associated CD22 in multiple B cell lines. Through ELISA using mammalian cell-expressed sub-domains of CD22 as binding antigen, we mapped the binding epitopes of the newly identified CD22 antibodies to be within the Ig-like domains 5 to 7 of CD22. Their epitopes do not overlap with those of several therapeutic antibodies currently in preclinical or clinical development. These antibodies have potential as cancer therapeutic candidates and research reagents.
Key words: human antibody, CD22, phage display, cancer, hematological
Introduction
Antibody therapy has gained popularity in the past decades.1 The predominant success has been with treatment of immunological disorders and cancer,2 particularly hematological malignancies. CD22 is involved in B cell development and signal transduction,3,4 and is among the cell surface antigens that have been identified as molecular targets for antibody cancer therapy. Increased expression of CD22 is seen in non-Hodgkin and other lymphomas.5 Three therapeutic antibody candidates targeting CD22, epratuzumab,6 inotuzumab ozogamicin,7 and the fully recombinant immunotoxin BL22,8 displayed efficacies in patients with B-cell malignacies. The unconjugated epratuzumab studied in clinical trials has the original murine CDRs grafted to a human antibody frame work. Inotuzumab ozogamicin is a humanized version of a murine antibody against the N terminus of CD22.9 The immunotoxins BL22,10,11 and HA22,12 which is an engineered version of BL22 with increased antitumor activity, maintained their murine origin. An improved version of HA22 is in development.13 There has been no report of a fully human anti-CD22 antibody with characteristics that warrant its further development even though such a molecule is highly desirable. We report here the identification and characterization of two fully human CD22 specific antibodies from a naive human Fab phage library. The candidates have apparent nM level affinities against purified CD22, specific binding to cell surface expressed CD22 with affinities in the 10–100 nM range, and target unique epitopes within the Ig-like domains 5 to 7 of CD22. These results suggest that the antibodies might have potential as both research and therapeutic agents.
Results
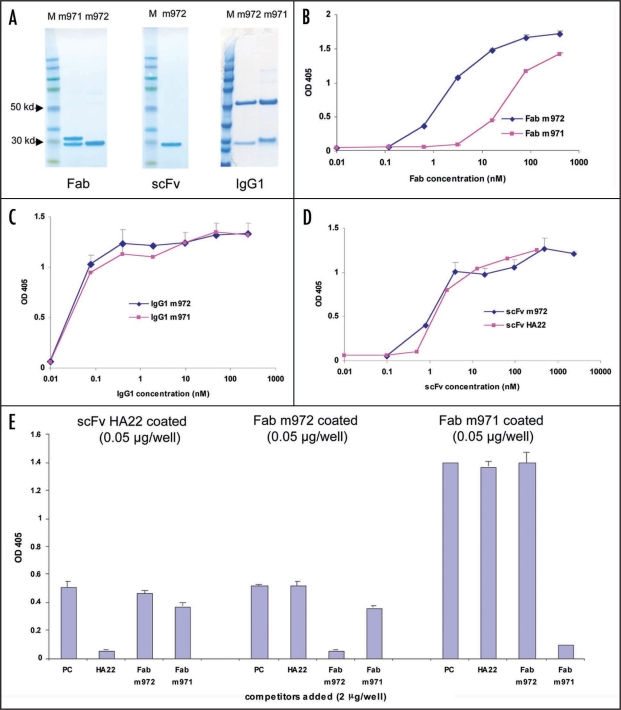
Two CD22-specific Fabs were isolated. Using CD22-Fc as the panning antigen and the plate format, we isolated one dominant clone, m971. Another clone, m972, was isolated in parallel using biotin labeled CD22 as the panning antigen and the streptavidin bead format. They were expressed and purified in Fab, IgG1 and, in the case of m972, scFv format (Fig. 1A). In Fab format, m972 bound CD22 ten times better than m971 with apparent EC50s of 2 and 20 nM respectively (Fig. 1B), although the difference was less significant for their IgG1s (EC50s < 1 nM) (Fig. 1C). This was probably due to the increased valency of IgG1 and the multimeric nature of CD22.15 M972 was further converted into the scFv format; it had a similar binding affinity as its Fab format and binding was comparable to that of scFv HA22 (Fig. 1D). The two Fabs both bound to CD22-Fc, but with different profiles. As measured by ELISA, M971 bound better to CD22-Fc than both m972 and HA22, whereas m972 bound better to its selecting antigen CD22 than m971 (Fig. 1B, D and E). This suggests that soluble CD22 purified from mammalian cells and CD22-Fc expressed from mammalian cells may adopt different conformations. It is not clear at this point if our observation has any correlation with cell surface expressed CD22, even though a report has suggested that CD22 may adopt highly flexible structures in domains 1 and 2.16 When a competition ELISA was performed among the two new Fabs and HA22 scFv, no significant cross competition was detected among them (Fig. 1E). This suggests that the two new antibodies have distinct epitopes on CD22.
Figure 1.
Characterization of the anti-CD22 antibodies in ELISA. The fully human CD22 antibodies in various formats were purified (A) and tested for binding to soluble CD22 in ELISA in their Fab (B), IgG1 (C) and scFv (D) formats. In (A), antibodies in indicated formats were run on a 4–12% gradient SDS-PAGE gel under reducing condition and stained with Coomassie blue. Note that the heavy and light chains of m971 have different molecular weight, whereas the heavy and light chains of m972 ran at the same position. M, molecular weight marker. In (D), only m972 scFv was tested in comparison to the established HA22 scFv. In (E), competition ELISA was performed. ScFv HA22, Fabs m972 and m971 (50 ng/well) were coated to the ELISA plate and a mixture of CD22-Fc (1 µg/well) and one of the indicated antibodies in either Fab format (for m971 and m972) or scFv format (HA22) was added to each well. PC, no competitors added. The amount of CD22-Fc bound by the coated scFv or Fab was then detected using goat anti-human Fc-IgG-HRP.
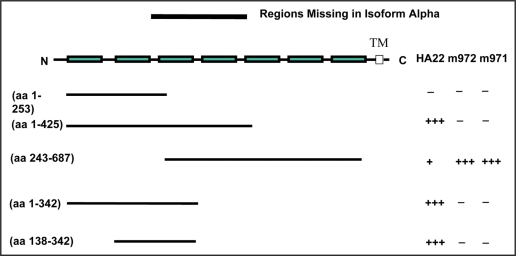
To better characterize the binding epitopes of the two antibodies, we expressed protein fragments containing different Ig-like domains of CD22 and evaluated them in an ELISA. We found that HA22 bound efficiently to CD22 fragments that included both domains 2 and 3, even though very weak binding was also detected against fragments containing only domain 3 (Fig. 2). This suggests that the HA22 epitope resides primarily in domain 3, and domain 2 is important in maintaining the integrity of the epitope. This is in agreement with the published data showing that RFB4, the parental IgG of HA22, competed partially with another mAb that binds to domain 3.17 The two new antibodies reacted only to the region including domains 5 to 7 (Fig. 2). Since this is a rather broad region, it is not surprising that the two new antibodies do not compete with each other (Fig. 1E).
Figure 2.
Epitope determination of the human anti-CD22 antibodies. Sub-domains of CD22 indicated were expressed transiently and the supernatant from transfected cells were added to ELISA plate wells coated with the three antibodies indicated. The bound CD22 domains were detected w/mouse anti-Myc-IgG-HRP. The seven boxes represent the seven Ig- like domains of CD22. Numbers in the brackets indicate the starting and ending amino acid residues of each domain construct. TM, trans-membrane sequence. The symbol “+” indicates a binding that is approximately 10% of a binding repre- sented by the symbol “+++.” “−”, no binding.
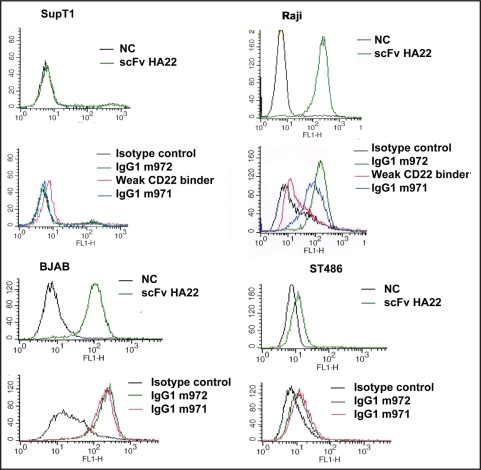
To test whether the new antibodies bind to native, cell-associated CD22 we performed the flow cytometry assay with B lymphocyte cell lines Raji, BJAB and ST486 (Fig. 3). Neither IgG1 bound to the T lymphocyte supT1 cells used as a negative control (Fig. 3). Both antibodies bound specifically as IgG1s to the B cell lines. Specifically, when used at the same concentration (10 µg/ml), m972 bound to Raji cells better than m971. In studies using BJAB and ST486 cells, when twice as much m971 was added (100 µg/ml versus 50 µg/ml of m972), similar bindings were achieved by both (Fig. 3). These data suggest that m972 has a higher affinity to cell surface-associated CD22 compared to m971, and CD22 purified from the mammalian cells is a more accurate representation of cell surface associated CD22 than CD22-Fc fusion proteins, at least when the epitopes of the IgGs are considered. It is noteworthy that the extent to which the two human IgGs bound to the T and B cell lines closely resembled that of HA22. This is another strong indication of specific bindings to cell surface CD22 by these antibodies.
Figure 3.
Flow cytometry detection of the human antibody binding to B lymphocytes. IgG1s m971, m972 and weak binder were used in flow cytometry to test their ability to recognize the native, cell surface-associated CD22. HA22 in its scFv format was used as the positive control. M396, a SARS-spike specific human IgG1 was used as the isotype control. An unrelated scFv was used as a negative control (NC) for HA22. SupT1 cells, which are T lymphocytes and do not have the B cell marker CD22 expressed were used as the negative control cell line. Raji, BAJB and ST486, which are B lymphoma cell lines were used as the testing cell lines. In each histogram, cell count is on the y-axis and the fluorescence intensity is on the x-axis. For each cell line, the upper panel shows the binding by scFv HA22 and the lower panel shows the binding by IgG1s of isolated human antibodies. For experiment with SupT1 and Raji cells, ten µg/ml of each of HA22, m971, m972 and weak CD22 binder were used. For experiment with BJAB and ST486 cells, HA22 concentration remained the same while 100 and 50 µg/ml were used for m971 and m972 respectively.
In studies done in parallel to those for m971 and m972, we isolated another antibody that bound purified CD22 domains 1 + 2. This molecule also recognized CD22-Fc in both Fab and IgG format specifically albeit weakly (data not shown). We named it “weak CD22 binder”. Since it failed to show specific binding to CD22 expressing B cells (Fig. 3), we terminated further investigation. However, the binding pattern of the weak binder did seem to suggest that the domains 1 + 2 region is highly flexible, and significant differences exist between the in vitro-expressed and cell surface-associated CD22 domains 1 + 2.
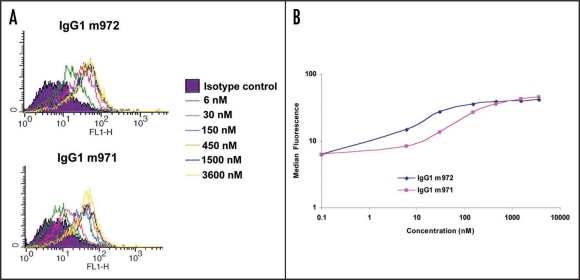
The binding characteristics of the two IgGs to cell surface- associated CD22 were further analyzed by using increasing concentrations of both IgGs against BJAB cells in flow cytometry assay. It is evident that the bindings of both IgGs to BJAB cells were saturable (Fig. 4A). This is another indication of specific binding. IgG1 m972 had an apparent affinity of approximately 15 nM, whereas m971 had an affinity of approximately 75 nM (Fig. 4B). This is in agreement with the flow cytometry data obtained with the other B cell lines showing better bindings by m972. However, the affinities obtained through flow cytometry were lower than those obtained through ELISA. This suggests that differences exist between cell surface-associated CD22 and soluble CD22, despite the fact that soluble CD22 is a better representation than CD22-Fc of the cell surface associated CD22.
Figure 4.
Affinity determination by flow cytometry. Increasing concentrations of both IgG1 m971 and m972 were incubated with BJAB cells in a flow cytometry assay as described in the Methods. (A) the histogram of the flow cytometry. (B) the median fluorescence intensities from the flow cytometry were plotted against the corresponding concentrations used for both IgG1s m971 and m972.
Finally, to assess the new antibodies' ability to down modulate the cell surface CD22, flow cytometry assay with HA22 was performed on Raji cells with or without pretreatment with the new IgG1s. Compared to the efficient internalization and down modulation of cell surface CD22 by HA22,18 only slight downmodulation was detected by IgG1m972. There was a 15% downmodulation of the cell surface CD22 after a one hour incubation with 10 µg/ml of m972, whereas IgG1 m971 and an isotype control IgG1 had no effect under the same experimental conditions (data not shown).
Discussion
We have identified two fully human anti-CD22 antibodies that have low nM apparent affinity in the ELISA assay and bind specifically to the cell surface-expressed CD22 in multiple B cell lines. The epitopes of these antibodies do not overlap with each other or with other CD22 antibodies currently under development for clinical use.
In clinical trials with humanized CD22 antibodies where significant improvement was seen in hairy cell leukemia (HCL) patients, limited numbers of infusion were normally required. Limited dosing might reduce the possible immune response to murine protein sequences in humanized antibodies, thus reducing adverse effects.6 In other types of blood cell cancers such as chronic lymphocytic leukemia (CLL), which is characterized by low surface expression of CD22, the results with anti-CD22 antibodies are not as promising as in HCL, which is characterized by high CD22 surface level.19 Therefore, more frequent infusions of optimal dosages might be necessary for treatment of CLL. Fully human antibodies may have advantages under these circumstances because immunogenicity tends to be reduced for human antibodies compared to humanized or chimeric versions.
Three anti-CD22 antibodies (epratuzumab, inotuzumab ozogamicin and BL22) that have been in clinical trials bind to the N terminus proximal regions of CD22. This area is close to the region through which CD22 interacts with its ligands and a multitude of other cell surface receptors. Due to the complex interaction schemes in different cell lines, CD22 may adopt different conformations and thus expose varying epitopes on different cell surfaces.16 This may lead to inconsistent binding by therapeutic antibodies. The weak binder identified through this study seems to add to the pool of data supporting this hypothesis. Our newly identified anti-CD22 human antibodies bind to non-overlapping epitopes in a region that is closer to the cell membrane. This region is not subjected to alternative splicing or involved in multiple physical and functional interactions with others. Antibodies targeting this region may exhibit more consistent, specific recognition of CD22 from various cell lines or tissues. Our antibodies provide a unique opportunity to test this hypothesis. Because their epitopes do not overlap with each other or with other anti-CD22 antibodies, the antibodies could be used as a combination with or without other anti-cancer drugs.
Epratuzumab is believed to exert its anti-cancer effect through multiple mechanisms including apoptosis, antibody dependent cell-mediated cytotoxicity (ADCC), and alteration of signal transduction.6 It induces rapid CD22 internalization.20 Both inotuzumab ozogamicin and HA22-based immunotoxins rely on rapid internalization to deliver the cytotoxic payload to the cytoplasma of cancer cells. While rapid internalization may be favorable for delivery of cytotoxic agents, it is a disadvantage if ADCC, which requires prolonged presence of antibodies on the cell surface, is a desired mechanism of action. In these preliminary studies, our antibodies showed very limited CD22 down modulation. This suggests that the antibodies may utilize ADCC, complement mediated cell (CDC) lysis and other effector function dependent mechanisms. However, careful evaluation in systemic studies using established protocols will be required to determine which pathways may be used to exert the most significant anticancer effect. These fully human antibodies should have favorable pharmacokinetics and immunogenicity profiles in patients, and should be further explored as promising candidates for treatment of lymphomas, as well as for investigation of the mechanisms of cancer transformation and therapeutic antibody functions.
Materials and Methods
Proteins.
Soluble CD22 was purchased from PeproTech (Rocky Hill, NJ). CD22-Fc was generated in-house. Briefly, the extracellular domain of CD22 protein was expressed as a fusion to human IgG1 Fc using pCDNA1.1-22-Fc as described previously.13 The plasmid was transfected into 293T cells by Lipofectamine reagent (Invitrogen, Carlsbad, CA), and the CD22-Fc protein was harvested from the culture supernatant and purified with a protein A column (GE Healthcare, Piscataway, NJ). For production of CD22 sub-domain proteins, DNA fragments encoding various domains were PCR-amplified and in the process EcoRI and XhoI restriction sites were introduced to the 5 and 3′ ends respectively. The DNA fragments were then digested with both enzymes and cloned into the pSecTag2C (Invitrogen) expression vector digested with the same two enzymes. Different CD22 domains were expressed by transient transfection into 293T cells using the polyfect transfection agent (Qiagen, Hilden, Germany) according to the manufacturer's protocol. Since the pSecTag vector has the murine kappa chain leader fused to N terminus of the protein expressed from it, the resulting fusion protein would be secreted to the supernatant. In addition, all the expressed CD22 domain proteins had c-Myc and His tags from the vector fused to their C termini. They were used for detection in ELISA and affinity purification. Cell culture supernatant containing the secreted CD22 fragments was used directly in ELISA assay, and purified CD22 fragments were used for selecting antibodies targeting specific CD22 regions. Specifically, the CD22 fragment containing Ig-like domains 1 and 2 was purified using the Ni-NTA-agarose column (Qiagen) for selecting CD22 specific antibodies targeting this region. Briefly, supernatant from 293T cells transfected with pSecTag2C-CD22 (domains 1 + 2) was buffer exchanged to PBS before it was loaded on the Ni-NTA-agarose column for native purification according to the manufacture's protocol. The final product was dialyzed against PBS. CD22 fragment purified as such was used in phage panning subsequently.
Cell lines.
T lymphoma cell line SupT1 and B lymphoma cell lines Raji, BJAB and ST486 were all from ATCC. SupT1 was maintained in RPMI + 10% FBS, whereas all the B cell lines were maintained in RPMI + 20% FBS.
Selection of CD22 antibody.
Two different panning procedures were used to isolate anti-CD22 antibody from a human naive Fab library.14 CD22-Fc and purified CD22 domains 1 + 2 were coated directly to Maxisorp plates (Nunc, Denmark) in PBS buffer at 4°C, o/n for the plate format panning and soluble CD22 was labeled first with EZ-link Sulfo-NHS-LC-Biotin (Pierce, Rockford, IL) for the streptavidin-conjugated magnetic bead format panning. For the plate format, approximately 1012 Fabs displayed on the surface of phage amplified from the large naive library were suspended in PBS with 2% dry milk and applied first to wells coated with irrelevant Fc fusion proteins for pre-absorption in the case of panning against CD22-Fc. After 1 hour at room temperature, phage suspension was transferred to the wells coated with CD22-Fc. After two-hour incubation at room temperature, the wells were washed and the phage was rescued with TG1 cells. For panning against CD22 domains 1 + 2, the pre-incubation step with irrelevant Fc fusion protein was omitted. For the bead format, biotin-labeled CD22 were first incubated with the phage in 1 ml of PBS + 2% dry milk suspension at room temperature for 2 hour. Fifteen µl of Dynabeads MyOne Streptavidin T1 (Invitrogen Dynal AS, Oslo, Norway) pre-blocked with PBS + 2% dry milk was added to the antigen/phage mixture for another one hour at room temperature. The beads were then washed and phage was rescued with TG1 cells. A total of four rounds were performed for each format. Monoclonal ELISA was then performed to select for positive clones. Two hundred clones were screened from each format. One dominant positive clone from each procedure was finally selected.
Fab, IgG1 and scFv expression and purification.
Plasmids of the positive clones were used to transform the E. coli strain HB2151 for Fab expression. The heavy and light chains of each clone were transferred to the pDR12 vector (kindly provided by Dennis Burton, Scripps Institute, La Jolla, CA) for making the IgG1 format of the two antibodies. To make the scFv format of the antibody, the DNA fragments encoding the heavy and light chains of the clone were linked through an overlapping PCR procedure. The resulting scFv gene had the heavy chain followed by a linker containing amino acids GGGGS, and the light chain. One SfiI restriction site was also introduced during the PCR procedure to each end of the scFv DNA fragment, which was cloned into pCom3x vector (kindly provided by Dennis Burton) digested with SfiI for protein expression. ScFv plasmid was used to transform also the E.coli strain HB2151 for expression. IgG1 plasmids were transiently transfected to 293 free style cells (Invitrogen) for IgG1 production. Fab and scFv were purified using the Ni-NTA agarose bead (Qiagen) and IgG1 with the protein A sepharose column (GE Healthcare, Piscataway, NJ).
ELISA.
CD22 or CD22-Fc in PBS was coated to the 96-well plate at 4°C for overnight. The plate was then washed with PBST (PBS + 0.05% Tween) and blocked with PBST supplemented with 5% dry milk. Fab, scFv or IgG1 diluted in PBST + milk was added to the wells. The Fab and scFv binding was detected using the anti-His-IgG-HRP (Qiagen) and the IgG1 binding was detected using the anti-human Fc-IgG-HRP (Sigma, St. Louis, MO). After washing, ABTS substrate (Roche, Mannheim, Germany) was added to each well and OD405 was recorded after five to ten minutes. For competition ELISA, one antibody was first coated to the ELISA plate. CD22-Fc was mixed with indicated Fabs or scFv in the PBST + 5% milk buffer and added to each well. The bound CD22-Fc was then detected with anti-human Fc-IgG-HRP.
Epitope mapping.
CD22 sub-domains secreted into the culture supernatant were used directly in ELISA assay. Briefly, the testing antibodies and anti-His antibody (Qiagen) were coated to the ELISA plates. Cell culture supernatant (100 µl) containing CD22 fragments with both His and c-Myc tags were added to the pre- coated wells. After washing, anti-C-Myc-IgG-HRP was added to each well to measure the amount of CD22 fragments captured either by anti-His antibody as a measurement of expression of each CD22 fragment, or by testing antibodies as a measurement of their epitopes.
Flow cytometry.
SupT1, Raji, BJAB and ST486 cells in suspension culture were collected by centrifugation and then re-suspended in ice-cold RPMI + 10% FBS medium in a density of 1 × 106/ml. ScFv or IgG1 at different concentrations were added to the cells. Cells were left on ice for half an hour before being washed with the same complete RPMI medium. For scFv HA22, a mixture of mouse anti-His and anti-mouse-IgG-FITC was added to the cells for another half an hour on ice. For IgG1, anti-human-IgG-FITC was added to the cells. The cells were then washed with RPMI medium twice and re-suspended in PBS and subjected to flow cytometry analysis using the FACScalibur (Becton Dickinson, San Jose, CA).
Acknowledgements
This project was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, and by federal funds from the National Cancer Institute, NIH, under contract N01-CO-12400.
Footnotes
Previously published online as a mAbs E-publication: http://www.landesbioscience.com/journals/mabs/article/8113
References
- 1.Reichert JM, Rosensweig CJ, Faden LB, Dewitz MC. Monoclonal antibody successes in the clinic. Nat Biotechnol. 2005;23:1073–1078. doi: 10.1038/nbt0905-1073. [DOI] [PubMed] [Google Scholar]
- 2.Reichert JM, Valge-Archer VE. Outlook—Development trends for monoclonal antibody cancer therapeutics. Nat Rev Drug Discov. 2007;6:349–356. doi: 10.1038/nrd2241. [DOI] [PubMed] [Google Scholar]
- 3.Tedder TF, Tuscano J, Sato S, Kehrl JH. CD22, a B lymphocyte-specific adhesion molecule that regulates antigen receptor signaling. Annu Rev Immunol. 1997;15:481–504. doi: 10.1146/annurev.immunol.15.1.481. [DOI] [PubMed] [Google Scholar]
- 4.Walker JA, Smith KJC. CD22: an inhibitory enigma. Immunology. 2007;123:314–325. doi: 10.1111/j.1365-2567.2007.02752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fanale MA, Younes A. Monoclonal antibodies in the treatment of non-Hodgkin's lymphoma. Drugs. 2007;67:333–350. doi: 10.2165/00003495-200767030-00002. [DOI] [PubMed] [Google Scholar]
- 6.Leonard JP, Goldenberg DM. Preclinical and clinical evaluation of epratuzumab (antiCD22 IgG) in B-cell malignancies. Oncogene. 2007;26:3704–3713. doi: 10.1038/sj.onc.1210370. [DOI] [PubMed] [Google Scholar]
- 7.DiJoseph JF, Dougher MM, Armellino DC, Evans DY, Damle NK. Therapeutic potential of CD22-specific antibody-targeted chemotherapy using inotuzumab ozogamicin (CMC-544) for the treatment of acute lymphoblastic leukemia. Leukemia. 2007;21:2240–2245. doi: 10.1038/sj.leu.2404866. [DOI] [PubMed] [Google Scholar]
- 8.Kreitman RJ, Squires DR, Stetler-Stevenson M, Noel P, Fitzgerald DJP, Wilson WH, Pastan I. Phase I trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with B-Cell malignancies. J Clin Oncol. 2005;23:6719–6729. doi: 10.1200/JCO.2005.11.437. [DOI] [PubMed] [Google Scholar]
- 9.DiJoseph JF, Armellino DC, Khandke K, Boghaert ER, Dougher M, Kunz A, et al. CMC-544: a CD22-targeted immunoconjugate of Calicheamicin for the treatment of non-Hodgkin's lymphoma. Eur J Cancer. 2002;38:150. [Google Scholar]
- 10.Campana D, Janossy G, Bofill M, Trejdosiewicz LK, Ma D, Hoffbrand AV, et al. Human B-Cell Development 1. Phenotypic Differences of Lymphocytes-B in the Bone-Marrow and Peripheral Lymphoid-Tissue. J Immunol. 1985;134:1524–1530. [PubMed] [Google Scholar]
- 11.Decker T, Wagner M, Oelsner M, Kreitman RJ, Pastan I, Peschel C, Licht T. BL22, a recombinant anti-CD22 immunotoxin, induces cell cycle arrest and apoptosis in B-cell lymphoma. Blood. 2004;104:237. [Google Scholar]
- 12.Bang S, Nagata S, Onda M, Kreitman RJ, Pastan I. HA22 (R490A) is a recombinant immunotoxin with increased antitumor activity without an increase in animal toxicity. Clin Cancer Res. 2005;11:1545–1550. doi: 10.1158/1078-0432.CCR-04-1939. [DOI] [PubMed] [Google Scholar]
- 13.Ho M, Kreitman RJ, Onda M, Pastan I. In vitro antibody evolution targeting germline hot spots to increase activity of an anti-CD22 immunotoxin. J Biol Chem. 2005;280:607–617. doi: 10.1074/jbc.M409783200. [DOI] [PubMed] [Google Scholar]
- 14.Zhu ZY, Chakraborti S, He Y, Roberts A, Sheahan T, Xiao XD, et al. Potent cross-reactive neutralization of SARS coronavirus isolates by human monoclonal antibodies. Proc Natl Acad Sci USA. 2007;104:12123–12128. doi: 10.1073/pnas.0701000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han S, Collins BE, Bengtson P, Paulson JC. Homomultimeric complexes of CD22 in B cells revealed by protein-glycan cross-linking. Nat Chem Biol. 2005;1:93–97. doi: 10.1038/nchembio713. [DOI] [PubMed] [Google Scholar]
- 16.Toba K, Hanawa H, Fuse I, Sakaue M, Watanabe K, Uesugi Y, et al. Difference in CD22 molecules in human B cells and basophils. Exp Hematol. 2002;30:205–211. doi: 10.1016/s0301-472x(01)00791-3. [DOI] [PubMed] [Google Scholar]
- 17.Stein R, Belisle E, Hansen HJ, Goldenberg DM. Epitope Specificity of the Anti-(B-Cell Lymphoma) Monoclonal-Antibody, Ll2. Cancer Immunol Immunother. 1993;37:293–298. doi: 10.1007/BF01518451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du X, Beers R, FitzGerald DJ, Pastan I. Differential cellular internalization of antiCD19 and -CD22 immunotoxins results in different cytotoxic activity. Cancer Res. 2008;68:6300–6305. doi: 10.1158/0008-5472.CAN-08-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kreitman RJ, Wilson WH, Bergeron K, Raggio M, Stetler-Stevenson M, FitzGerald DJ, Pastan I. Efficacy of the anti-CD22 recombinant immunotoxin BL22 in chemotherapy-resistant hairy-cell leukemia. N Engl J Med. 2001;345:241–247. doi: 10.1056/NEJM200107263450402. [DOI] [PubMed] [Google Scholar]
- 20.Carnahan J, Wang P, Kendall R, Chen C, Hu S, Boone T, et al. Epratuzumab, a humanized monoclonal antibody targeting CD22: Characterization of in vitro properties. Clin Cancer Res. 2003;9:3982–3990. [PubMed] [Google Scholar]