Abstract
Full-length antibodies and antibodies that ferry a cargo to target cells are desired biopharmaceuticals. We describe the production of full-length IgGs and IgG-toxin fusion proteins in E. coli. In the presented examples of anti CD30 and anti EGF-receptor antibodies, the antibody heavy and light chains or toxin fusions thereof were expressed in separate bacterial cultures, where they accumulated as insoluble inclusion bodies. Following refolding and purification, high yields (up to 50 mg/L of shake flask culture) of highly purified (>90%) full-length antibodies and antibody-toxin fusions were obtained. The bacterially produced antibodies, named “Inclonals,” equaled the performance of the same IgGs that were produced using conventional mammalian cell culture in binding properties as well as in cell killing potency. The rapid and cost effective IgG production process and the high quality of the resultant product may make the bacterial production of full-length IgG and IgG-drug fusion proteins an attractive option for antibody production and a significant contribution to recombinant antibody technology.
Key words: IgG, IgG-toxin fusion protein, CD30, EGFR, PE38, inclusion bodies, refolding
Introduction
Antibodies are among the most powerful tools in biological and biomedical research and are presently the leading category of biopharmaceuticals with annual sales exceeding $20 billion. Currently over 20 therapeutic antibodies are FDA-approved, and hundreds more are in late stages of clinical development.1 Although many formats of recombinant antibodies and antibody fragments populate the pipeline, the antibody market is dominated by full-length IgG antibodies both in research, diagnostic and clinical applications.
Unfortunately, many cancers are resistant to treatment with naked (unarmed) antibodies. Attaching a cytotoxic moiety to the antibody can provide several logs-fold improvement of potency in cell killing efficacy. Immunoconjugates are made by attaching chemotherapy drugs, radioisotopes or toxins to the antibody. Antibody-drug conjugates and antibody-toxin fusion proteins are also making headway in the clinical pipeline.2,3 However, conventional mammalian cell-based IgG production systems are not capable of expressing toxic proteins. Antibody-toxin conjugates were originally made by chemical conjugation that, with a few exceptions, yielded heterogeneous products that contained a mixture of species with different molar ratios of drug to antibody, linked at different sites, each with distinct in vivo pharmacokinetic, efficacy and safety profiles. The unfavorable in vivo effects associated with heterogeneity in the drug load and sites of attachment in antibody-drug conjugates could compromise their promise as cancer therapeutics.4
Full-length monoclonal antibodies have traditionally been produced in mammalian cell culture. However, due to its simplicity and reduced production time and cost, Escherichia coli (E. coli) is the system of choice for the expression of recombinant proteins, including most recombinant antibody derivatives. Early, largely unsuccessful attempts to produce IgGs in bacteria were reported over 20 years ago.5,6 With advances in technology, full-length antibodies were recently obtained in E. coli by directing secretion of the antibody heavy and light chains to the bacterial periplasm.7–9 With regard to E. coli-produced full length IgGs, two main obstacles remained unsolved: first is the purity of the final product that contains partially assembled species, and second is the limited yields, with approximately 1 mg (range of 0.2–1 mg/L9) antibody being produced from 1 liter of low density shake flask cultures.
To overcome these obstacles, we have developed a highly efficient production method for full-length IgG and IgG-toxin fusion proteins in E. coli, named “Inclonals.”
Results
Production of chimeric IgGs in E. coli.
The first model antibody was an anti CD30 antibody, T427.10 T427 Inclonal IgG1 was cloned into the pHAK expression vectors (Supplementary Fig. 1) and produced in E. coli as described in Materials and Methods. Fractions from the purification process are shown in Fig. 1A and B. As shown, a high yield of highly purified preparation of chimeric T427 Inclonal was obtained. From 1 liter of shake flask culture we routinely obtain 100–200 mg of solubilized inclusion body protein. Refolding was initiated after mixing 50 mg of heavy chain and 50 mg of light chain inclusion bodies protein and reducing the mixture with 1,4-dithioerythritol (DTE). After refolding, dialysis and protein-A purification, up to 15 mg of pure (>90% according to densitometry of the SDS gel) IgG were obtained, which correspond to about 45 mg pure IgG per liter of heavy chain E. coli culture.
Figure 1.
Expression and purification of T427 Inclonal in E. coli. (A) 12% SDS/PAGE. Lane 1, un-induced E. coli culture. Lane 2, induced heavy-chain. Lane 3, induced light-chain Lane 4, unpurified refolded IgG. Lane 5, Protein-A purified IgG. M, MW marker, in kDa, Lane 6, cetuximab. Lane 7, protein-A purified T427 Inclonal. Lanes 1–5 were analyzed under reducing conditions while lanes 6–7 were not. Proteins were visualized by staining with GelCode Blue®. (B) Immunoblot using HRP-conjugated anti human antibody and ECL development. The lane arrangement is as in (A), except lane E = cetuximab.
Evaluation of the bacterially produced antibody.
The bacterially produced Inclonal was compared to mammalian-cell produced IgG by gel-filtration chromatography, by measuring stability in serum, by antigen binding properties and by cell killing activity.
An aliquot of the purified IgG was analyzed by gel-filtration chromatography on a TSK3000 column (Fig. 2). As shown, the T427 Inclonal (calculated MW 147,500) eluted from the column as a monomer (free of aggregates). The control mammalian-cell produced mAb cetuximab (MW 151,800) migrates as a slightly larger protein probably due to post-translational modifications (glycosylation) that are absent in our E. coli produced IgG. Cetuximab and the mammalian cell produced chT427 IgG migrate similarly in gel filtration (not shown).
Figure 2.
Analysis of IgGs by gel filtration chromatography. IgG samples were separated on a TSK3000 column. The arrows mark the migration pattern of commercial size markers on the column.
To evaluate the stability of the Inclonal IgG T427 we compared its serum stability in 37°C to that of mammalian-cell produced chimeric T427 IgG (T427 chIgG) that was prepared essentially as described.11 As shown (Fig. 3), the mammalian cell produced chT427 IgG and the T427 Inclonal were equally stable, losing no binding activity over the test period of four days at 37°C.
Figure 3.
Stability in serum. Analysis of the stability of mammalian-cells produced T427 (lower graph) and of the T427 Inclonal (upper graph) upon incubation in bovine serum. IgGs were diluted to a final concentration of 30 µg/ml in 100% bovine serum and incubated at 37°C for the indicated time periods. Residual binding activity to MBP-CD30 of each fraction was evaluated by ELISA as described in materials and methods.
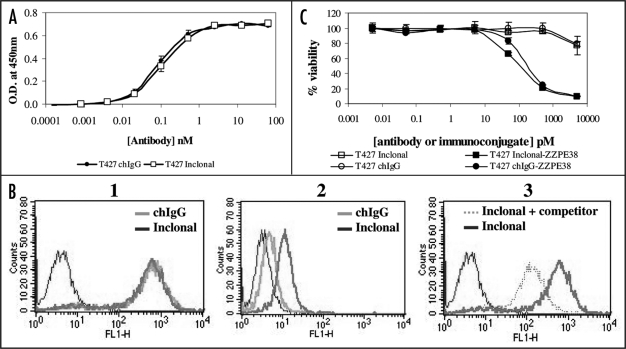
Antigen binding was studied by ELISA and by flow cytometry. As shown (Fig. 4A), the T427 Inclonal bound soluble antigen in ELISA with a similar avidity to the corresponding T427 chIgG that was produced in mammalian cell culture. Similarly, identical binding properties could be observed in the flow cytometry analysis on CD30-expressing cells (Fig. 4B1). Binding specificity could be demonstrated by the competition of the T427 Inclonal binding signal by a T427(dsFv)-PE38 recombinant immunotoxin (prepared as described in Supplementary methods), as shown in (Fig. 4B3).
Figure 4.
Evaluation of T427 Inclonal. Binding properties: (A) Binding to MBP-CD30 in ELISA. Detection is with HRP-conjugated anti human IgG. (B) FACS analysis. (1) Stable A431/CD30 transfected cells were incubated with 10 nM of chT427-IgG made in mammalian cells or with T427 Inclonal. (2) FACS analysis of T427 Inclonal binding in the presence of X30 molar excess of T427(dsFv)-PE38 immunotoxin as competitor. Binding was detected using FITC-conjugated anti human antibody. (C) Specific cytotoxicity of T427-ZZPE38. A431/CD30 cells were incubated for 48 h with the indicated concentration of IgG-ZZPE38 immunoconjugates or the IgGs alone. The relative number of viable cells was determined using an enzymatic MTT assay. Each point represents the mean of a set of data determined in triplicate in three independent experiments. Error bars represent the standard deviation of the data.
The ability of the Inclonal antibodies to target tumor cells in vitro was evaluated by forming a complex with an antibody-binding toxin fusion protein (ZZ-PE38) (as described in reference 11). The cytotoxicity evaluation also revealed that the T427 Inclonal parallels the performance of the mammalian cell produced antibody (Fig. 4C).
As an additional example, we produced an Inclonal derivative of the anti EGF receptor antibody 225. MAb 225 is the parental mouse monoclonal antibody from which the therapeutic antibody cetuximab was derived.12 We compared the 225 Inclonal to cetuximab for antigen binding properties and by for cell killing activity as ZZ-PE38 immunocomplexes. As shown (Supplementary Fig. 5), the 225 Inclonal specifically bound EGFR expressing cells with about ×10 lower avidity than that of cetuximab. Similarly, the 225 Inclonal-ZZ-PE38 immunocomplex had cytotoxic activity on both high EGFR expressing A431 cell line and on low EGFR expressing 293 cell line, which was about ×10 less potent than the cetuximab-ZZ-PE38 immunocomplex. This difference is in accordance with the reported ×10 affinity increase reported for cetuximab in comparison to the 225 mAb.12
Figure 5.
IgG-toxin fusion proteins. (A) Schematic representation of the Inclonals that were produced in this study. (B) Immunoblot of protein-A-purified T427 Inclonals under non-reducing conditions. Lane 1, IgG; Lane 2, IgG-(di)-PE38; Lane 3, IgG-(tetra)-PE38. (C) Immunoblot of protein-A-purified T427 Inclonal-PE38 fusion proteins under reducing conditions. Lane 1, IgG-(di)-PE38; Lane 2, IgG-(tetra)-PE38. M, MW marker, in kDa.
The Inclonal-PE38 fusion proteins production.
By applying the Inclonals technology, we generate full-length IgGs that are genetically fused to a cytotoxic moiety. We prepared PE38 fusion proteins of the T427 Inclonal. Two derivatives were prepared; a T427(di)-PE38 derivative, with PE38 fused to the antibody heavy chain, and T427(tetra)-PE38, with PE38 fused to both the antibody heavy and light chains. The Inclonal-toxin fusion derivatives differ in their molecular weight (∼225 kDa for the di-toxin and ∼300 kDa for the tetra toxin) and in the number of toxin molecules payload delivered for each binding event (Fig. 5A). Both T427(di)-PE38 and T427(tetra)-PE38 were produced at a high purity (Fig. 5B and 5C), and at a high yield, similar to that we obtained for the IgG Inclonals.
Evaluation of the Inclonal-PE38 fusion proteins.
These novel T427(di)-PE38 and T427(tetra)-PE38 proteins were evaluated for their binding properties and for their cell-killing activity. As shown (Fig. 6A), the apparent binding affinity as evaluated from the ELISA signal for both T427(di)-PE38 and T427(tetra)-PE38 is about 0.2 nM, which is similar to that of the T427 Inclonal and T427 chIgG (that are shown in Fig. 4A). Both IgGs bound with an apparent avidity, which was x10 higher than the affinity of the corresponding monovalent recombinant immunotoxin T427(dsFv)-PE38.
Figure 6.
Evaluation of the T427 Inclonal-toxin fusion proteins. (A) Binding to MBP-CD30 in ELISA. Detection is with HRP-conjugated anti human IgG. (B) Specific cytotoxicity: A431/CD30 cells were incubated for 48 h with the indicated concentration of recombinant PE38 fusion proteins. The relative number of viable cells was determined using an enzymatic MTT assay. Each point represents the mean of a set of data determined in triplicate in three independent experiments. Error bars represent the standard deviation of the data.
The cell killing potential of T427(di)-PE38 and T427(tetra)-PE38 Inclonal-toxin fusion proteins was tested on cultured CD30-expressing cells. As shown in Fig. 6B, both molecules inhibited the growth of the target cells with an IC50 of ∼30 pM, while the monovalent immunotoxin T427(dsFv)-PE38 had an IC50 of ∼60 pM.
Discussion
This study demonstrates an expression and purification protocol we developed for producing full-length IgGs and IgG-toxin fusion proteins, by refolding E. coli-produced inclusion bodies of the antibody heavy and light chain. Our modified expression-refolding system enables an effective production of full length IgGs in E. coli. By applying this novel system we successfully obtained two antibodies: the anti-CD30 T427 antibody and the anti-EGFR 225 antibody. The production process of the antibody chains from inclusion bodies revealed high quantity of over 200 mg of relatively pure protein. The entire refolding and purification process yielded up to 50 mg of IgG protein from 1 liter of shake flask culture, yields that were not reported before using bacterial expression systems for IgG production in low density culture. These production yields could benefit research laboratories that, in contrast to industrial laboratories, are generally not equipped with high density fermentors. The second important benefit of this system is the purity of the final product; following protein-A purification, the monomeric form of the antibody is notably the main form that was obtained. The purified protein is almost free of partially assembled species that were observed in previous studies.8,9 The advantage of the E. coli production system in time savings was considerable. The entire process in the mammalian system, (transfection, selection of a highly-expressing clone, expansion of the clone, IgG purification) required about eight weeks, while in the bacterial system, the production process was completed in about 8–9 days. Inclonals equaled the performance of the same IgGs that were produced using conventional mammalian cell culture in binding properties, as well as in their potential to deliver toxins to cultured target cells. Moreover, the Inclonals method provided us the opportunity to generate full-length IgG that is genetically fused to a cytotoxic moiety, and consequently to explore IgG-enzyme fusion proteins.
Our antibody production system thus provides several advantages over other systems. First, this system has the advantages of the bacterial expression systems (simple, cheaper, faster and easier to scale up compared to mammalian cell culture). Second, the bacterial produced antibodies are aglycosylated, and can be used where effector functions are either not required or are actually detrimental. Third, the separate expression of the antibody heavy and light chains enables mixing different heavy and light chains which can give rise to combinatorial shuffling in the protein level to obtain desired antigen specificities and affinity properties. The fourth and most significant advantage of production of targeting molecules in a non-mammalian host is the ability to express a cytotoxic moiety fused to the molecule as a single polypeptide. Refolding of therapeutic proteins is well established and in general refolded E. coli-produced proteins have a low endotoxin level compared to proteins that are recovered from the bacterial periplasm.
Our Inclonal-fusion technology resolves the issue of conjugate heterogeneity and should be applicable to production of a wide range of cytotoxic proteins. For research purposes, there is currently a great need to generate protein-specific affinity reagents to explore the human proteome. High-throughput methods to generate renewable antibodies are still immature.13 Antibody-enzyme or antibody-fluorophore fusion proteins that can be generated by the Inclonals technology may become very useful for such purposes. Cost-effective production of immunoconjugates, which are widely studied as anticancer treatments, is needed. We believe that our rapid and cost effective IgG and IgG-fusion protein production process and the high quality of the resultant product may make the bacterial production of full-length IgG and IgG-fusion proteins a viable and attractive option for antibody production for research and hopefully for clinical applications.
Materials and Methods: Construction of Vectors for Expression of Inclonals
Heavy chain vectors: the VH variable domain of anti CD30 antibody T427 with the C-region of human IgG1 was subcloned from pMAZ-IgH-T427 (described in supplementary methods) into a T7-based, IPTG-inducible bacterial expression vector as follows: the entire heavy chain was amplified by PCR using plasmid pMAZ-IgH-T427 as template with primers CMV-Seq and CMV-antiseq-EcoRI-REV (All the PCR primers are described in Supplementary Table 1). The PCR product was digested with PstI and EcoRI and cloned into a pRB98Amp-T427VH(C44)-PE38 vector that was linearized using the same enzymes. The resulting plasmid, pHAK-IgH-T427 can be used to express the heavy chain of T427 in a chimeric IgG1 format in E. coli. VH domains can be exchanged into this plasmid as NdeI-NheI fragments.
A similar plasmid for the expression of the heavy chain of the anti EGFR antibody 225 in E. coli was constructed as follows: the VH variable domain was recovered by PCR using plasmid pCMV/H6myc/cyto-225(Fv)14 as template with primers 225VH-NdeI-FOR and 225VH-NheI-REV. The PCR product was digested with NdeI and NheI and cloned into a pHAK-IgH-T427 vector (described above) that was linearized using the same enzymes. The resulting plasmid was named pHAK-IgH-225.
Light chain vectors: the light chain of anti CD30 antibody T427 with the human C-kappa region was subcloned from pMAZ-IgL-T427 (described in supplementary methods) into a T7-based, IPTG-inducible bacterial expression vector as follows: the entire light chain was amplified by PCR using plasmid pMAZ-IgL-T427 as template with primers CMV-Seq and CMV-antiseq-EcoRI-REV. The PCR product was digested with PstI and EcoRI and cloned into a pRB98Amp-T427VL(C105) plasmid vector15 that was linearized using the same enzymes. The resulting plasmid, pHAK-IgL-T427 can be used to express the light chain of T427 in a chimeric IgG1 format in E. coli. VL domains can be exchanged into this plasmid as NdeI-BsiWI fragments.
A similar plasmid for the expression of the light chain of the anti EGFR antibody 225 in E. coli was constructed as follows: the V-Kappa variable domain was recovered by PCR using plasmid pCMV/H6myc/cyto-225(Fv)14 as template with primers 225VK-NdeI-FOR and 225VK-BsiWI-REV. the PCR product was digested with NdeI and BsiWI and cloned into a pHAK-IgL-T427 vector (described above) that was linearized using the same enzymes. The resulting plasmid was named pHAK-IgH-225.
Construction of vectors for expression of IgG-PE38 fusion.
The heavy or light chain-PE38 fusion protein expression vectors were constructed on the backbone of pHAK vectors that were modified by insertion of HindIII and EcoRI cloning site at the 3′ end of the antibody constant regions as follows: For the heavy chain vector, the cloning site was inserted by PCR using plasmid pHAK-IgH as template with primers RGD/TAT-BsrGI-FOR and CH3-HindIII-EcoRI-REV. For the heavy chain vector, pHAK-IgL was used as template with primers BsiWI-Back-IgL and Cκ-HindIII-EcoRI-REV. The PCR products were digested with BsrGI and EcoRI for the heavy chain and with SacI-EcoRI for the light chain, respectively, and cloned into a pHAK-IgH vector and pHAK-IgL vector respectively that were linearized using the same enzymes. The resulting vectors were linearized with HindIII and EcoRI and ligated with the PE38 DNA fragment that was recovered form plasmid pRB98Amp-T427VH(C44)-PE38 using the same enzymes. The resulting vectors were named pHAK-IgH-PE38 and pHAK-IgL-PE38.
Expression of Inclonals in E. coli.
The Inclonals and Inclonal-PE38 fusion proteins were expressed in E. coli BL21(DE3) pUBS500 cells16 that were transformed with the expression vectors. For the production of IgGs, cells were transformed with pHAK-IgH and pHAK-IgL. For the production of IgG-(di) PE38, cells were transformed with pHAK-IgH-PE38 and pHAK-IgL. For the production of IgG-(tetra)PE38, cells were transformed with pHAK-IgH-PE38 and pHAK-IgL-PE38. Cells were grown in SB medium (35 gr/L tryptone (Difco, USA), 20 gr/L yeast extract (Difco, USA), 5 gr/L NaCl, 6.3 gr/L glycerol (Frutarom, Israel), 12.5 gr/L K2HPO4, 3.8 gr/L KH2PO4, 0.48 gr/L MgSO4, 0.4% (w/v) glucose) supplemented with 100 µg/ml ampicillin and 50 µg/ml kanamycin at 37°C shaking at 250 RPM. The bacterial cultures were induced for protein expression in the late exponential growth phase (OD600 of 2.5) with 1 mM isopropyl-1-thio-β-D-galactopyranoside for 3 h at 37°C. The recombinant proteins accumulated as insoluble inclusion bodies and were isolated from lysed bacteria cells by centrifugation. From 500 ml of culture about 3 gr of wet cell paste was collected. The cells were suspended in 50 mM Tris (HCl) pH 8.0, 20 mM EDTA, using a tissuemizer and further processed as described.17 The inclusion bodies were completely solubilized in 6 M guanidine hydrochloride, 50 mM Tris (HCl) pH 8.0, 20 mM EDTA, mixed, reduced and refolded essentially as described.15 After refolding, the protein was dialyzed against phosphate buffer pH 7.4 (20 mM containing 77% Na2HPO4 and 23% NaH2PO4). The refolded active protein was then filter sterilized using a 0.45 µm filter and separated from contaminating bacterial proteins, excess light chains and from improperly folded protein by protein-A chromatography. Purified IgG was stored at 4°C. Typically, from a refolding initiated by mixing 50 mg of heavy chain with 50 mg of light chain protein, we obtain ∼12.5 mg of pure Inclonal.
The anti EGFR 225 Inclonal was produced in the same way using cultures of cells carrying pHAK-IgH-225 and pHAK-IgL-225.
Preparation of IgG-ZZ-PE38 immunocomplexes.
The immunocomplex of T427 or 225 IgGs with ZZ-PE38 fusion protein was carried out by mixing IgGs with ZZ-PE38 fusion protein and purifying the immunocomplex by Superdex 200 (Amersham Pharmacia Biotech, now GE healthcare, USA) gel filtration chromatograph essentially as described.11
Gel filtration chromatography.
Analytical separation of chimeric IgGs was carried out by gel-filtration chromatography using a 30 ml TSK3000 column (TosoHaas, Japan) on a fast protein liquid chromatography (FPLC), (Pharmacia LKB-Pump-P500) according to supplier's recommendations. About 200 micrograms of sample were loaded in 500 µl with PBS as buffer at a flow rate of 0.5 ml/min.
Evaluation of IgG stability in serum.
To compare the stabilities of an Inclonal IgG T427 to that of the corresponding chT427 IgG that was produced in mammalian cell culture, a serum stability assay was carried out as follows: The IgGs were diluted to a final concentration of 30 µg/ml in 100% bovine serum (Beit Haemek, Israel) and incubated at 37°C for the indicated time periods. Residual binding activity to MBP-CD30 of each fraction was evaluated by ELISA as describes below.
Evaluation of antigen binding by ELISA and whole-cell ELISA.
Antigen binding by chimeric IgGs was tested in ELISA as follows: ELISA plates were coated with a solution of 5 µg/ml MBP-CD30 in PBS at 4°C for 20 h and blocked with 3% (v/v) non-fat milk in PBS for 1–2 h at 37°C. All subsequent steps were done at room temperature (25°C). Protein-A purified IgGs were applied onto the plates in a five-fold dilution series and tested for their affinity to MBP-CD30. Following incubation the plates were washed thee times with PBST. HRP-conjugated goat anti human antibodies were used as secondary antibodies diluted ×5,000 dilution in PBST. The ELISA was developed using the chromogenic HRP substrate TMB and color development was terminated with 50 µl/well of 1 M H2SO4. The results were plotted as absorbance at 450 nm and the binding-avidity was roughly estimated as the IgG concentration that generates 50% of the maximal signal.
Cellular EGFR binding by 225 Inclonal and cetuximab, was tested by whole-cell ELISA as follows; the human epidermoid carcinoma A431 cells were seeded in 96-well plate at a density of 2×104 cells/well in DMEM supplemented with 10% FBS for 16 h. The medium was aspirated and the cells were fixed with 3% glutaraldehyde for 15 minutes at 25°C. The wells were blocked with 3% (v/v) non-fat milk in PBS for 1–2 h at 37°C. Next, IgGs were added to the wells at a 5 fold dilution series in PBS +3% BSA and incubated for 1.5 h at 25°C. After cells were washed three time with PBS +3% BSA, 100 µl of HRP-conjugated goat anti human antibodies (×5000 dilution in PBS + 3% BSA) was added for 1 h at 25°C. After another washing cycle, detection of cell bound antibodies was performed by addition of 100 µl of the chromogenic HRP substrate TMB to each well and color development was terminated with 50 µl/well of 1 M H2SO4. Absorbance was measured at 450 nm using a microplate reader.
Flow cytometry.
Binding analysis to CD30 expressed on A431/CD30 transfected cells18 with bacterial or mammalian produced chT427 IgG1 was tested by flow cytometry. Approximately 5 × 105 cells in immunotubes (5 ml polystyrene tubes, Nunc, Sweden) were used in each experiment. After trypsinization, cells were washed once in 2% fetal calf serum in PBS (FACS buffer). Next, the chimeric IgGs were added at a final concentration of 10 nM in PBS + 3% BSA and the cells were incubated for 90 min at 4°C. The cells were then washed three times FACS buffer and FITC-labeled goat anti human antibodies (x50 dilution in PBS + 3% BSA) were added to the appropriate tubes for 45 min at 4°C. Detection of bound antibodies was done by flow cytometry on a FACS-Calibur (Becton Dickinson, CA) and results were analyzed with the CELLQuest program (Becton Dickinson). To confirm specificity, antibodies were incubated with or without a ×30 fold excess of competing protein during the 90 min incubation period.
Cell-viability assay.
The in vitro cell-killing activities of chimeric IgG-ZZ-PE38 immunocomplexes and of IgG-PE38 fusion proteins were measured by an MTT assay. Tested cells were seeded in 96-well plates at a density of 1 × 104 cells/well in DMEM supplemented with 10% FBS. Immunocomplexes, IgG-PE38 fusion proteins or control proteins were added (in triplicate) in a 10-fold dilution series and the cells were incubated for 48 h at 37°C in 5% CO2 atmosphere. After 48 h, the media was replaced by fresh media (100 µl per well) containing 1 mg/ml MTT (Thiazolyl Blue Tetrazoliam Bromide, dissolved in PBS) reagent and the cells were incubated for another 4 h. MTT-formazan crystals were dissolved by the addition of 20% SDS, 50% DMF, pH 4.7 (100 µl per well) and incubation for 16 h at 37°C. Absorbance at 570 nm was recorded on an automated microtiter plate reader. The results were expressed as percentage of living cells relatively to the untreated controls that were processed simultaneously using the following equation: (OD570 of treated sample/ OD570 of untreated sample) ×100. The IC50 values were defined as the immunocomplexes or the IgG-PE38 fusion protein concentrations that inhibited cell growth by 50%.
Supplementary Material
Acknowledgments
We thank Dr. Ira Pastan (LMB, NCI, NIH) for the expression vectors of anti CD30 immunotoxin T427(dsFv)-PE38, the pRB98-Amp expression vector for recombinant immunotoxins, A431-CD30 cells and an expression vector for CD30 extracellular domain. We thank Prof. Winfried Wels (Georg Speyer Haus, Frankfurt, Germany) for the 225 scFv clone. This study was supported in part by a research grant from the Israel Cancer Research fund (ICRF).
Abbreviations
- PE38
truncated form of Pseudomonas exotoxin A
- scFv
single-chain Fv composed of VH connected to VL through a short peptide linker
- dsFv
disulfide-stabilized Fv fragment
- IC50
concentration required to cause 50% inhibition of the measured phenotype
Note
Supplementary materials can be found at: www.landesbioscience.com/supplement/HakimMABS1-3-Sup.pdf
Footnotes
Previously published online as a mAbs E-publication: www.landesbioscience.com/journals/mabs/article/8492
References
- 1.Maggon K. Monoclonal antibody “gold rush.”. Curr Med Chem. 2007;14:1978–1987. doi: 10.2174/092986707781368504. [DOI] [PubMed] [Google Scholar]
- 2.Carter PJ, Senter PD. Antibody-drug conjugates for cancer therapy. Cancer J. 2008;14:154–169. doi: 10.1097/PPO.0b013e318172d704. [DOI] [PubMed] [Google Scholar]
- 3.Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ. Immunotoxin therapy of cancer. Nat Rev Cancer. 2006;6:559–565. doi: 10.1038/nrc1891. [DOI] [PubMed] [Google Scholar]
- 4.Junutula JR, Raab H, Clark S, Bhakta S, Leipold DD, Weir S, et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat Biotechnol. 2008;26:925–932. doi: 10.1038/nbt.1480. [DOI] [PubMed] [Google Scholar]
- 5.Cabilly S, Riggs AD, Pande H, Shively JE, Holmes WE, Rey M, et al. Generation of antibody activity from immunoglobulin polypeptide chains produced in Escherichia coli. Proc Natl Acad Sci U S A. 1984;81:3273–3277. doi: 10.1073/pnas.81.11.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boss MA, Kenten JH, Wood CR, Emtage JS. Assembly of functional antibodies from immunoglobulin heavy and light chains synthesised in E. coli. Nucleic Acids Res. 1984;12:3791–3806. doi: 10.1093/nar/12.9.3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazor Y, Van Blarcom T, Iverson BL, Georgiou G. E-clonal antibodies: selection of full-length IgG antibodies using bacterial periplasmic display. Nature protocols. 2008;3:1766–1777. doi: 10.1038/nprot.2008.176. [DOI] [PubMed] [Google Scholar]
- 8.Simmons LC, Reilly D, Klimowski L, Raju TS, Meng G, Sims P, et al. Expression of full-length immunoglobulins in Escherichia coli: Rapid and efficient production of aglycosylated antibodies. J Immunol Methods. 2002;263:133–147. doi: 10.1016/s0022-1759(02)00036-4. [DOI] [PubMed] [Google Scholar]
- 9.Mazor Y, Van Blarcom T, Mabry R, Iverson BL, Georgiou G. Isolation of engineered, full-length antibodies from libraries expressed in Escherichia coli. Nat Biotechnol. 2007;25:563–565. doi: 10.1038/nbt1296. [DOI] [PubMed] [Google Scholar]
- 10.Nagata S, Numata Y, Onda M, Ise T, Hahn Y, Lee B, et al. Rapid grouping of monoclonal antibodies based on their topographical epitopes by a label-free competitive immunoassay. J Immunol Methods. 2004;292:141–155. doi: 10.1016/j.jim.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Mazor Y, Barnea I, Keydar I, Benhar I. Antibody internalization studied using a novel IgG binding toxin fusion. J Immunol Methods. 2007;321:41–59. doi: 10.1016/j.jim.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Rowinsky EK. The erbB family: Targets for therapeutic development against cancer and therapeutic strategies using monoclonal antibodies and tyrosine kinase inhibitors. Annu Rev Med. 2004;55:433–457. doi: 10.1146/annurev.med.55.091902.104433. [DOI] [PubMed] [Google Scholar]
- 13.Uhlen M, Graslund S, Sundstrom M. A pilot project to generate affinity reagents to human proteins. Nat Methods. 2008;5:854–855. doi: 10.1038/nmeth1008-854. [DOI] [PubMed] [Google Scholar]
- 14.Shaki-Loewenstein S, Zfania R, Hyland S, Wels WS, Benhar I. A universal strategy for stable intracellular antibodies. J Immunol Methods. 2005;303:19–39. doi: 10.1016/j.jim.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Nagata S, Onda M, Numata Y, Santora K, Beers R, Kreitman RJ, et al. Novel anti-CD30 recombinant immunotoxins containing disulfide-stabilized Fv fragments. Clin Cancer Res. 2002;8:2345–2355. [PubMed] [Google Scholar]
- 16.Brinkmann U, Mattes RE, Buckel P. High-level expression of recombinant genes in Escherichia coli is dependent on the availability of the dnaY gene product. Gene. 1989;85:109–114. doi: 10.1016/0378-1119(89)90470-8. [DOI] [PubMed] [Google Scholar]
- 17.Buchner J, Pastan I, Brinkmann U. A method for increasing the yield of properly folded recombinant fusion proteins: Single-chain immunotoxins from renaturation of bacterial inclusion bodies. Anal Biochem. 1992;205:263–270. doi: 10.1016/0003-2697(92)90433-8. [DOI] [PubMed] [Google Scholar]
- 18.Rozemuller H, Chowdhury PS, Pastan I, Kreitman RJ. Isolation of new anti-CD30 scFvs from DNA-immunized mice by phage display and biologic activity of recombinant immunotoxins produced by fusion with truncated pseudomonas exotoxin. Int J Cancer. 2001;92:861–870. doi: 10.1002/ijc.1266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.