Abstract
Asthma represents a syndrome of airway inflammatory diseases with complex pathology. The immunologic pathogenesis is being increasingly revealed and provides opportunity for targeted biological intervention. Current experience with immunomodulators as targeted therapy in asthma is described in this literature review. Targeted therapies have included strategies to activate dendritic cells through the TLR-9 receptors, to interrupt the action of TH2 cytokines with cytokine blockers and monoclonal antibodies, to promote development of TH1 responses, to block IgE mediated pathways and to block TNFα. Omalizumab is the only biological therapy that has an approved indication in asthma at this time. An improved understanding of the heterogeneity of asthma should allow for specific targeting of different disease phenotypes specific therapies including immunomodulators.
Key words: cytokine blockers, dendritic cell, monoclonal antibodies, immunomodulators, TH2 cells, TH1 cells, airway inflammation, IgE, omalizumab
The Pathobiology of Allergic Asthma
Asthma affects 5% of the population, making it one of the most common chronic diseases worldwide.1 Asthma represents a complex syndrome broadly defined by inflammation of the airways associated with airway hyperresponsiveness and mucous hypersecretion with clinical features including shortness of breath, mucus production and wheezing, together with objectively measurable reversible airway narrowing. Our increased understanding of the pathophysiology of asthma in recent years is leading to new therapeutic approaches including monoclonal antibodies and other biologic agents that could have profound repercussions for the treatment of this condition.
The pathological changes seen in the asthmatic airway are multiple and complex. The epithelium lining the asthmatic airway exhibits sloughing/denudation, ciliary dysfunction, goblet cell hyperplasia and mucous gland hypertrophy. The mucus produced by the asthmatic epithelium exhibits increased elasticity, increased viscosity, increased content of mucin, cellular debris and surfactant dysfunction. There is fibrosis in the sub-epithelial compartment, and the submucosal tissues also demonstrate a significant inflammatory cellular infiltrate characterized by lymphocytes, eosinophils, neutrophils, increased numbers of mast cells and new blood vessel formation (angiogenesis). Abnormalities of the airway smooth muscle layer include increased mass, consisting of both hypertrophy and hyperplasia and increased contractility together with mast cell infiltration and angiogenesis. In a majority of patients these pathological changes are seen during active disease, but largely reverse during periods of disease control. In some patients failure to resolve or repair these changes is observed with many of the above changes becoming fixed, a process referred to as airway remodeling.1–3
The immune responses underpinning the pathologic changes seen in the asthmatic airway are also complex and involve both the innate and the adaptive immune systems. Among the processes involved are epithelial cell disruption and activation with cytokine production, dendritic cell activation and antigen presentation, activation and expansion of TH2 cells with cytokine secretion, activation of resident inflammatory cells, including mast cells, and recruitment from the blood stream of other inflammatory cells including lymphocytes, eosinophils and neutrophils which also release mediators and cytokines.1–3
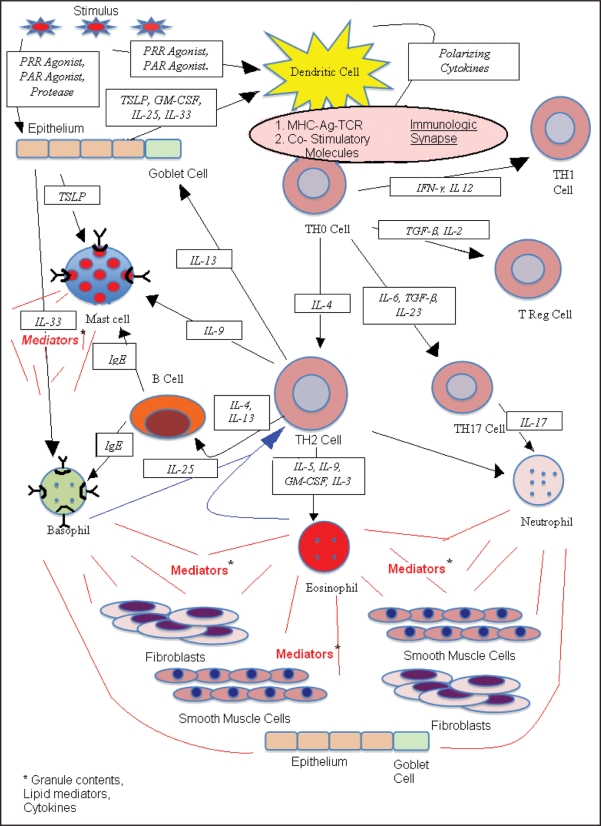
The airway inflammatory response in asthma is conceptualized as beginning in the airway lumen (Fig. 1). Here allergens, viruses or other pathogens interact with airway epithelial cells and dendritic cells. Some aeroallergens have enzymatic properties capable of directly cleaving tight junctions between epithelial cells or activating the epithelial cells through protease activated receptors. The activated epithelium and other tissue cells release factors including cytokines and chemokines that can prime or otherwise influence the response of the dendritic cell4 and other cells of the innate (basophils, eosinophils and mast cells) and of the adaptive immune systems (B and T lymphocytes).5 Both airway epithelial cells and dendritic cells carry surface pattern recognition receptors for pathogens including Toll-Like receptors (TLRs), nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) and retinoic inducible gene (RIG) receptors which recognize surface characteristics of pathogens and, upon ligation, initiate responses of the innate immune system. In addition to pattern recognition receptors, antigen uptake by the dendritic cell may occur through either nonspecific mechanisms including macropinocytosis, or through a highly antigen-specific mechanism using immunoglobulin Fc receptors. For example, in sensitized individuals, expression of the high affinity receptor for IgE (FcεR1) on airway dendritic cells probably strongly facilitates the processing of specific allergen, captured by bound IgE.6–8 The dendritic cell internalizes and processes antigen into fragments which are then cognately presented in the context of MHC molecules to naïve (TH0) CD4 lymphocytes. Factors associated with the antigen, the presence of TLR ligands and factors from the activated epithelium can influence the dendritic cell activation status.5 The patterns of co-stimulatory molecule expression, the ligand-receptor interactions between the dendritic cell and the lymphocyte at the immunological synapse, and cytokines released by the activated dendritic cell combine to direct the subsequent differentiation of the antigen-specific TH0 cell. The signals that the TH0 cell receives lead it to differentiate along one of several possible lineages including TH1, TH2, TH17 and T regulatory cells. The presence of IL-4 appears to favor differentiation of TH0 cells into TH2 type cells, and has an inhibitory effect on the development of a TH1 response. IL-12 and IFNγ have a negative developmental effect on TH2 phenotype cells, while preferentially driving the differentiation of TH0 cells towards a TH1 phenotype. Factors favoring the development of TH17 cells indicate a primary role for IL-6 with subsidiary roles for TGFβ and IL-23. Factors favoring the development of regulatory T-cells continue to be elucidated, but also appear to include TGFβ.
Figure 1.
Schematic depiction of the components of the airway allergic inflammatory response. PRR (pattern recognition receptor); PAR (protease activated receptor); TSLP (thymic Stromal Lymophopoietin); GM-CSF (granulocyte-macrophage colony stimulating factor). Arrows indicate the direction in which the identified signal is acting. Red lines indicate the discharge of cellular mediators (including granular contents, lipid mediators and cytokines).
Immunomodulation in Allergic Asthma
The antigen presenting cell, or dendritic cell, plays a key role in initiating and propagating the allergic inflammation and much of the abnormal pathology can be linked to cytokine products of activated proliferating TH2 cells, basophils and mast cells. This knowledge has exposed many potential targets for immune modulation as described below. Strategies have included approaches aimed at skewing the dendritic cell towards promotion of TH1 responses by TLR activation, direct targeting of CD4 positive TH2 cells, anti-TH2 or pro-TH1 cytokine-based biologic therapies or targeting of specific effector cells such as mast cells or eosinophils. There is increasing evidence for the role of cytokines derived from resident tissue cells in amplifying TH2 type allergic inflammation. Therapeutic agents aimed at interrupting such antigen-independent pathways will undoubtedly be soon explored. Anti-cytokine therapies might theoretically include (1) receptor-specific non-activating/blocking antibodies which prevent cytokine binding, (2) soluble receptors which would competitively inhibit binding of cytokine to cell surface cytokine receptors or (3) other compounds, such as mutant ligands, designed to block the cytokine receptor. The discussion of immunomodulatory strategies below follows the process of allergic inflammation from its beginning at the dendritic cell, through the innate immune system, the adaptive immune system and ultimately effector cells.
Dendritic Cell Based Therapies
TLR-9 agonists.
The dendritic cell has been described as a sophisticated information transfer system linking the environment with the adaptive immune system.9 Hence, the dendritic cell interacts with antigens through a number of important mechanisms including pattern recognition receptors such as TLRs (Fig. 2A). A large number of animal studies have demonstrated activation of TLR-9 through the use of CpG rich unmethylated DNA segements commonly found in bacteria. These are also known as immunostimulatory DNA sequences (ISS) and can result in skewing of immune responses towards TH1.10–13
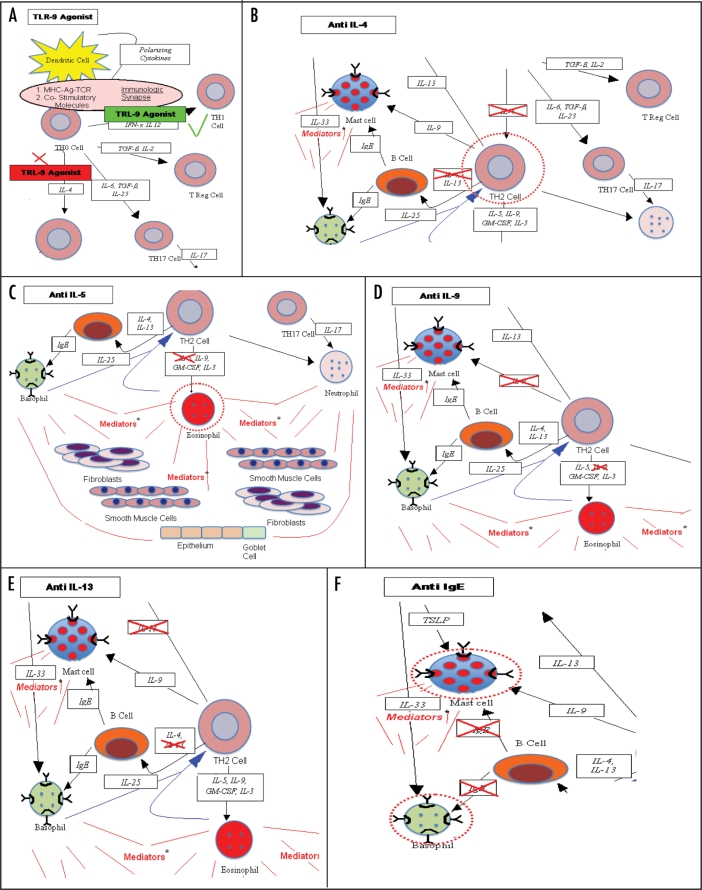
Figure 2.
(A) TLR 9 agonists act on dendritic cells in conjunction with antigen and result in a preferential activation of antigen specific TH12 cells (green line) and inhibition of development of antigen specific TH2 cells (red X). (B) Anti IL-4 agents block the development of TH2 cells, whether the source of the IL-4 is from activated basophils or eosinophils or from the stimulated TH0 cells. This in turn has the potential to diminish production of all TH2 products-indicated by broken red circle surrounding the TH2 cell. (C) Anti IL-5 agents block the development and release from the bone marrow of eosinophils. This has the potential to result in less tissue eosinophilia in allergic inflammation and less exposure to mediators from activated eosinophils-indicated by broken red circle surrounding the eosinophil. (D) IL-9 is a product of activated TH2 cells which can directly activate mast cells. Anti IL-9 could inhibit this. (E) IL-13 is a product of activated Th2 cells with activities in determining isotype switch to IgE of allergen specific B cells, direct activation mast cells and effects on airway goblet cell activity and mucus production. Anti IL-13 has the potential to inhibit these various activities. (F) Anti-IgE results in loss of high-affinity IgE receptors of mast cell and basophils with result diminished capacity for activation after allergen exposure. Anti-IgE reduces high affinity IgE receptors on dendritic cells which may in turn decrease allergen processing and presentation.
Human studies have now been undertaken with TLR-9 agonist ISS covalently linked to allergen, delivered as a series of subcutaneous injections, with a goal of ameliorating allergic inflammatory disease. Creticos et al. reported a randomized, double-blind, placebo-controlled Phase II trial of ragweed Amb-a 1, a major ragweed allergen, bound to a TLR-9 agonist (six weekly injections versus placebo) in the treatment of ragweed-associated seasonal allergic rhinitis.14 They observed a diminution in peak seasonal symptoms of allergic rhinoconjunctivitis in the year in which treatment was administered and during the following year's ragweed season, as well as reduction in the need for allergy medications. An accompanying report looking at nasal biopsies of these patients demonstrated increased amounts of IFNγ suggesting TH1-skewed immune responses, but there was no decrease in the amount of IL-4 or eosinophils in the nasal biopsies.15 A separate study reported a diminished ex-vivo ragweed-specific TH2 responses and a transient TH1 biasing effect of this therapy in patients with ragweed allergic rhinitis.16 The initial proof of concept clinical trial was followed by a larger multicenter randomized controlled clinical trial. Improvement was observed in the treatment groups, again over two years, but a higher treatment dose group apparently showed no incremental benefit over those receiving a lower dose equivalent to what had been studied in the first trial. Enthusiasm has waned somewhat for this single-allergen immunotherapy approach, but it is believed that additional studies are being designed and other studies are ongoing to investigate therapy of allergic asthma by TLR-9 agonists alone or conjugated to allergen.13
The promotion of TH2 type allergic inflammation by cytokines released from resident tissue cells of the innate immune system, such as epithelial cells, is an area of active investigation. Thymic stromal lymphopoietin (TSLP), derived largely from activated epithelial cells, influences the expression of co-stimulatory molecules on dendritic cells, particularly OX-40 ligand. This represents a TH2 polarising signal which preferentially drives naïve TH0 CD4 T-cells towards an allergic inflammatory TH2 phenotype.17–18 TSLP can also cause immature cord blood-derived mast cells to secrete increased amounts of the TH2 cytokines IL-5 and IL-13 and certain chemokines, as well as demonstrate an enhanced rate of mast cell maturation.19 More recently, a novel IL-1 family member cytokine, IL-33, has also been demonstrated to increase TH2 allergic inflammation. IL-33 has a very high level of expression in high endothelial venules of lymphoid tissue, is thought to play a role in the chemotaxis of TH2 cells, and was shown to be capable of increasing cytokine production from TH2 polarized lymphocytes.20 IL-33 has been shown to cause a direct activation of basophils resulting in increased secretion of IL-4, IL-13, IL-8, as well as enhanced FCεR1 dependent mediator release and cytokine production. It is also capable of activating human eosinophils.21 Targeting TSLP, IL-33 and other allergen-independent cytokine pathways which promote TH2 type allergic inflammatory disease might offer an important potential to interrupt chronic allergic inflammation, and these approaches are currently under investigation in animal models of asthma.
T-Cell Blocking Therapies
Daclizumab (anti-IL-2 receptor α subunit/anti-Tac, anti-CD25) monoclonal antibody acts to decrease T-cell proliferation and cytokine (TH1 and TH2) production, and is FDA approved for use in the prophylaxis of renal allograft rejection. It decreases the binding of IL-2 to its receptor and has greater effects on activated than resting T-cells. Regulatory T-cells, which also use IL-2 for proliferation and survival, do not appear to be functionally inhibited in patients treated with daclizumab.22–24 A double blind placebo controlled trial in patients with moderate to severe persistent asthma of daclizumab, administered IV every two weeks, has shown improvement in asthma control (both impairment and risk) including improvements in pulmonary function and deceased peripheral blood eosinophils.25 This proof of concept study will need to be followed by larger trials in asthma to more fully define the role of this agent therapeutically.
Keliximab is a primatized anti-CD4 monoclonal antibody that has been studied in patients with steroid dependent asthma as a single infusion, with a four week follow up. There was a dose-dependent decrease in peripheral CD4 counts and a decrease in mitogen-induced T-cell proliferation. The highest dose used resulted in an increase in morning and evening peak expiratory flow rates, but there were no significant changes in asthma symptoms.26,27 Additional studies have not been reported in asthma.
Earlier T-cell based strategies in asthma have included evaluation of calcinuerin inhibitors cyclosporine and tacrolimus. In patients with chronic severe asthma, treatment with cyclosporine resulted in modest improvement.28
TH2 Cytokine Blocking Therapies
The cytokines produced by TH2 type lymphocytes, basophils and mast cells (IL-4, IL-5, IL-9 and IL-13) have been linked to many of the pathologic findings in asthma detailed above. Such knowledge has led to the development of several anti-cytokine agents with potential utility in interrupting the TH2 dependent allergic inflammation characteristic of asthma. Human studies so far have included attempts to target most of the major TH2 cytokines including IL-4, IL-5, IL-9, IL-13, and joint targeting of IL-4 and IL-13.
Targeting IL-4.
IL-4 has been demonstrated to have an important role in the development of allergic inflammation at several levels and is an attractive target for therapy. In the presence of dendritic cell-presented allergen, IL-4 (possibly basophil-derived) strongly influences the differentiation of TH0 cells into TH2 cells. Furthermore, along with IL-13, IL-4 is a primary signal influencing the B lymphocyte to undergo isotype switch from IgM to IgE. IL-4 has also been demonstrated to upregulate the high affinity and low affinity IgE receptor expression on mast cells and basophils (Fig. 2B). Finally, IL-4 can also contribute to inflammatory cell recruitment via vascular cell adhesion molecule-1/very late antigen-4 dependent mechanisms, and can also directly contribute to goblet cell hyperplasia. In mouse models of asthma, many studies have shown that IL-4 removal or blockade profoundly abrogates the allergic inflammatory response and other features of asthma.29
The solubilized IL-4 receptor fragment altrakincept, consisting of an extracellular portion of human IL-4R-α chain that competitively inhibits the binding of IL-4 to its receptor, appeared promising in early human studies of mild to moderate asthma. Delivered by nebulizer, it appeared safe and well-tolerated and had a serum half-life of about five days. After a single dose it resulted in a reduction in exhaled nitric oxide and stabilization of asthma symptoms, despite inhaled corticosteroid withdrawal.30 In a subsequent 12-week proof of concept study that included 62 atopic patients with mild-to-moderate persistent asthma, weekly administration by nebulizer safely permitted withdrawal of inhaled steroid while maintaining asthma control. Thus, in contrast to the control group, patients treated with soluble IL-4 receptor showed no decline in FEV1 or increase in symptoms after inhaled corticosteroid therapy withdrawal. However, the study discontinuation rate due to asthma exacerbations was similar between the soluble IL-4 receptor and placebo groups.31 While no further reports of clinical studies have been published, it is understood that a larger, Phase III trial failed to confirm this benefit or efficacy suggested by the earlier, smaller trials.32
IL-4 and IL-13 overlap in several functions and share a common α-chain in their receptors. Recent research has suggested that the soluble IL-4R under certain conditions may even enhance IL-13 responses. This observation may be relevant to the soluble IL-4 receptor's ultimate lack of efficacy in clinical trials.33
An alternative approach to targeting IL-4 has been the use of an anti-IL-4 humanized monoclonal antibody, pascolizumab. This antibody effectively blocked IL-4 responses in vitro, and showed a favorable in-vivo pharmacokinetic profile in cynomolgus monkeys. A Phase II clinical trial in humans with asthma yielded unimpressive results, and further development was discontinued.34
Targeting IL-5.
IL-5 plays an important role in the differentiation of CD34 positive hematopoietic precursor cells into eosinophils, their subsequent maturation and passage out of the bone marrow into peripheral blood. IL-5 also induces activation of mature eosinophils and promotes their survival (Fig. 2C).35 As eosinophils express the pro-fibrotic growth factor TGFβ, IL-5 has also been linked to airway remodeling.36 A series of animal studies highlighted the important role of IL-5 in asthma,37,38 and demonstrated that removal of IL-5 resulted in improved parameters of asthmatic disease activity.39,40
In humans, a 12-week double-blind, placebo-controlled, randomized controlled trial (DBPCRCT) of the anti-IL-5 monoclonal antibody mepolizumab in patients with mild to moderate asthma was reported. While the study showed a rapid, dose-dependent and sustained reduction in eosinophil counts in blood and sputum, no differences in bronchial hyperresponsiveness, late phase allergic responses or other asthma outcomes were identifiable between the two treatment groups.41
A separate study in patients with severe persistent asthma of another anti-IL-5 monoclonal antibody, rezlizumab, yielded similar findings. That is, eosinophil numbers were decreased in peripheral blood, but changes in asthma outcomes were not observed over ten weeks of treatment.42 A subsequent study using mepolizumab confirmed the suppression of eosinophilia in blood, in bone marrow and in airway lavage fluid, with a limited reduction in the number of the eosinophils seen in airway biopsies.43 The reduction in airway eosinophil levels was associated with decreased expression of eosinophil TGFβ mRNA, decreased levels of TGFβ in bronchoalveolar lavage fluid (BAL), as well as decreased levels of tenascin, lumican and procollagen 3 in the bronchial subepithelial basement membrane.44 These findings in patients suggest that anti-IL-5 therapies might have potential in regulating tissue remodeling in asthma.
A possible explanation for the observations of less impressive reductions of eosinophils in the airways than in the blood or BAL might be that airway eosinophils do not express the IL-5 receptor making them less amenable to modulation by this therapy than circulating eosinophils.45,46 Specific strategies to reduce tissue eosinophils in the airways might prove more beneficial in asthma treatment; these might include targeting either the shared beta chain between IL-3 and IL-5 or eotaxin. Anti-IL-5 monoclonal antibodies may be useful in other diseases characterized by high levels of eosinophils in blood and tissues, including hyper-eosinophilic syndrome (HES), eosinophilic esophagitis (EE) and other eosinophilic enteritides, and chronic rhinosinusitis with nasal polyps. Preliminary data in patients with HES and EE treated with anti-IL5 have revealed promising results including improvement in clinical parameters and levels of tissue in eosinophilia.47–50 A recent randomized, double-blind, placebo-controlled trial in patients with HES demonstrated an anti-IL-5 therapy resulted in clinical stabilization, decreased peripheral blood eosinophil levels and decreased oral steroid requirements.51 Two recent small, proof of concept studies report use of mepolizumab in a subset of asthmatic patient who demonstrate persistent blood and sputum eosinophilia despite treatment with systemic corticosteroids.52,53 It is felt that this select subgroup might account for only approximately 5% of all adult-onset asthmatics.54 Both of these studies report a decrease in asthma exacerbations when compared to a control group of asthmatics, but there was no change in lung function tests, asthma symptoms or quality of life assessments.52,53 This finding indicates that one particular phenotype of asthmatics might stand to benefit from use of an anti-IL-5 monoclonal antibody.
Targeting IL-9.
IL-9 has been demonstrated to have a direct effect on the development and activation of human mast cells, to contribute to goblet cell hyperplasia in the airways and to other aspects of airway remodeling (Fig. 2D).36 In mouse models of asthma, blocking IL-9 reduces allergen-induced airway inflammation and airway hyperresponsiveness. Early human studies of an anti-IL-9 monoclonal antibody (MEDI-528) have been conducted. These include two Phase I dosing studies that have been completed in healthy subjects without major adverse events. Phase II studies are currently in progress in patients with symptomatic moderate to severe asthma, but trial results are not yet available.4
Targeting IL-13.
IL-13 shares many functional properties with IL-4, stemming from the fact that they share a common receptor sub-unit, the α-receptor of the IL-4 receptor (IL-4Rα). Even though the precise molecular mechanism by which IL-13 achieves downstream effects separate from IL-4 has not yet been elucidated, it has been clearly demonstrated to have distinct functions.55 IL-13, in addition to its role in promoting IgE isotype switching by B cells, also has important effects on epithelial cell maturation, goblet cell hyperplasia and mucous production, generation of extracellular matrix proteins and enhanced contractility of airway smooth muscle cells. IL-13 is also involved in the recruitment of eosinophils, monocytes, macrophages and T-cells (Fig. 2E).56,57 These properties make it a key potential target in the treatment of allergic inflammatory diseases including asthma.58 In animal models including mice and primates, reduction of lung inflammation, decreased airway responsiveness and diminished mucus production have been associated with use of anti-IL-13 monoclonal antibodies or a soluble form of IL-13 receptor.57,59,60
Human anti-IL-13 specific monoclonal antibodies are under development and some are in Phase I and II trials. A Phase I clinical trial with CAT-354 showed that increasing single doses of intravenously administered anti-IL-13 monoclonal antibody in 34 patients with mild asthma were well-tolerated at all doses with no identified safety concerns.4 No outcome data related to asthma improvement are yet available.
Joint targeting of IL-4 and IL-13.
Both IL-4 and IL-13 use the IL-4Rα as a component of their receptor, and this chain is integral to transmission of signals by both cytokines. Blocking this shared component of the receptor has the potential to inhibit signaling by both IL-4 and IL-13, two potent TH2 cytokines with several overlapping properties.
An IL-4 mutant protein, pitakinra, that inhibits the effects of both IL-4 and IL-13 through its ability to bind to the IL-4R α-chain, has been developed both as a subcutaneous injection and as an inhaled therapy. A Phase III study of the inhaled formulation in allergen-induced asthma in humans, involving twice daily inhalation for 27 days, demonstrated decreases in the late phase allergic responses, decreases in exhaled nitric oxide and improved pulmonary function in asthmatics. In contrast to results from animal studies, bronchial hyperresponsiveness did not change.61 Further studies are under way.
Promoting TH1 Cytokines
As mentioned, IL-12 and IFNγ have a negative developmental effect on TH2 phenotype cells, while preferentially driving the differentiation of TH0 cells towards a TH1 phenotype. An alternative biological approach to the treatment of allergic asthma therefore might include use of recombinant TH1 cytokines, such as IL-12, with a goal of downregulating TH2 type responses.
In animal models of allergic asthma, administration of IL-12 during sensitization suppresses allergen-induced TH2 cellular responses in favor of TH1 responses, inhibits airway hyperresponsiveness and results in diminished airway eosinophilia after allergen challenge. In humans, a clinical trial involving injection of recombinant human IL-12 in patients with mild asthma resulted in a decrease in the number of circulating blood eosinophils after allergen challenge, but not in sputum eosinophilia, the late phase asthmatic responses or in airway hyperresponsiveness. This therapy was accompanied by flu-like symptoms, abnormal liver function tests and cardiac arrhythmia.62
TH17 Cells as a Target
TH17 cells have been identified as a distinct T-helper cell lineage that follows a distinct differentiation pattern requiring IL-6 and TGFβ.63 This novel T-helper subset is characterized by the production of IL-17 cytokines that produce tissue inflammation by inducing the release of pro-inflammatory and neutrophil immobilizing cytokines. TH17 cells have been demonstrated experimentally to have an important role in the development of autoimmune diseases, but may also contribute to the pathogenesis of classically recognized TH2 mediated allergic diseases.64 Experimental evidence suggests that IL-17 decreases tissue eosinophil recruitment and bronchial hyperreactivity, but may on the other hand increase neutrophil infiltration and increase mucus proteins.65 Additionally, more recent evidence suggests that IL-17 is associated with a steroid-resistant phenotype in asthma.66 These observations may eventually lead to targeting this pathway in the treatment of severe neutrophilic asthma.
Targeting IL-25.
Interleukin 25 (IL-25, IL-17E) is also a member of the IL-17 family, but is distinct from all the other IL-17 family members in that it has been shown to provoke TH2 cell mediated inflammatory responses in animal studies. Animal studies have also shown that a neutralizing antibody against IL-25 significantly abrogates airway hyperresponsiveness, reduces IL-5 and IL-13 production, reduces tissue eosinophil infiltration, goblet cell hyperplasia and serum IgE.67 In humans, it has been shown that eosinophils and basophils likely represent the primary source of IL-25, and that its major affect may be upon TH2 memory cells.68 While human studies have not yet been reported, this might be a potential therapeutic target.
Anti-IgE
Omalizumab, an IgE-specific humanized IgG1 monoclonal antibody binds to the Fc-region of the IgE molecule, prevents IgE from interacting with high or low-affinity IgE receptors (FcεR1 and FcεR11) and results in rapid decreases in levels of circulating free IgE. Omalizumab does not directly bind to receptor-bound IgE, but downregulates FcεR1 on circulating basophils, on skin mast cells, and on circulating dendritic cells. It has the potential to inhibit allergen-induced mast cell activation regardless of the allergen specificity of the IgE molecules (Fig. 2F). The mechanisms of action of omalizumab might also include reduced presentation of allergen by dendritic cells to T-cells. Early studies in asthma showed that this antibody inhibited both early asthmatic responses and late asthmatic responses to inhaled allergen in patients with asthma.69
Demonstration of the anti-inflammatory properties of omalizumab was provided in a 16-week placebo-controlled bronchial biopsy and sputum study of 45 patients with corticosteroid naive mild persistent asthma. Omalizumab-treated subjects demonstrated a reduction in sputum and tissue eosinophils, a reduction in the number of IgE positive and FcεR1 positive cells in the submucosa, a decrease in the number of cells staining positive for IL-4, and a decreased overall number of CD4 and CD8 lymphocytes. However, perhaps surprisingly, bronchial hyperresponsiveness to methacholine was not significantly changed.70
Omalizumab is administered by subcutaneous injection. Although the level of circulating free IgE decreases rapidly after the first dose of omalizumab, up to 16 weeks of treatment might be required before optimal clinical effects are seen. Large placebo-controlled clinical trials in adults, adolescents and children with poorly-controlled IgE-mediated asthma have shown that omalizumab improves symptom control, decreases the frequency of asthma exacerbations and allows patients to be managed with lower doses of inhaled corticosteroids.71,72 Recent studies have demonstrated efficacy of omalizumab in patients with more severe asthma, and in pooled analyses of several large clinical trials, omalizumab significantly reduced asthma exacerbations (by 38%), emergency department visits (by 61%) and hospital admissions (by 52%) and unscheduled doctor visits (by 47%) when compared to control subjects.73,74 Omalizumab has also been studied as a treatment for other allergic diseases including allergic rhinoconjunctivitis,75,76 as adjunctive therapy with allergen immunotherapy,77,78 and as a treatment for chronic urticaria and angioedema,79–82 atopic dermatitis,83,84 allergic bronchopulmonary aspergillosis,85,86 Churg-Stauss syndrome87,88 and latex allergy.89 Further study is ongoing; none of these conditions are approved indications for use of this drug.
Lumiliximab (antiCD23).
Lumiliximab is a primatized macaque/human monoclonal antibody directed against the low affinity IgE receptor CD23 (FCεRII) which is expressed on several cell types including monocytes, alveolar macrophages, B cells and dendritic cells of allergic individuals. CD23 is felt to have a role in the regulation of IgE production and IgE-mediated inflammatory processes, and its activation can result in increased antigen presentation by B cells and increased production of proinflammatory cytokines. A Phase I single dose trial in patients with allergic asthma resulted in decreased IgE levels by 40%, and in vitro studies showed reduced allergen-specific PBMC proliferation and reduced production of proinflammatory cytokines (IL-1β and TNFα).90 Additional studies are needed to determine the true clinical efficacy of this agent in asthma or other allergic diseases.
TNFα based Therapy
Recent studies have suggested TNFα may serve as a marker of severity in asthma and possibly as a target for biological therapy of asthma.91 TNFα is important in the innate immune response. It is a cytokine principally produced by macrophages in response to activation of membrane bound pattern recognition molecules, such as toll-like receptors, but is also produced by several other pro-inflammatory cells including monocytes, dendritic cells, B lymphocytes, T lymphocytes, neutrophils, mast cells and eosinophils, as well as structural cells including fibroblasts, epithelial cells and smooth muscle cells. TNFα has been linked to several chronic inflammatory diseases including rheumatoid arthritis and Crohn disease in which anti-TNFα therapy has proved useful as a treatment.
The possibility that TNFα contributes to the inflammatory response seen in the asthmatic airway is supported by several observations. A biopsy study showed that TNFα mRNA is up to 30-fold higher in the airways of severe asthmatics compared to well-controlled asthmatics. This finding was paralleled by increased levels of TNFα protein in bronchoalveolar lavage fluid and mucosal biopsies.92 Administration of recombinant TNFα to normal subjects led to development of airway hyperresponsiveness and airway neutrophilia.93 Silvestri et al. examined the level of four cytokines TNFα, IL-8, IL-6 and IL-13 in the circulation of severe, mild/moderate asthmatics and normal controls.94 They report that TNFα and IL-8 levels are higher in those with severe asthma and are inversely correlated with baseline forced expiratory volume. They also report correlations between serum TNFα levels, exhaled nitric oxide and circulating neutrophil counts. They suggest that circulating levels of TNFα and IL-8 may serve as biomarkers to identify those severe asthmatic patients in whom anti-TNFα therapy may be efficacious.94
Based on these observations and a clear unmet clinical need in corticosteroid refractory asthma, a small number of recent clinical trials have evaluated the efficacy of anti-TNFα therapy in asthma.95 Biologic agents targeting the TNFα axis include infliximab (a chimeric mouse/humanized monoclonal antibody), etanercept (a soluble fusion protein combining TNFα receptors with an FC fragment of human IgG1) and adalimumab (a fully human monoclonal antibody).
In a 12-week open-label uncontrolled study of etanercept in patients with severe asthma, Howarth et al. reported a significant improvement in methacholine airway hyperresponsiveness, an improvement in FEV1 and in improvement in quality of life.92 This was followed by a randomized controlled crossover trial of etanercept in ten patients with severe asthma in which it was confirmed that treatment resulted in an improvement in airway hyperresponsiveness, FEV1 and asthma-related quality of life. There was no effect of etanercept therapy on the number of sputum eosinophils or neutrophils. It was, however, shown that membrane associated TNFα on circulating mononuclear cells appeared to be a predictor of the therapeutic response to etanercept.96 A subsequent 12 week long randomized, placebo-controlled, paralleled group study of etanercept in a patients with severe asthma demonstrated no beneficial effect.97 In a randomized placebo-controlled trial, Erin et al. reported the efficacy of infliximab in patient's with moderate asthma.98 No improvement in morning peak flow occurred, but there were decreases in diurnal variation of peak expiratory flow rate, and a 50% reduction in the number of mild asthma exacerbations. There were no significant improvements in lung function.98 It has been stated that unpublished longer-term randomized, placebo-controlled, paralleled group studies of patient with moderate severe asthma have questioned the efficacy of anti-TNFα therapy in asthma as discussed by Brightling et al.91
Efalizumab is a humanized IgG1 monoclonal antibody against lymphocyte function antigen-1 (LFA-1) alpha chain (CD11a) that can block trafficking of leukocytes from the circulation to sites of inflammation by interrupting LFA-1/intercellular adhesion molecule-1 interactions. In a DBPCRCT study of patients with mild allergic asthma, statistically significant differences in late phase allergic responses were not seen after allergen inhalational challenge.99
Conclusion
Recent increases in our understanding of the pathobiology of asthma, and of the innate and adaptive immune responses underlying those changes, including the role of various cellular elements and cytokines, has resulted in the identification of a large number of potential therapeutic targets for biologic agents in asthma. Many such therapies have been developed and subjected to clinical study, but, to date at least, the vast majority of these have not received FDA approval for treatment of asthma or other allergic diseases. Omalizumab is a notable exception. Asthma is clearly a complex disease or syndrome of diseases, and the possibility that separate populations of asthmatic patients are responsive to different biological therapies emphasizes the likely importance of tailoring novel therapies to individual patients. It is anticipated that, as the heterogeneity of asthma becomes more fully understood, new targeted biologic therapies will realize their potential as therapeutic agents. This is a very exciting time in the treatment of allergic diseases, and the future seems full of promise.
Acknowledgements
Neil Fahy assisted with illustrations.
Abbreviations
- TH
T helper cell
- TLR
toll-like receptor
- TNF
tumor necrosis factor
- NOD
nucleotide-binding oligomerization domain
- RIG
retinoic inducible gene
- FCεR1
crystalizable fragment of IgE receptor type 1
- MHC
major histocompatibility complex
- CD
cluster of differentiation
- IL
interleukin
- IFN
interferon
- TGF
transforming growth factor
- CpG
cytosine phosphate guanosine
- ISS
immunostimulatory sequences
- Amb-a 1
ambrosia artemesia first allergen
- TSLP
thymic stromal lymphopoietin
- OX-40
a secondary co-stimulatory molecule and member of the tumor necrosis receptor super family
- Daclizumab
monoclonal antidody against the alpha sub-unit of the IL-2 receptor, or CD25 or Tac
- FDA
food and drug administration
- Keliximab (IDEC CE9.1)
monoclonal antibody against CD4
- Altrakincept
soluble extracellular component of the α chain of IL-4 receptor
- Pascolizumab
monoclonal antibody against IL-4
- Mepolizumab
monoclonal antibody against IL-5
- Rezlizumab
monoclonal antibody against IL-5
- HES
hypereosinophilc syndrome
- EE
eosinophilic esophagitis
- Medi 528
monoclonal antibody against IL-9
- CAT-354
monoclonal antibody against IL-13
- Pitakinra
mutant protein that binds the shared alpha chain of IL-4 and IL-13
- Omalizumab
monoclonal antibody against IgE
- Lumilixumab (IDEC 152)
monoclonal antibody against the low affinity receptor got IgE (CD23)
- Infliximab
monoclonal antibody against TNFα
- Etanercept
fusion protein containing TNFα receptor and Fc portion of human IgG1
- Efalizumab
monoclonal antibody against LFA-1 alpha chain (CD11a)
- LFA
lymphocyte function antigen
- DBPCRCT
double-blind, placebo-controlled, randomized clinical trial
Footnotes
Previously published online as a mAbs E-publication: http://www.landesbioscience.com/journals/mabs/article/8352
References
- 1.Busse WW, Lemanske R., Jr Asthma. N Engl J Med. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 2.Pascual RM, Peters SP. Airway remodeling contributes to the progressive loss of lung function in asthma: an overview. J Allergy Clin Immunol. 2005;116:477–486. doi: 10.1016/j.jaci.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454:445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holgate ST, Polosa R. Treatment strategies for allergy and asthma. Nat Rev Immunol. 2008;8:218–230. doi: 10.1038/nri2262. [DOI] [PubMed] [Google Scholar]
- 5.Schleimer RP, Kato A, Kern R, Kuperman D, Avila PC. Epithelium: at the interface of innate and adaptive immune responses. J Allergy Clin Immunol. 2007;120:1279–1284. doi: 10.1016/j.jaci.2007.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambrecht BN. Lung dendritic cells: targets for therapy in allergic disease. Curr Mol Med. 2008;8:393–400. doi: 10.2174/156652408785160916. [DOI] [PubMed] [Google Scholar]
- 7.Maurer D, Ebner C, Reininger B, Fiebiger E, Kraft D, Kinet JP, et al. The high affinity IgE receptor (FcepsilonRI) mediates IgE-dependent allergen presentation. J Immunol. 1995;154:6285–6290. [PubMed] [Google Scholar]
- 8.Maurer D, Ebner C, Reininger B, Petzelbauer P, Fiebiger E, Stingl G. Mechanisms of FcepsilonRI-IgE-facilitated allergen presentation by dendritic cells. Adv Exp Med Biol. 1997;417:175–178. [PubMed] [Google Scholar]
- 9.Holt PG, Upham JW. The role of dendritic cells in asthma. Curr Opin Allergy Clin Immunol. 2004;4:39–44. doi: 10.1097/00130832-200402000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5:471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 11.Bhattacharjee RN, Akira S. Modifying toll-like receptor 9 signaling for therapeutic use. Mini Rev Med Chem. 2006;6:287–291. doi: 10.2174/138955706776073411. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi T, Raz E. TLR9-based immunotherapy for allergic disease. Am J Med. 2006;119:897. doi: 10.1016/j.amjmed.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 13.Kline JN. Eat dirt: CpG DNA and immunomodulation of asthma. Proc Am Thorac Soc. 2007;4:283–288. doi: 10.1513/pats.200701-019AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Creticos PS, Schroeder JT, Hamilton RG, Balcer-Whaley SL, Khattignavong AP, Lindblad R, et al. Immunotherapy with a ragweed-toll-like receptor 9 agonist vaccine for allergic rhinitis. N Engl J Med. 2006;355:1445–1455. doi: 10.1056/NEJMoa052916. [DOI] [PubMed] [Google Scholar]
- 15.Tulic MK, Fiset PO, Christodoulopoulos P, Vaillancourt P, Desrosiers M, Lavigne F, et al. Amb a 1-immunostimulatory oligodeoxynucleotide conjugate immunotherapy decreases the nasal inflammatory response. J Allergy Clin Immunol. 2004;113:235–241. doi: 10.1016/j.jaci.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Simons FE, Shikishima Y, Van Nest G, Eiden JJ, HayGlass KT. Selective immune redirection in humans with ragweed allergy by injecting Amb a 1 linked to immunostimulatory DNA. J Allergy Clin Immunol. 2004;113:1144–1151. doi: 10.1016/j.jaci.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 18.Gilliet M, Soumelis V, Watanabe N, Hanabuchi S, Antonenko S, de Waal-Malefyt R, et al. Human dendritic cells activated by TSLP and CD40L induce proallergic cytotoxic T cells. J Exp Med. 2003;197:1059–1063. doi: 10.1084/jem.20030240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allakhverdi Z, Comeau MR, Jessup HK, Yoon BR, Brewer A, Chartier S, et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204:253–258. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komai-Koma M, Xu D, Li Y, McKenzie AN, McInnes IB, Liew FY. IL-33 is a chemoattractant for human Th2 cells. Eur J Immunol. 2007;37:2779–2786. doi: 10.1002/eji.200737547. [DOI] [PubMed] [Google Scholar]
- 21.Pecaric-Petkovic T, Didichenko SA, Kaempfer S, Spiegl N, Dahinden CA. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1-family member IL-33. Blood. 2009;113:1526–1534. doi: 10.1182/blood-2008-05-157818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bielekova B, Catalfamo M, Reichert-Scrivner S, Packer A, Cerna M, Waldmann TA, et al. Regulatory CD56(bright) natural killer cells mediate immunomodulatory effects of IL-2Ralpha-targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci USA. 2006;103:5941–5946. doi: 10.1073/pnas.0601335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kreijveld E, Koenen HJ, Klasen IS, Hilbrands LB, Joosten I. Following anti-CD25 treatment, a functional CD4+CD25+ regulatory T-cell pool is present in renal transplant recipients. Am J Transplant. 2007;7:249–255. doi: 10.1111/j.1600-6143.2006.01604.x. [DOI] [PubMed] [Google Scholar]
- 24.Pillai V, Ortega SB, Wang CK, Karandikar NJ. Transient regulatory T-cells: a state attained by all activated human T-cells. Clin Immunol. 2007;123:18–29. doi: 10.1016/j.clim.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Busse WW, Israel E, Nelson HS, Baker JW, Charous BL, Young DY, et al. Daclizumab Improves Asthma Control in Patients with Moderate to Severe Persistent Asthma. Am J Respir Crit Care Med. 2008;178:1002–1008. doi: 10.1164/rccm.200708-1200OC. [DOI] [PubMed] [Google Scholar]
- 26.Kon OM, Sihra BS, Compton CH, Leonard TB, Kay AB, Barnes NC. Randomised, dose-ranging, placebo-controlled study of chimeric antibody to CD4 (keliximab) in chronic severe asthma. Lancet. 1998;352:1109–1113. doi: 10.1016/S0140-6736(97)12261-9. [DOI] [PubMed] [Google Scholar]
- 27.Kon OM, Sihra BS, Loh LC, Barkans J, Compton CH, Barnes NC, et al. The effects of an anti-CD4 monoclonal antibody, keliximab, on peripheral blood CD4+ T-cells in asthma. Eur Respir J. 2001;18:45–52. doi: 10.1183/09031936.01.00064101. [DOI] [PubMed] [Google Scholar]
- 28.Corrigan CJ, Hartnell A, Kay AB. T lymphocyte activation in acute severe asthma. Lancet. 1988;1:1129–1132. doi: 10.1016/s0140-6736(88)91951-4. [DOI] [PubMed] [Google Scholar]
- 29.Nakajima H, Takatsu K. Role of cytokines in allergic airway inflammation. Int Arch Allergy Immunol. 2007;142:265–273. doi: 10.1159/000097357. [DOI] [PubMed] [Google Scholar]
- 30.Borish LC, Nelson HS, Corren J, Bensch G, Busse WW, Whitmore JB, et al. Efficacy of soluble IL-4 receptor for the treatment of adults with asthma. J Allergy Clin Immunol. 2001;107:963–970. doi: 10.1067/mai.2001.115624. [DOI] [PubMed] [Google Scholar]
- 31.Borish LC, Nelson HS, Lanz MJ, Claussen L, Whitmore JB, Agosti JM, et al. Interleukin-4 receptor in moderate atopic asthma. A phase I/II randomized, placebo-controlled trial. Am J Respir Crit Care Med. 1999;160:1816–1823. doi: 10.1164/ajrccm.160.6.9808146. [DOI] [PubMed] [Google Scholar]
- 32.Adcock IM, Caramori G, Chung KF. New targets for drug development in asthma. Lancet. 2008;372:1073–1087. doi: 10.1016/S0140-6736(08)61449-X. [DOI] [PubMed] [Google Scholar]
- 33.Andrews AL, Holloway JW, Holgate ST, Davies DE. IL-4 receptor alpha is an important modulator of IL-4 and IL-13 receptor binding: implications for the development of therapeutic targets. J Immunol. 2006;176:7456–7461. doi: 10.4049/jimmunol.176.12.7456. [DOI] [PubMed] [Google Scholar]
- 34.Hart TK, Blackburn MN, Brigham-Burke M, Dede K, Al-Mahdi N, Zia-Amirhosseini P, et al. Preclinical efficacy and safety of pascolizumab (SB 240683): a humanized anti-interleukin-4 antibody with therapeutic potential in asthma. Clin Exp Immunol. 2002;130:93–100. doi: 10.1046/j.1365-2249.2002.01973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 36.Doherty T, Broide D. Cytokines and growth factors in airway remodeling in asthma. Curr Opin Immunol. 2007;19:676–680. doi: 10.1016/j.coi.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 37.Dent LA, Strath M, Mellor AL, Sanderson CJ. Eosinophilia in transgenic mice expressing interleukin 5. J Exp Med. 1990;172:1425–1431. doi: 10.1084/jem.172.5.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JJ, McGarry MP, Farmer SC, Denzler KL, Larson KA, Carrigan PE, et al. Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J Exp Med. 1997;185:2143–2156. doi: 10.1084/jem.185.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Oosterhout AJ, Ladenius AR, Savelkoul HF, Van Ark I, Delsman KC, Nijkamp FP. Effect of anti-IL-5 and IL-5 on airway hyperreactivity and eosinophils in guinea pigs. Am Rev Respir Dis. 1993;147:548–552. doi: 10.1164/ajrccm/147.3.548. [DOI] [PubMed] [Google Scholar]
- 40.Akutsu I, Kojima T, Kariyone A, Fukuda T, Makino S, Takatsu K. Antibody against interleukin-5 prevents antigen-induced eosinophil infiltration and bronchial hyperreactivity in the guinea pig airways. Immunol Lett. 1995;45:109–116. doi: 10.1016/0165-2478(94)00241-i. [DOI] [PubMed] [Google Scholar]
- 41.Leckie MJ, ten Brinke A, Khan J, Diamant Z, O'Connor BJ, Walls CM, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000;356:2144–2148. doi: 10.1016/s0140-6736(00)03496-6. [DOI] [PubMed] [Google Scholar]
- 42.Kips JC, O'Connor BJ, Langley SJ, Woodcock A, Kerstjens HA, Postma DS, et al. Effect of SCH55700, a humanized anti-human interleukin-5 antibody, in severe persistent asthma: a pilot study. Am J Respir Crit Care Med. 2003;167:1655–1659. doi: 10.1164/rccm.200206-525OC. [DOI] [PubMed] [Google Scholar]
- 43.Flood-Page P, Swenson C, Faiferman I, Matthews J, Williams M, Brannick L, et al. A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am J Respir Crit Care Med. 2007;176:1062–1071. doi: 10.1164/rccm.200701-085OC. [DOI] [PubMed] [Google Scholar]
- 44.Flood-Page P, Menzies-Gow A, Phipps S, Ying S, Wangoo A, Ludwig MS, et al. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003;112:1029–1036. doi: 10.1172/JCI17974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu LY, Sedgwick JB, Bates ME, Vrtis RF, Gern JE, Kita H, et al. Decreased expression of membrane IL-5 receptor alpha on human eosinophils: II. IL-5 down-modulates its receptor via a proteinase-mediated process. J Immunol. 2002;169:6459–6466. doi: 10.4049/jimmunol.169.11.6459. [DOI] [PubMed] [Google Scholar]
- 46.Liu LY, Sedgwick JB, Bates ME, Vrtis RF, Gern JE, Kita H, et al. Decreased expression of membrane IL-5 receptor alpha on human eosinophils: I. Loss of membrane IL-5 receptor alpha on airway eosinophils and increased soluble IL-5 receptor alpha in the airway after allergen challenge. J Immunol. 2002;169:6452–6458. doi: 10.4049/jimmunol.169.11.6452. [DOI] [PubMed] [Google Scholar]
- 47.Plotz SG, Simon HU, Darsow U, Simon D, Vassina E, Yousefi S, et al. Use of an antiinterleukin-5 antibody in the hypereosinophilic syndrome with eosinophilic dermatitis. N Engl J Med. 2003;349:2334–2339. doi: 10.1056/NEJMoa031261. [DOI] [PubMed] [Google Scholar]
- 48.Garrett JK, Jameson SC, Thomson B, Collins MH, Wagoner LE, Freese DK, et al. Antiinterleukin-5 (mepolizumab) therapy for hypereosinophilic syndromes. J Allergy Clin Immunol. 2004;113:115–119. doi: 10.1016/j.jaci.2003.10.049. [DOI] [PubMed] [Google Scholar]
- 49.Klion AD, Law MA, Noel P, Kim YJ, Haverty TP, Nutman TB. Safety and efficacy of the monoclonal anti-interleukin-5 antibody SCH55700 in the treatment of patients with hypereosinophilic syndrome. Blood. 2004;103:2939–2941. doi: 10.1182/blood-2003-10-3620. [DOI] [PubMed] [Google Scholar]
- 50.Stein ML, Collins MH, Villanueva JM, Kushner JP, Putnam PE, Buckmeier BK, et al. Anti-IL-5 (mepolizumab) therapy for eosinophilic esophagitis. J Allergy Clin Immunol. 2006;118:1312–1319. doi: 10.1016/j.jaci.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 51.Rothenberg ME, Klion AD, Roufosse FE, Kahn JE, Weller PF, Simon HU, et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N Engl J Med. 2008;358:1215–1228. doi: 10.1056/NEJMoa070812. [DOI] [PubMed] [Google Scholar]
- 52.Nair P, Pizzichini MMM, Kjarsgaard M, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360:985–993. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 53.Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–984. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wenzel SE. Eosinophils in Asthma—Closing the Loop or Opening the Door? N Engl J Med. 2009;360:1026–1028. doi: 10.1056/NEJMe0900334. [DOI] [PubMed] [Google Scholar]
- 55.Hershey GK. IL-13 receptors and signaling pathways: an evolving web. J Allergy Clin Immunol. 2003;111:677–690. doi: 10.1067/mai.2003.1333. [DOI] [PubMed] [Google Scholar]
- 56.Wills-Karp M. Interleukin-13 in asthma pathogenesis. Immunol Rev. 2004;202:175–190. doi: 10.1111/j.0105-2896.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- 57.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 58.Wynn TA. IL-13 effector functions. Annu Rev Immunol. 2003;21:425–456. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- 59.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang G, Volk A, Petley T, Emmell E, Giles-Komar J, Shang X, et al. Anti-IL-13 monoclonal antibody inhibits airway hyperresponsiveness, inflammation and airway remodeling. Cytokine. 2004;28:224–232. doi: 10.1016/j.cyto.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 61.Wenzel S, Wilbraham D, Fuller R, Getz EB, Longphre M. Effect of an interleukin-4 variant on late phase asthmatic response to allergen challenge in asthmatic patients: results of two phase 2a studies. Lancet. 2007;370:1422–1431. doi: 10.1016/S0140-6736(07)61600-6. [DOI] [PubMed] [Google Scholar]
- 62.Bryan SA, O'Connor BJ, Matti S, Leckie MJ, Kanabar V, Khan J, et al. Effects of recombinant human interleukin-12 on eosinophils, airway hyper-responsiveness and the late asthmatic response. Lancet. 2000;356:2149–2153. doi: 10.1016/S0140-6736(00)03497-8. [DOI] [PubMed] [Google Scholar]
- 63.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 64.Mills KH. Induction, function and regulation of IL-17-producing T cells. Eur J Immunol. 2008;38:2636–2649. doi: 10.1002/eji.200838535. [DOI] [PubMed] [Google Scholar]
- 65.Wang YH, Liu YJ. The IL-17 cytokine family and their role in allergic inflammation. Curr Opin Immunol. 2008 doi: 10.1016/j.coi.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008;181:4089–4097. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ballantyne SJ, Barlow JL, Jolin HE, Nath P, Williams AS, Chung KF, et al. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J Allergy Clin Immunol. 2007;120:1324–1331. doi: 10.1016/j.jaci.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 68.Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLPDC-activated Th2 memory cells. J Exp Med. 2007;204:1837–1847. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fahy JV, Fleming HE, Wong HH, Liu JT, Su JQ, Reimann J, et al. The effect of an anti-IgE monoclonal antibody on the early- and late-phase responses to allergen inhalation in asthmatic subjects. Am J Respir Crit Care Med. 1997;155:1828–1834. doi: 10.1164/ajrccm.155.6.9196082. [DOI] [PubMed] [Google Scholar]
- 70.Djukanovic R, Wilson SJ, Kraft M, Jarjour NN, Steel M, Chung KF, et al. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am J Respir Crit Care Med. 2004;170:583–593. doi: 10.1164/rccm.200312-1651OC. [DOI] [PubMed] [Google Scholar]
- 71.Busse W, Corren J, Lanier BQ, McAlary M, Fowler-Taylor A, Cioppa GD, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108:184–190. doi: 10.1067/mai.2001.117880. [DOI] [PubMed] [Google Scholar]
- 72.Soler M, Matz J, Townley R, Buhl R, O'Brien J, Fox H, et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J. 2001;18:254–261. doi: 10.1183/09031936.01.00092101. [DOI] [PubMed] [Google Scholar]
- 73.Bousquet J, Cabrera P, Berkman N, Buhl R, Holgate S, Wenzel S, et al. The effect of treatment with omalizumab, an anti-IgE antibody, on asthma exacerbations and emergency medical visits in patients with severe persistent asthma. Allergy. 2005;60:302–308. doi: 10.1111/j.1398-9995.2004.00770.x. [DOI] [PubMed] [Google Scholar]
- 74.Corren J, Casale T, Deniz Y, Ashby M. Omalizumab, a recombinant humanized anti-IgE antibody, reduces asthma-related emergency room visits and hospitalizations in patients with allergic asthma. J Allergy Clin Immunol. 2003;111:87–90. doi: 10.1067/mai.2003.49. [DOI] [PubMed] [Google Scholar]
- 75.Okubo K, Nagakura T. Anti-IgE antibody therapy for Japanese cedar pollinosis: omalizumab update. Allergol Int. 2008;57:205–209. doi: 10.2332/allergolint.R-08-164. [DOI] [PubMed] [Google Scholar]
- 76.Verbruggen K, Van Cauwenberge P, Bachert C. Anti-IgE for the treatment of allergic rhinitis—and eventually nasal polyps? Int Arch Allergy Immunol. 2009;148:87–98. doi: 10.1159/000155739. [DOI] [PubMed] [Google Scholar]
- 77.Stock P, Rolinck-Werninghaus C, Wahn U, Hamelmann E. The role of anti-IgE therapy in combination with allergen specific immunotherapy for seasonal allergic rhinitis. BioDrugs. 2007;21:403–410. doi: 10.2165/00063030-200721060-00007. [DOI] [PubMed] [Google Scholar]
- 78.Kopp MV, Hamelmann E, Zielen S, Kamin W, Bergmann KC, Sieder C, et al. Combination of omalizumab and specific immunotherapy is superior to immunotherapy in patients with seasonal allergic rhinoconjunctivitis and co-morbid seasonal allergic asthma. Clin Exp Allergy. 2009;39:271–279. doi: 10.1111/j.1365-2222.2008.03121.x. [DOI] [PubMed] [Google Scholar]
- 79.Spector SL, Tan RA. Therapeutic alternatives for chronic urticaria: additional reports on omalizumab. Ann Allergy Asthma Immunol. 2008;101:647. doi: 10.1016/S1081-1206(10)60232-3. [DOI] [PubMed] [Google Scholar]
- 80.Kaplan AP, Joseph K, Maykut RJ, Geba GP, Zeldin RK. Treatment of chronic autoimmune urticaria with omalizumab. J Allergy Clin Immunol. 2008;122:569–573. doi: 10.1016/j.jaci.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 81.Godse KV. Omalizumab in severe chronic urticaria. Indian J Dermatol Venereol Leprol. 2008;74:157–158. doi: 10.4103/0378-6323.39708. [DOI] [PubMed] [Google Scholar]
- 82.Spector SL, Tan RA. Omalizumab also successful in chronic urticaria. J Allergy Clin Immunol. 2008;121:784–785. doi: 10.1016/j.jaci.2007.12.1174. [DOI] [PubMed] [Google Scholar]
- 83.Andres C, Belloni B, Mempel M, Ring J. Omalizumab for patients with severe and therapy-refractory atopic eczema? Curr Allergy Asthma Rep. 2008;8:179–180. doi: 10.1007/s11882-008-0029-3. [DOI] [PubMed] [Google Scholar]
- 84.Sheinkopf LE, Rafi AW, Do LT, Katz RM, Klaustermeyer WB. Efficacy of omalizumab in the treatment of atopic dermatitis: a pilot study. Allergy Asthma Proc. 2008;29:530–537. doi: 10.2500/aap.2008.29.3160. [DOI] [PubMed] [Google Scholar]
- 85.Kanu A, Patel K. Treatment of allergic bronchopulmonary aspergillosis (ABPA) in CF with anti-IgE antibody (omalizumab) Pediatr Pulmonol. 2008;43:1249–1251. doi: 10.1002/ppul.20907. [DOI] [PubMed] [Google Scholar]
- 86.Zirbes JM, Milla CE. Steroid-sparing effect of omalizumab for allergic bronchopulmonary aspergillosis and cystic fibrosis. Pediatr Pulmonol. 2008;43:607–610. doi: 10.1002/ppul.20804. [DOI] [PubMed] [Google Scholar]
- 87.Giavina-Bianchi P, Giavina-Bianchi M, Agondi R, Kalil J. Omalizumab and Churg-Strauss syndrome. J Allergy Clin Immunol. 2008;122:217–218. doi: 10.1016/j.jaci.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 88.Pabst S, Tiyerili V, Grohe C. Apparent response to anti-IgE therapy in two patients with refractory “forme fruste” of Churg-Strauss syndrome. Thorax. 2008;63:747–748. doi: 10.1136/thx.2006.076513. [DOI] [PubMed] [Google Scholar]
- 89.Leynadier F, Doudou O, Gaouar H, Le Gros V, Bourdeix I, Guyomarch-Cocco L, et al. Effect of omalizumab in health care workers with occupational latex allergy. J Allergy Clin Immunol. 2004;113:360–361. doi: 10.1016/j.jaci.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 90.Poole JA, Meng J, Reff M, Spellman MC, Rosenwasser LJ. Anti-CD23 monoclonal antibody, lumiliximab, inhibited allergen-induced responses in antigen-presenting cells and T cells from atopic subjects. J Allergy Clin Immunol. 2005;116:780–788. doi: 10.1016/j.jaci.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 91.Brightling C, Berry M, Amrani Y. Targeting TNFalpha: a novel therapeutic approach for asthma. J Allergy Clin Immunol. 2008;121:5–10. doi: 10.1016/j.jaci.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Howarth PH, Babu KS, Arshad HS, Lau L, Buckley M, McConnell W, et al. Tumour necrosis factor (TNFalpha) as a novel therapeutic target in symptomatic corticosteroid dependent asthma. Thorax. 2005;60:1012–1018. doi: 10.1136/thx.2005.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thomas PS, Heywood G. Effects of inhaled tumour necrosis factor alpha in subjects with mild asthma. Thorax. 2002;57:774–778. doi: 10.1136/thorax.57.9.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Silvestri M, Bontempelli M, Giacomelli M, Malerba M, Rossi GA, Di Stefano A, et al. High serum levels of tumour necrosis factor-alpha and interleukin-8 in severe asthma: markers of systemic inflammation? Clin Exp Allergy. 2006;36:1373–1381. doi: 10.1111/j.1365-2222.2006.02502.x. [DOI] [PubMed] [Google Scholar]
- 95.Berry MA, Hargadon B, Shelley M, Parker D, Shaw DE, Green RH, et al. Evidence of a role of tumor necrosis factor alpha in refractory asthma. N Engl J Med. 2006;354:697–708. doi: 10.1056/NEJMoa050580. [DOI] [PubMed] [Google Scholar]
- 96.Morjaria J, Chauhan A, Bobu K, Mehta R, Smith S, North M. Assessment of a soluble TNFalpha fusion protein (etanercept) as a novel therapeutic agent for severe refractory asthma. Proc Am Thorac Soc. 2006;3:16. [Google Scholar]
- 97.Morjaria JB, Chauhan AJ, Babu KS, Polosa R, Davies DE, Holgate ST. The role of a soluble TNFalpha receptor fusion protein (etanercept) in corticosteroid refractory asthma: a double blind, randomised, placebo controlled trial. Thorax. 2008;63:584–591. doi: 10.1136/thx.2007.086314. [DOI] [PubMed] [Google Scholar]
- 98.Erin EM, Leaker BR, Nicholson GC, Tan AJ, Green LM, Neighbour H, et al. The effects of a monoclonal antibody directed against tumor necrosis factor-alpha in asthma. Am J Respir Crit Care Med. 2006;174:753–762. doi: 10.1164/rccm.200601-072OC. [DOI] [PubMed] [Google Scholar]
- 99.Gauvreau GM, Becker AB, Boulet LP, Chakir J, Fick RB, Greene WL, et al. The effects of an anti-CD11a mAb, efalizumab, on allergen-induced airway responses and airway inflammation in subjects with atopic asthma. J Allergy Clin Immunol. 2003;112:331–338. doi: 10.1067/mai.2003.1689. [DOI] [PubMed] [Google Scholar]